Indoline derivative as well as preparation method and application thereof
A technology of indoline and compounds, applied in the field of medicinal chemistry, to achieve the effects of easy purification, reduction of reaction steps, and cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
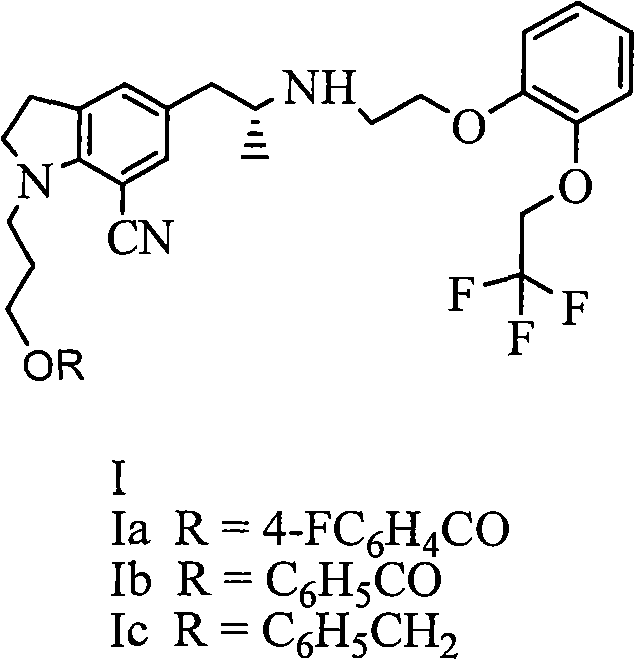
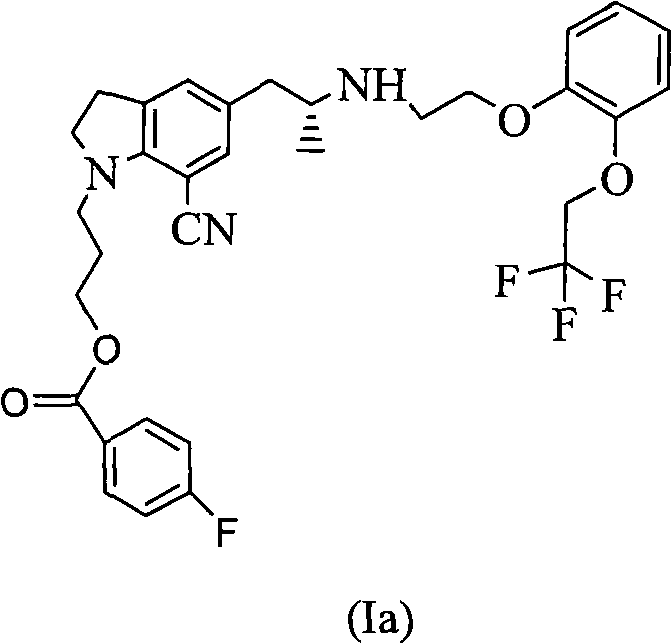
[0029] Embodiment 1: the preparation of compound (Ia)
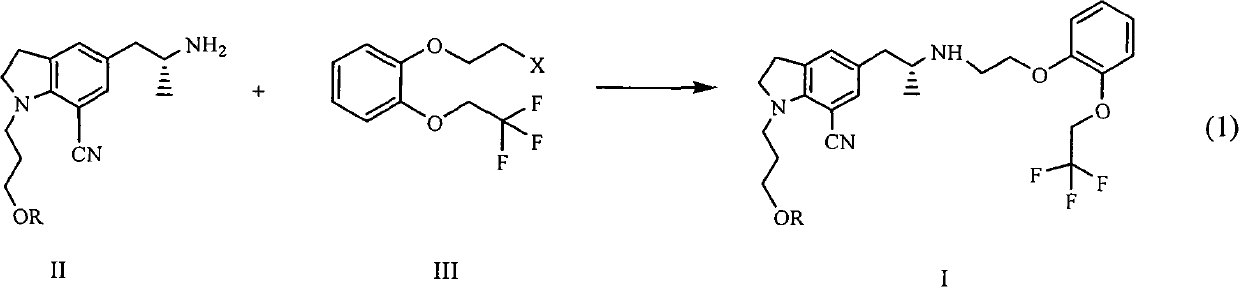
[0030] With reference to reaction formula (1), 20 grams of tartrate salt of compound II (R=4-fluorobenzoyl), 10 grams of potassium carbonate, 1 gram of tetrabutylammonium bromide, and 0.5 grams of potassium iodide are placed in a reaction flask, and 120ml of water was heated to 80°C, 14g of compound III (X=Br) was added dropwise, and the reaction was continued at 80°C for 6 hours. Extract with ethyl acetate, wash with sodium bicarbonate, wash with saturated brine, dry over magnesium sulfate, and concentrate. The oil was dissolved in isopropanol, 4.2 g of oxalic acid dihydrate was added, and a solid was precipitated to obtain 22 g of a white solid.
[0031] Mp 135-137°C
[0032] 1 NMR spectrum (DMSO-d6): δppm 1.1-1.2 (3H, d), 2.0-2.1 (2H, m), 2.5-2.6 (1H, dd), 2.8-2.9 (2H, t), 2.96-3.0 (1H , dd), 3.3-3.5 (3H, m), 3.5-3.7 (4H, m), 4.2-4.3 (2H, t), 4.3-4.4 (2H, t), 4.6-4.7 (2H, m), 4.8 -5.2 (1H, broad peak), 6.9-7.15 (6...
Embodiment 2
[0033] Embodiment 2: the preparation of silodosin
[0034] 8 g of compound (I a), dissolved in 100 ml of DMSO, added 12 ml of 5 mol / L NaOH, slowly added 30% H 2 o 2 7 g, and then at 30° C., the reaction was completed in 4 hours. Extract with ethyl acetate, combine the organic layers, wash the organic layer with 2N HCl, neutralize the obtained aqueous layer with sodium hydroxide, extract with ethyl acetate, wash with saturated sodium bicarbonate, dry over anhydrous sodium sulfate, and reduce pressure Concentrate, then dissolve with ethyl acetate, cool and crystallize, filter, and dry 5 g (87%), the purity is >99%.
[0035] Mp105~108℃
[0036] [α] 20 D =-16.2 C=1, MeOH
[0037] 1 NMR spectrum (DMSO-d6): δppm 0.9-1.0 (3H, d), 1.5-1.6 (1H, s), 1.6-1.7 (2H, m), 2.3-2.4 (1H, dd), 2.6-2.7 (1H ,dd), 2.8-3.0(5H,m), 3.1-3.2(2H,m), 3.3-3.4(2H,m), 3.4-3.5(2H,t), 4.0-4.1(2H,t), 4.2 -4.3(1H, s), 4.6-4.8(2H, t), 6.9-7.15(6H, m), 7.2-7.3(1H, s), 7.5-7.6(1H, s)
Embodiment 3
[0038] Embodiment 3: the preparation of compound (Ia)
[0039] With reference to reaction formula (1), 20 grams of tartrate salt of compound II (R=4-fluorobenzoyl), 10 grams of potassium carbonate, 1 gram of tetrabutylammonium bromide, and 0.5 grams of potassium iodide are placed in a reaction flask, and 120ml of water was heated to 80°C, 16g of compound III (X=OMs) was added dropwise, and the mixture was reacted at 80°C for another 6 hours after dropping. Extract with ethyl acetate, wash with sodium bicarbonate, wash with saturated brine, dry over magnesium sulfate, and concentrate. The oil was dissolved in isopropanol, 4.2 g of oxalic acid dihydrate was added, and a solid was precipitated to obtain 20 g of a white solid.
[0040] Mp 133-135°C
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com