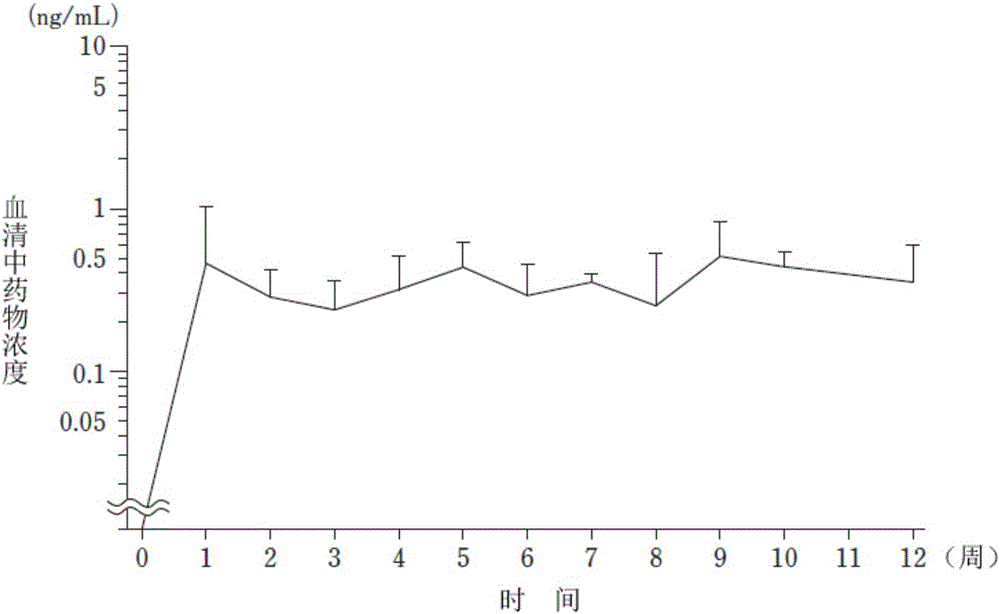
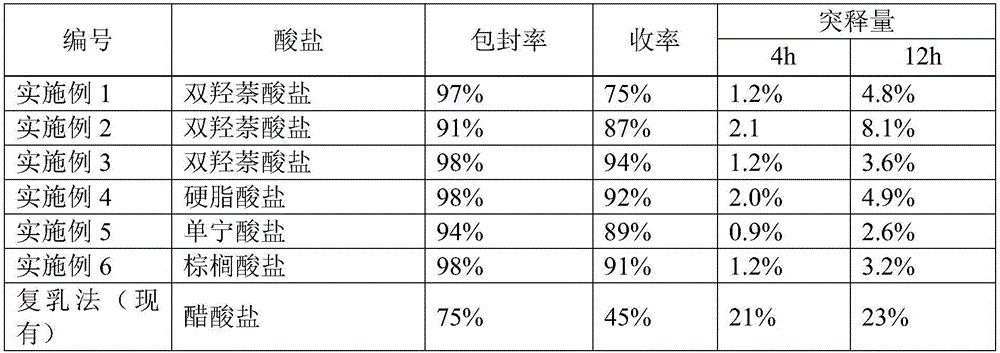
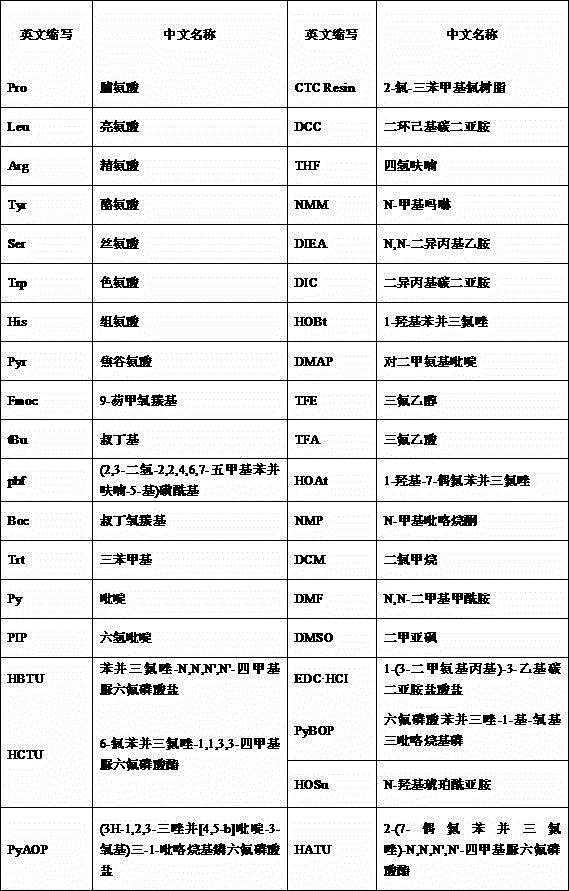
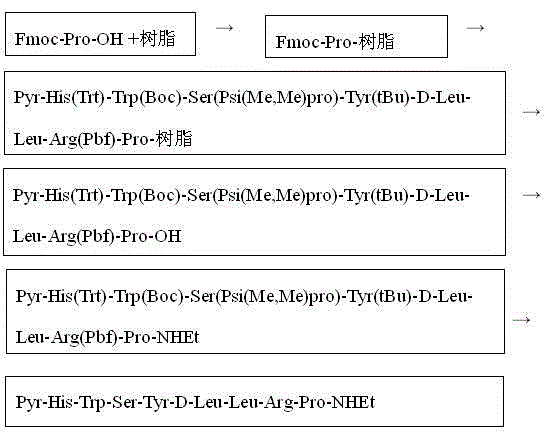
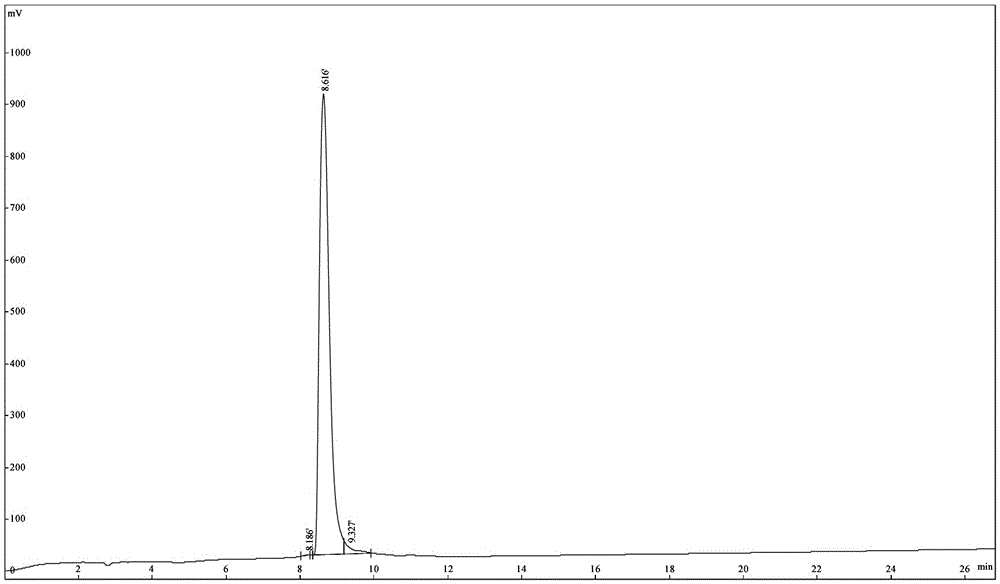
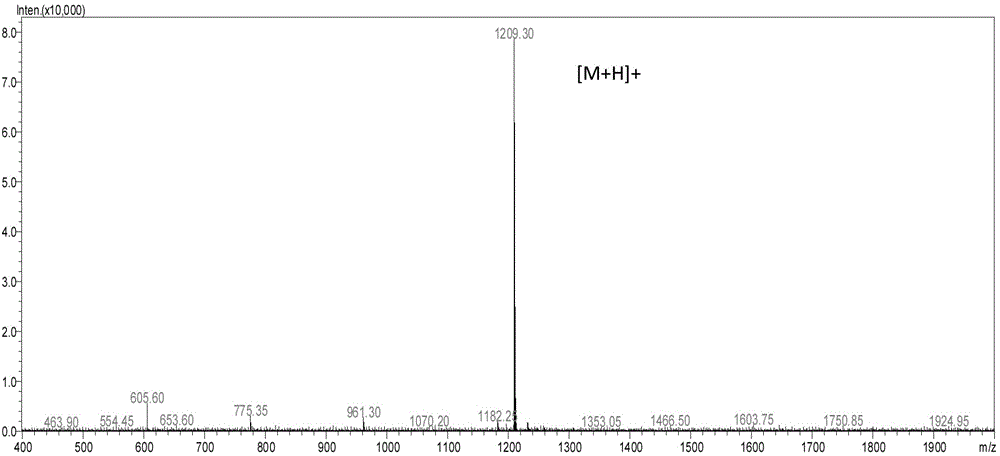
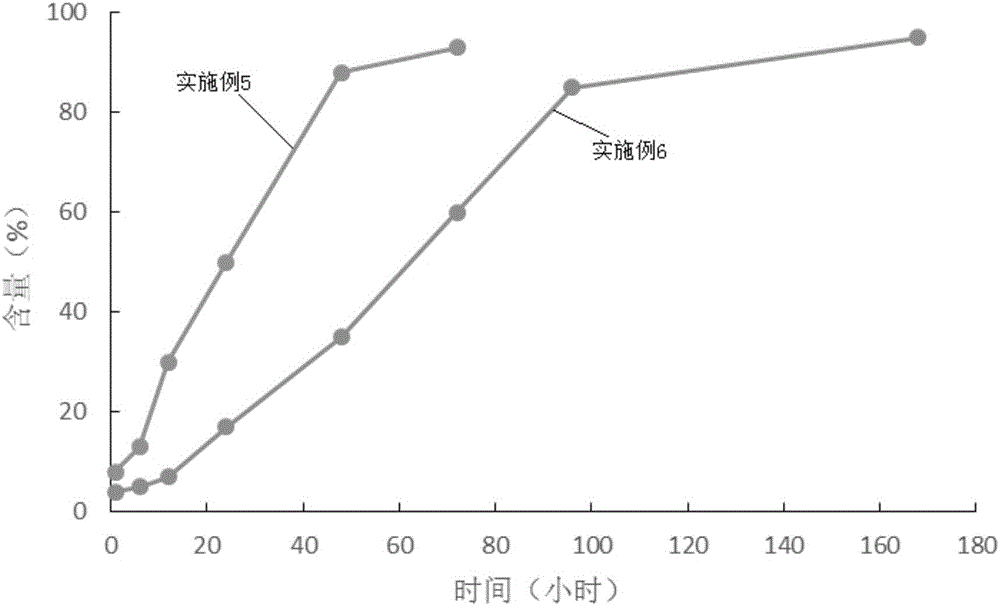
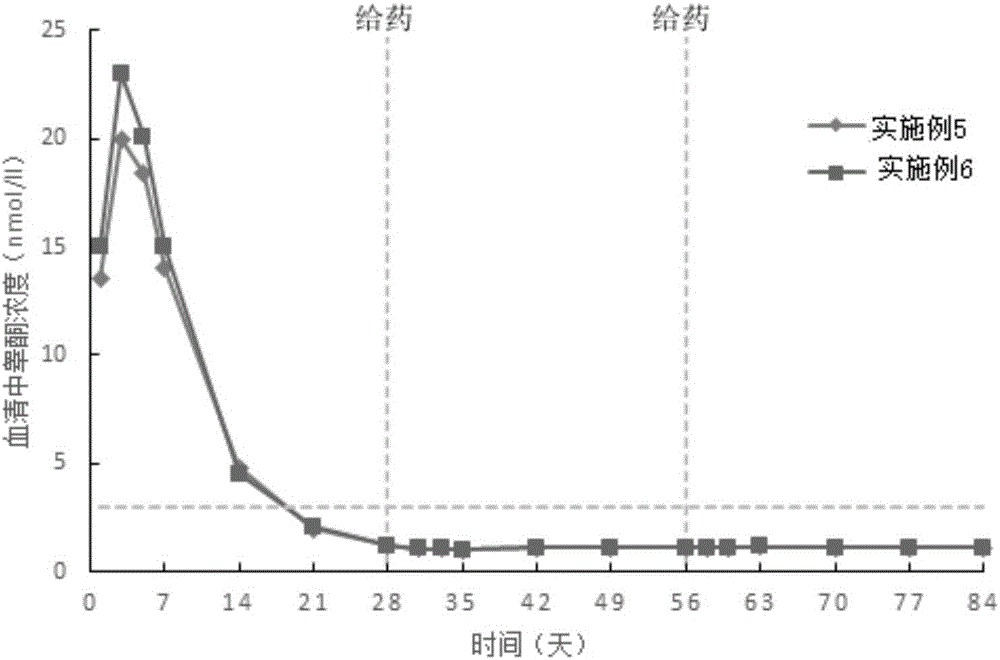
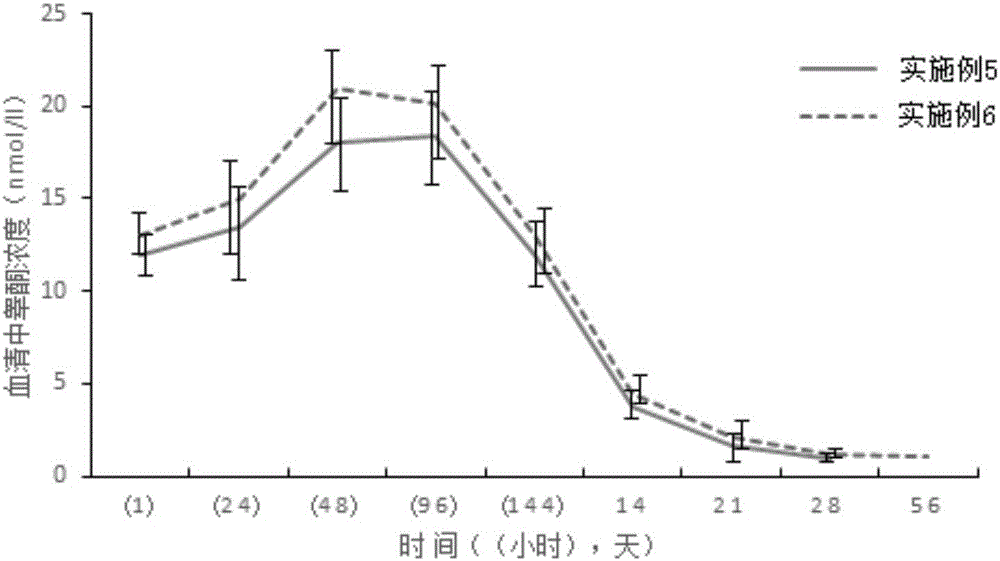
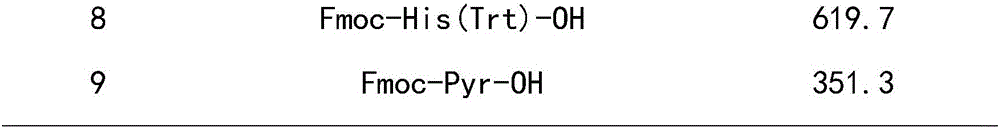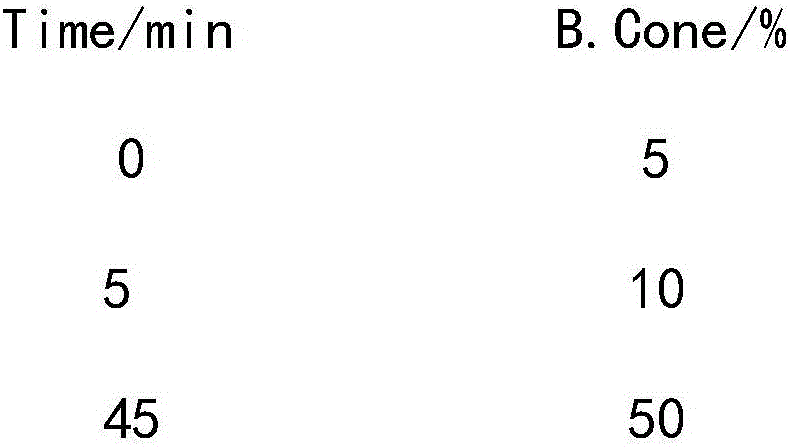Patents
Literature
66 results about "Leuprorelin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
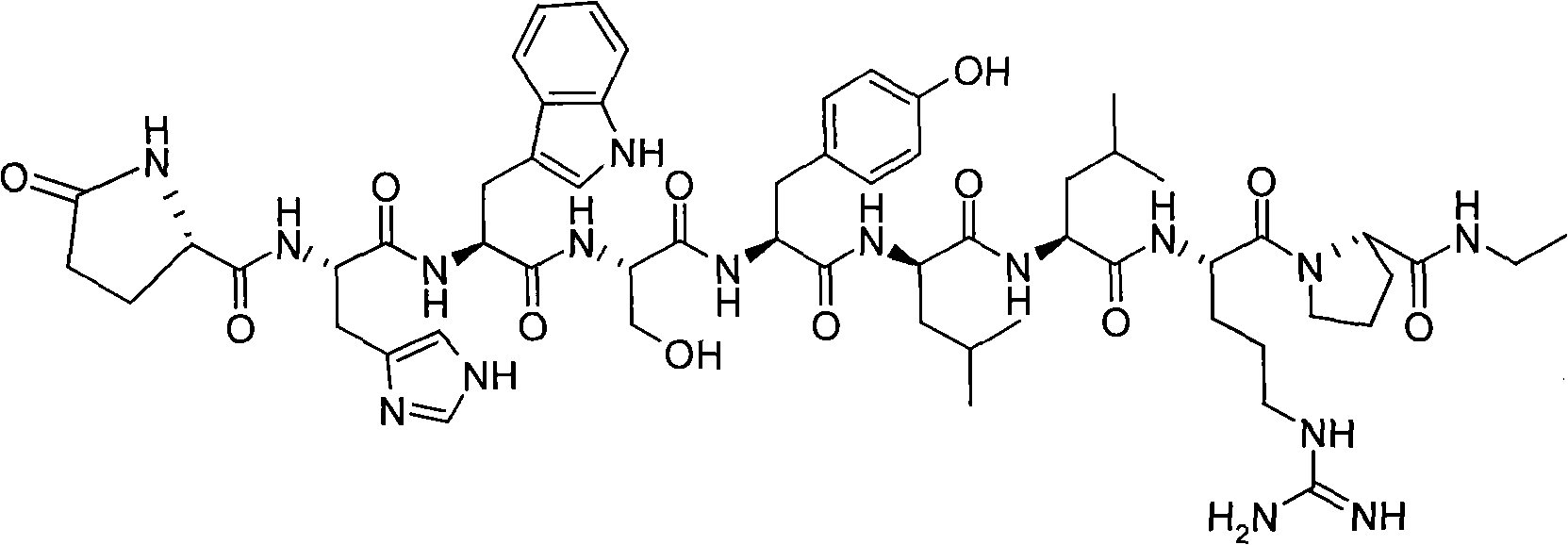
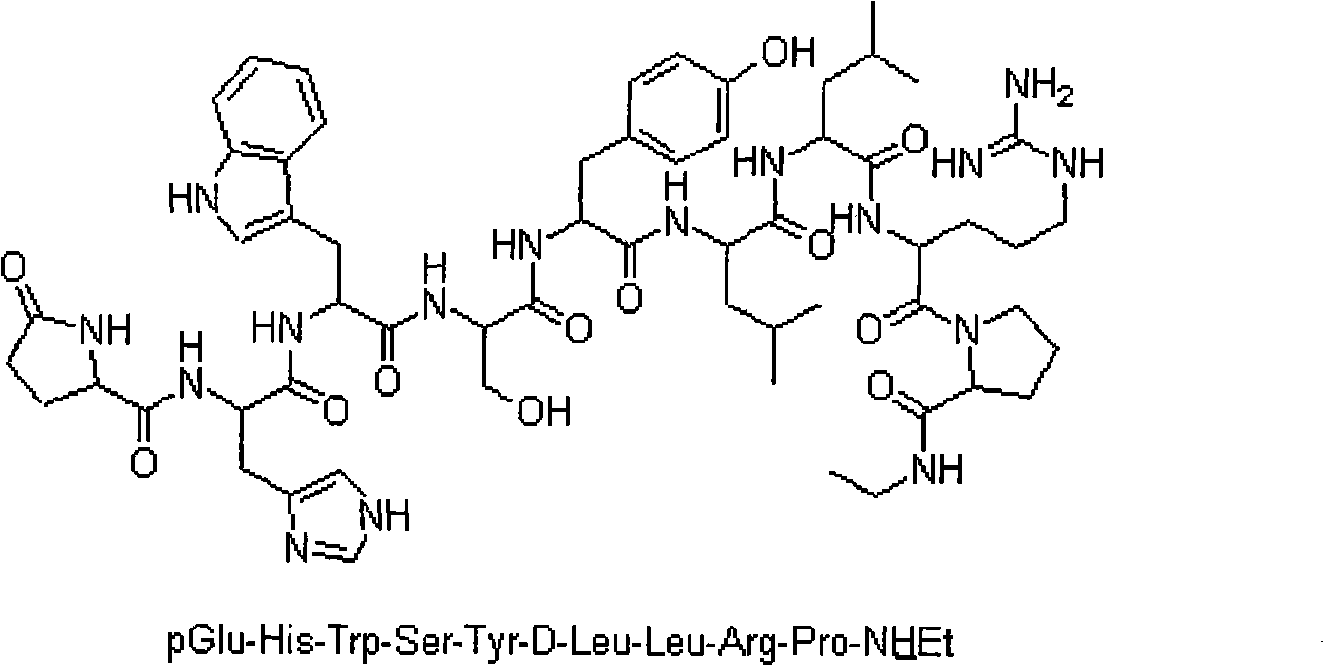
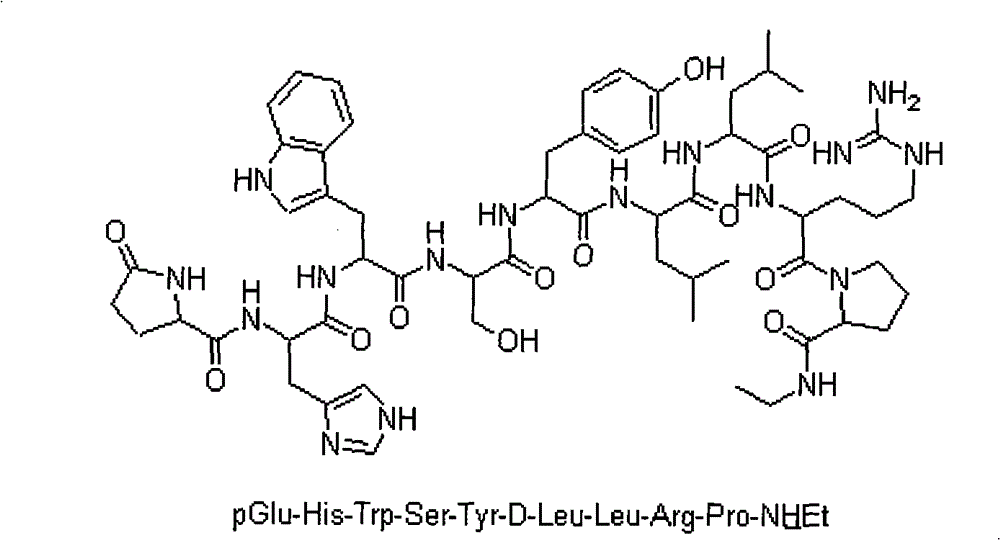
Leuprolide is used to treat advanced prostate cancer in men.
Therapy of Prostate Cancer With Ctla-4 Antibodies and Hormonal Therapy
InactiveUS20080279865A1Peptide/protein ingredientsAntibody ingredientsHistrelinAntiendomysial antibodies
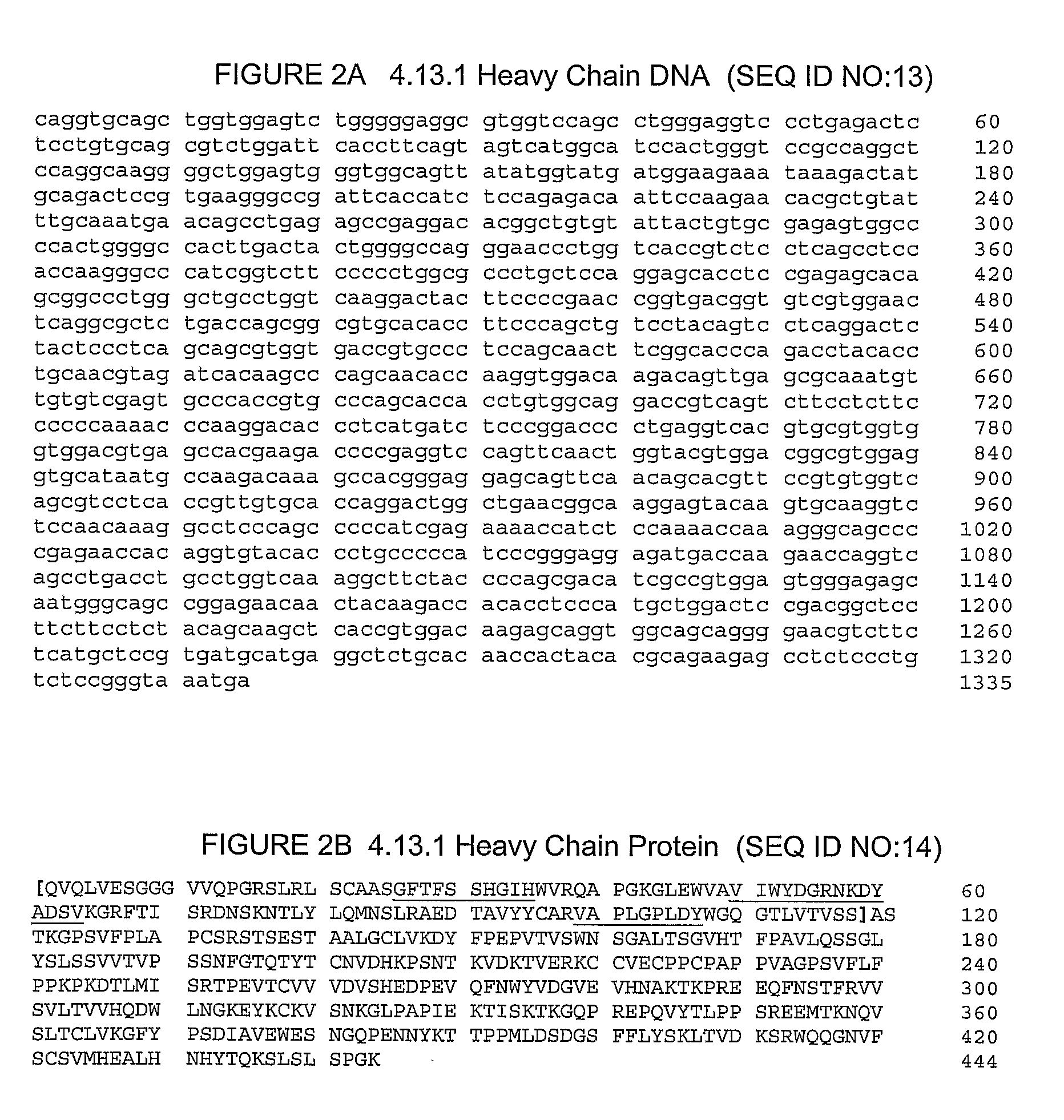
The invention relates to methods for treating prostate cancer comprising administration of an anti-CTLA4 antibody, or an antigen-binding portion thereof, particularly a human antibody to human CTLA4, e.g., antibody 3.1.1, 4.1.1, 4.8.1, 4.10.2, 4.13.1, 4.14.3, 6.1.1, ticilimumab (also known as 11.2.1), 11.6.1, 11.7.1, 12.3.1.1, 12.9.1.1, and ipilimumab (also known as MDX-010 and 10D1), in combination with hormonal therapy. Hormonal therapy agents include, inter alia, an anti-androgen (e.g., megestrol, cyproterone, flutamide, nilutamide, and bicalutamide), a GnRH antagonist (e.g., abarelix and histrelin), and a LH-RH agonist (e.g., leuprolide, goserelin, and buserelin). The invention relates to neoadjuvant therapy, adjuvant therapy, therapy for rising PSA, first-line therapy, second-line therapy, and third-line therapy of prostate cancer, whether localized or metastasized.
Owner:PFIZER PFIZER PRODS
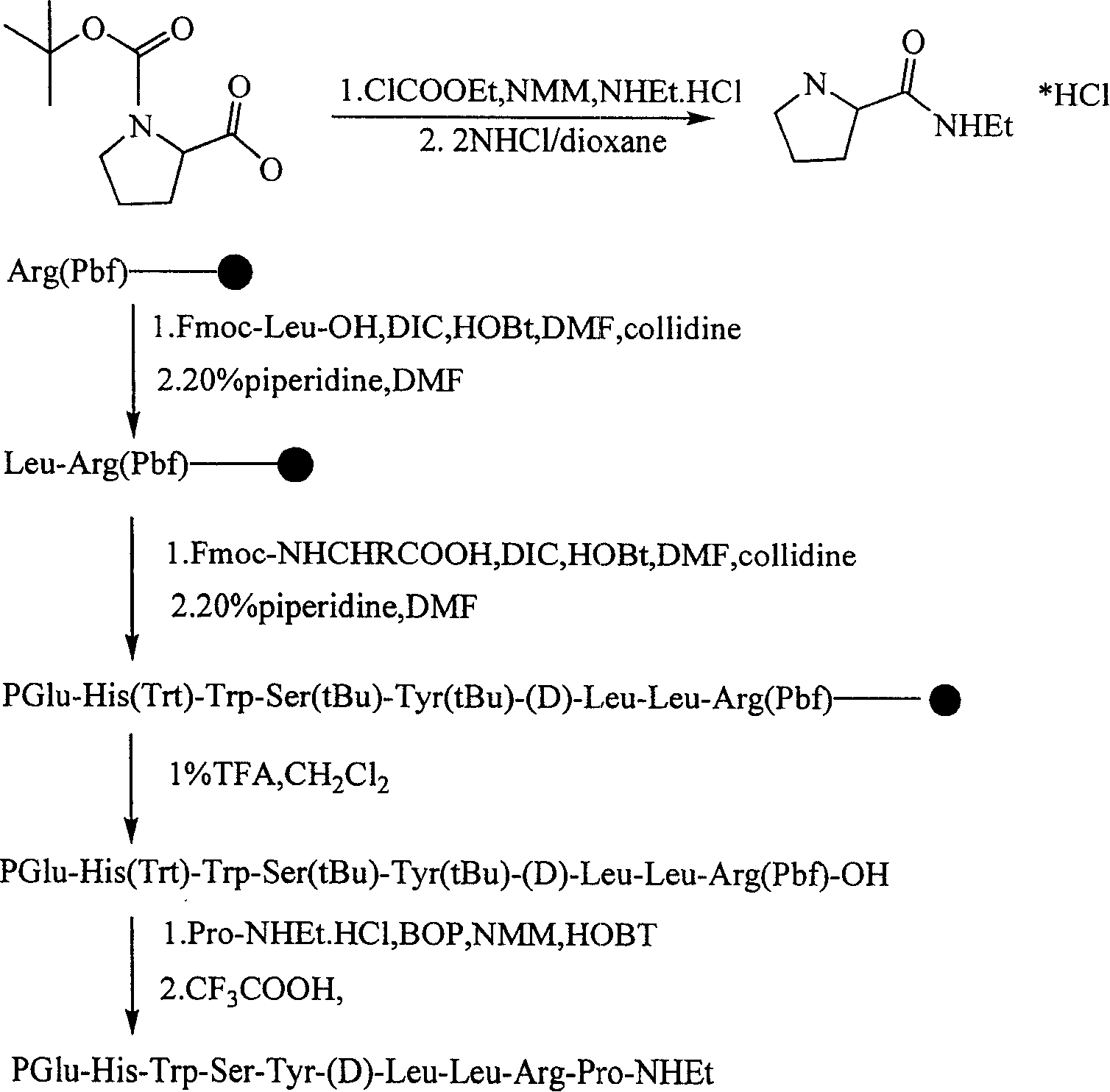
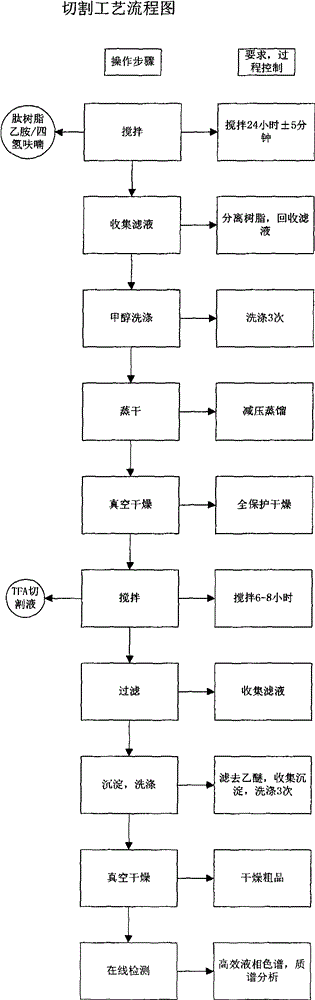
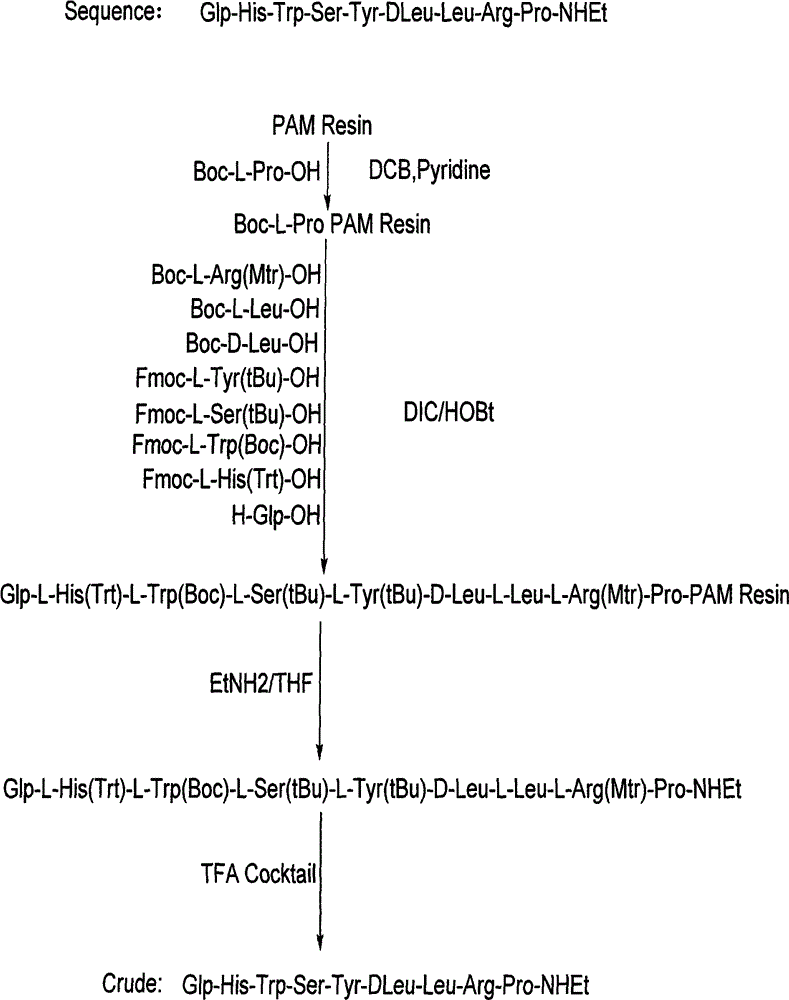
Solid phase polypeptide synthesis preparation method for leuprorelin
ActiveCN1865280AConvenient sourceReduce usagePeptide preparation methodsBulk chemical productionHydrogen fluorideLeuprorelin
The invention discloses a bright-ala-ruilin preparing method of solid-phase polypeptide, which comprises the following steps: adopting Wang resin or CTC resin as original material to connect amino with protective group to produce protective nonapeptide resin; removing Fmoc-protective group sequently; proceeding side-chain protective group synchronizingly and cutting peptide; connecting ethylamine through ethylamine-to-HOBT to produce crude product; proceeding separation and purifying through C18 (or C8) pillar to produce fine bright-ala-ruilin. The invention avoids the utility of poisonous agent, which improves the purifying, peptide connecting and obtaining rate.
Owner:SHANGHAI SOHO YIMING PHARMA
Novel sustained release polymer
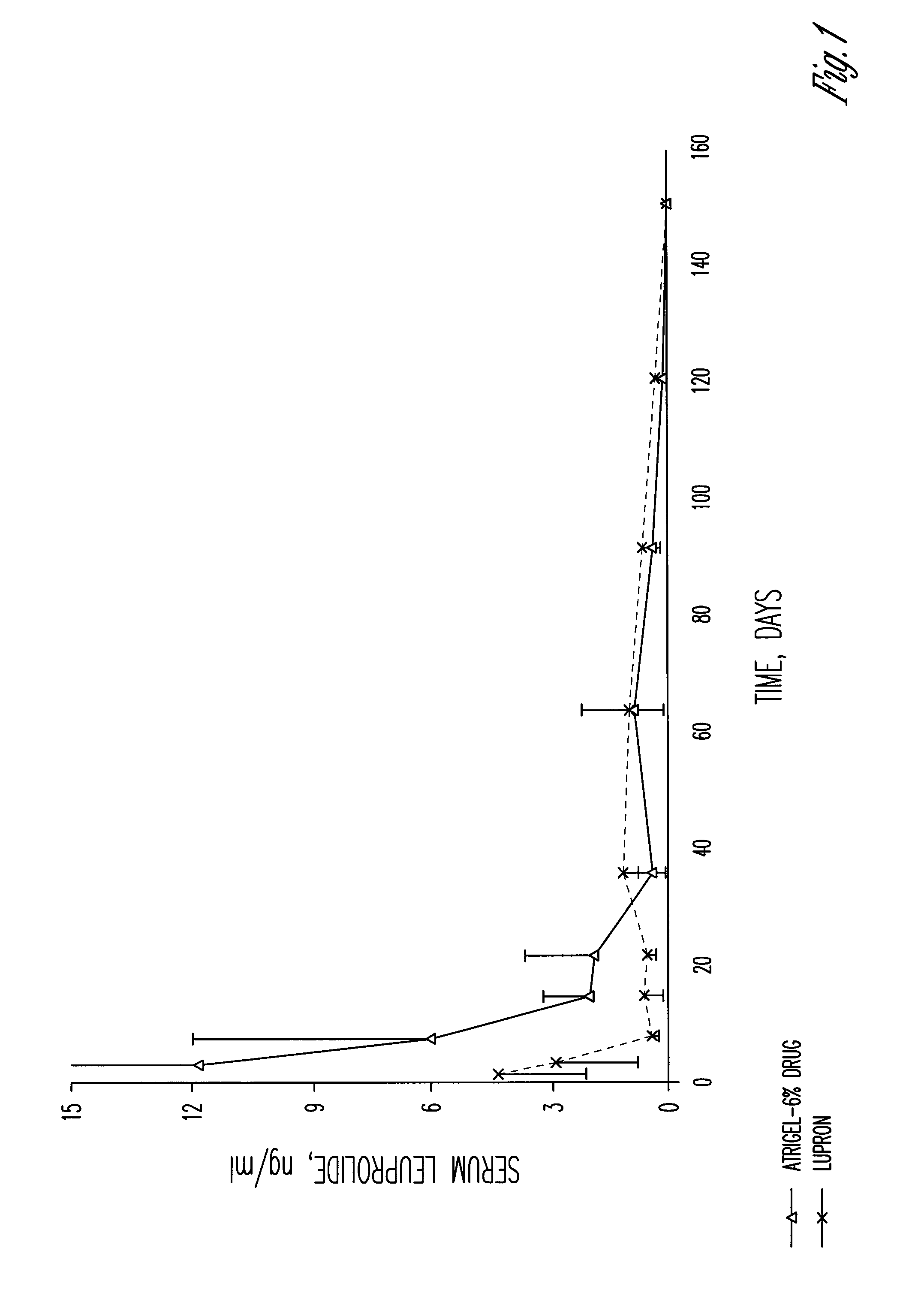
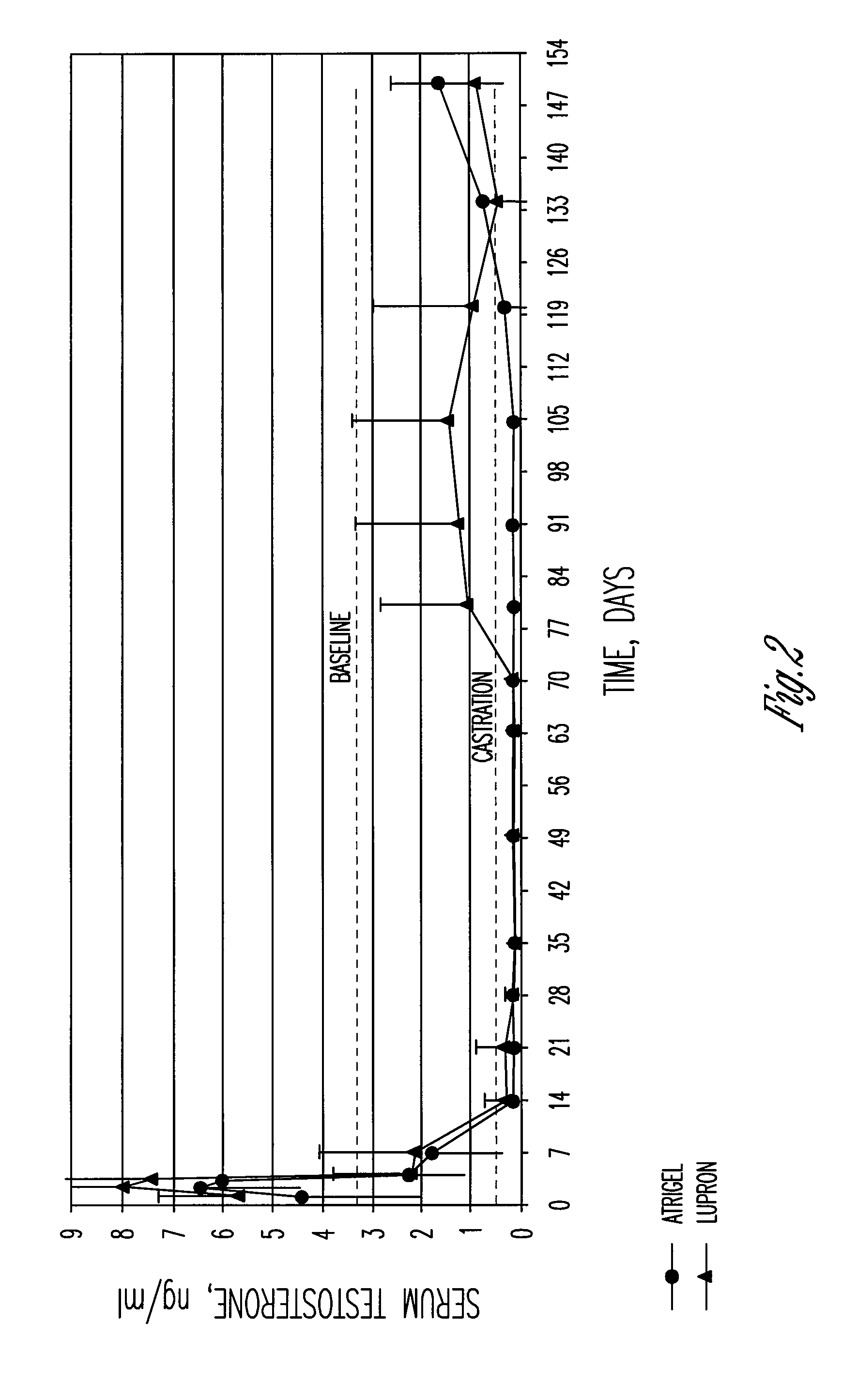
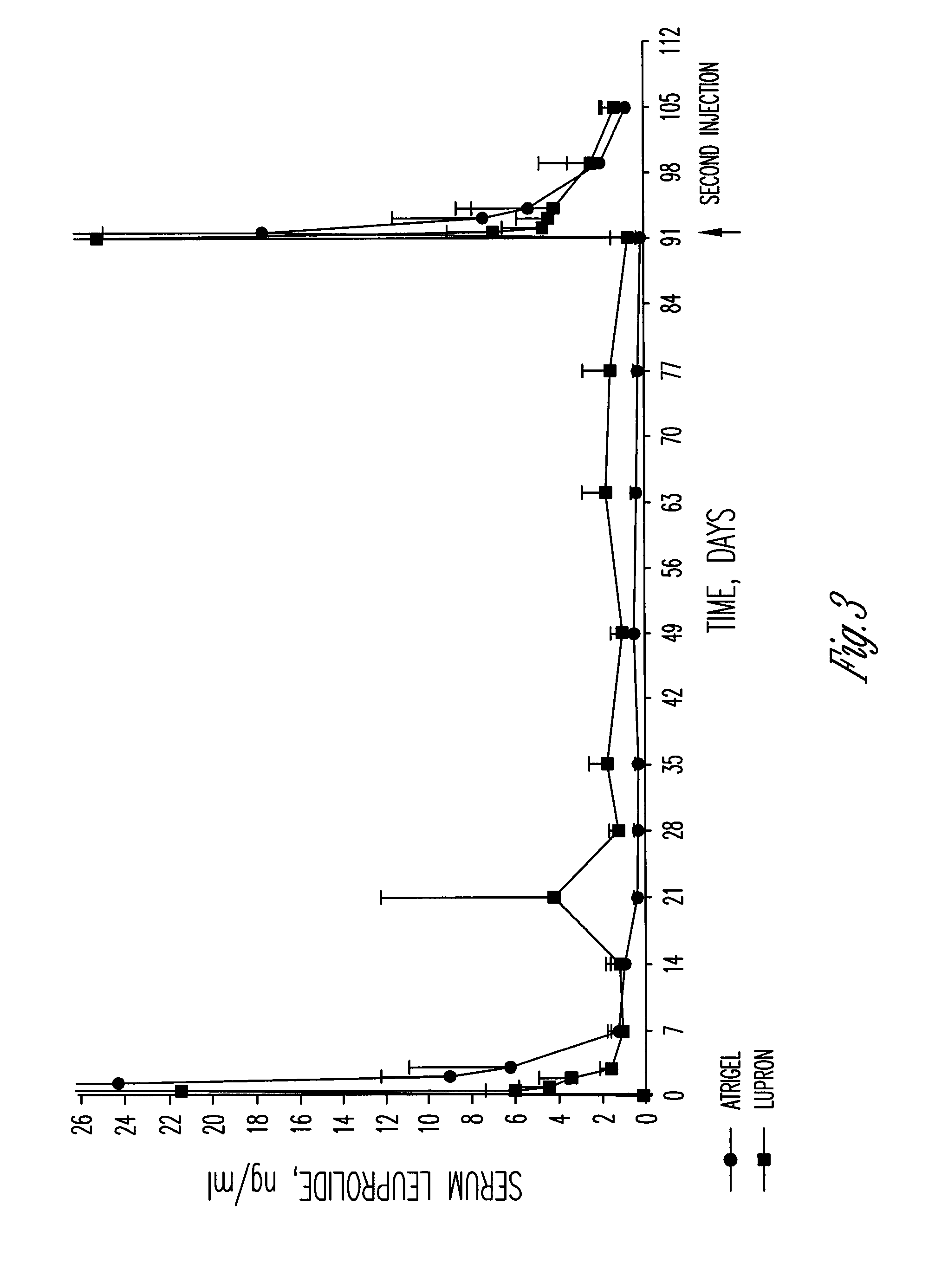
A polymer and a method for its preparation are provided. The polymer comprises poly(lactide), poly(lactide / glycolide) or poly(lactic acid / glycolic acid) segments bonded by ester linkages to both ends of an alkanediol core unit. The polymer is for use in a controlled release formulation for a medicament, preferably leuprolide acetate. The controlled release formulation is administered to a patient as a subcutaneous depot of a flowable composition comprising the polymer, a biocompatible solvent, and the medicament. Controlled release formulations comprising the polymer release leuprolide for treatment of prostate cancer patients over periods of 3-6 months.
Owner:TOLMAR INC
Solid phase synthesis method of leuprorelin
InactiveCN101407540AAvoid the process of alkaline aminolysisHigh yieldPeptide preparation methodsBulk chemical productionLeuprorelinFreeze-drying
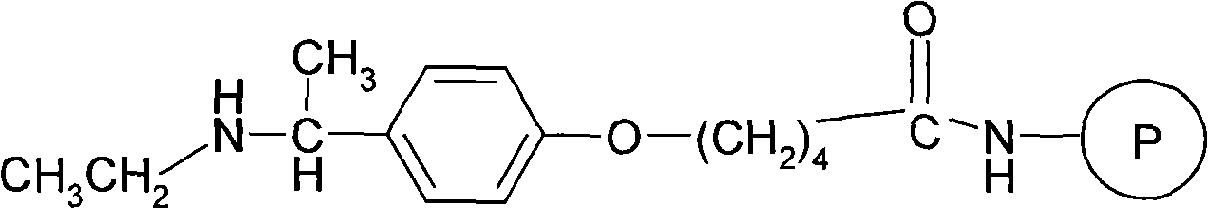
The invention relates to a solid phase synthesis method of leuprorelin, which uses 4-((1-N-Alkylamino)ethyl) phenoxy-butyramide resin as a solid phase carrier to carry out procedure reaction, and peptide whole protecting resin can be obtained by sequentially carrying out condensation reaction to connect protecting amino-acid; crude peptide can be obtained by cutting the resin by trifluoroacetic acid, and the crude peptide is dissolved in an acetic acid water solution of 5 percent, and the leuprorelin can be obtained by purifying, leaching, condensing and freeze drying the opposite phase high performance liquid chromatograph columns of C18 fillers. The invention uses a novel solid phase resin, and products decorated by peptide chain carbon end alkyl can be obtained directly after finishing assembling the peptide chain by the solid phase synthesis technology and using acid hydrolysis to cut the peptide from the resin after, and the solid phase synthesis method of the leuprorelin is convenient in operation, reduces synthesis steps for synthesizing the leuprorelin greatly and improves the yield.
Owner:ADLAI NORTYE BIOPHARMA CO LTD
Leuprolide acetate preparing method
InactiveCN105622726AIncrease lossReduce lossLuteinising hormone-releasing hormonePeptide preparation methodsLeuprorelinFiltration
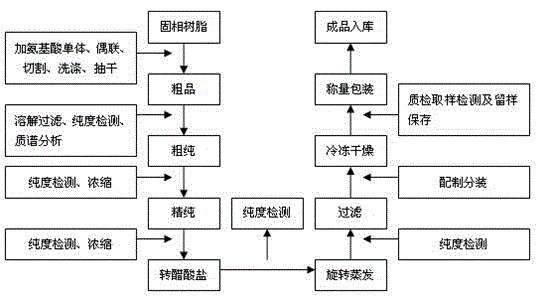
The invention relates to a leuprolide acetate preparing method to mainly solve the technical problems of existing leuprolide acetate preparing methods that the production process is complicated, the period is long, the cost is high, the yield is low, and salt conversion is difficult.According to the technical scheme, a leuprorelin crude product is obtained through solid-phase synthesis, the leuprorelin crude product is dissolved, filtered and detected, and then coarse purification, fine purification, concentration, salt conversion, recondensation and filtration are conducted on the leuprorelin crude product by means of an invert high liquid-phase chromatographic system, and finally freeze drying is conducted to obtain a white flocculent powdery product.The purification preparing method is suitable for laboratory research and development as well as large-scale industrial production.
Owner:GL BIOCHEM SHANGHAI +1
Method for preparing leuprorelin acetate, product and application
ActiveCN102464702AAvoid pollutionImprove securityPeptide/protein ingredientsLuteinising hormone-releasing hormoneSolid phasesChemistry
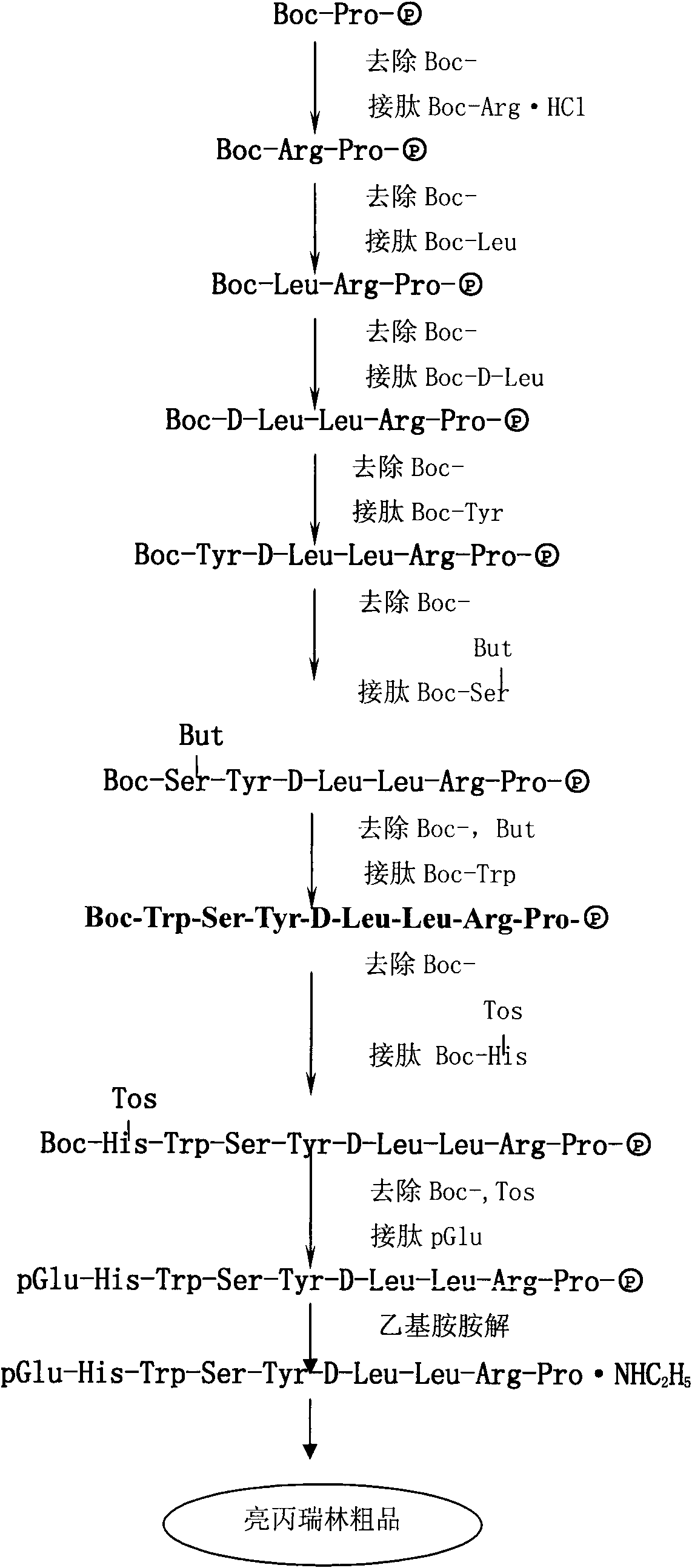
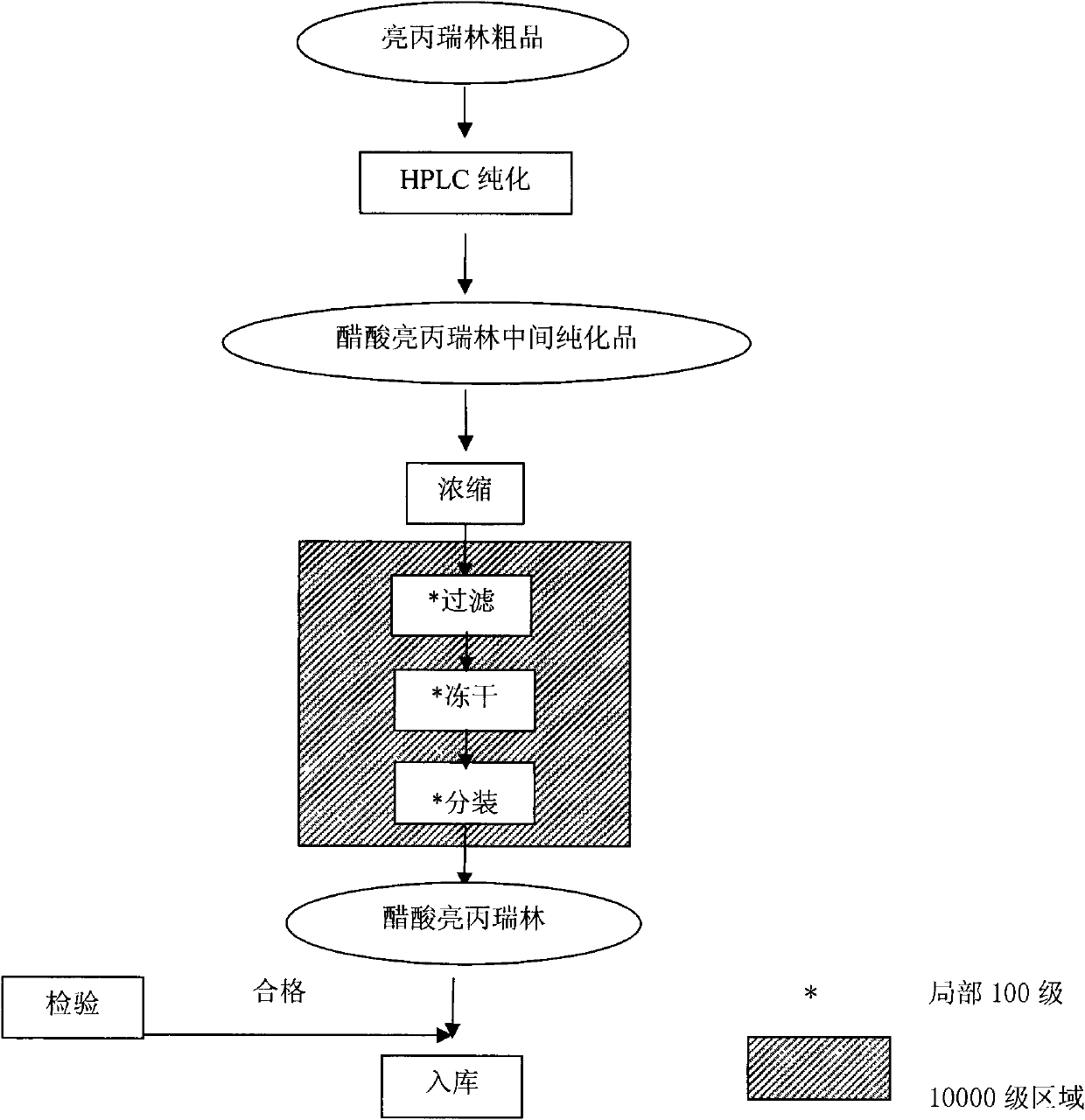
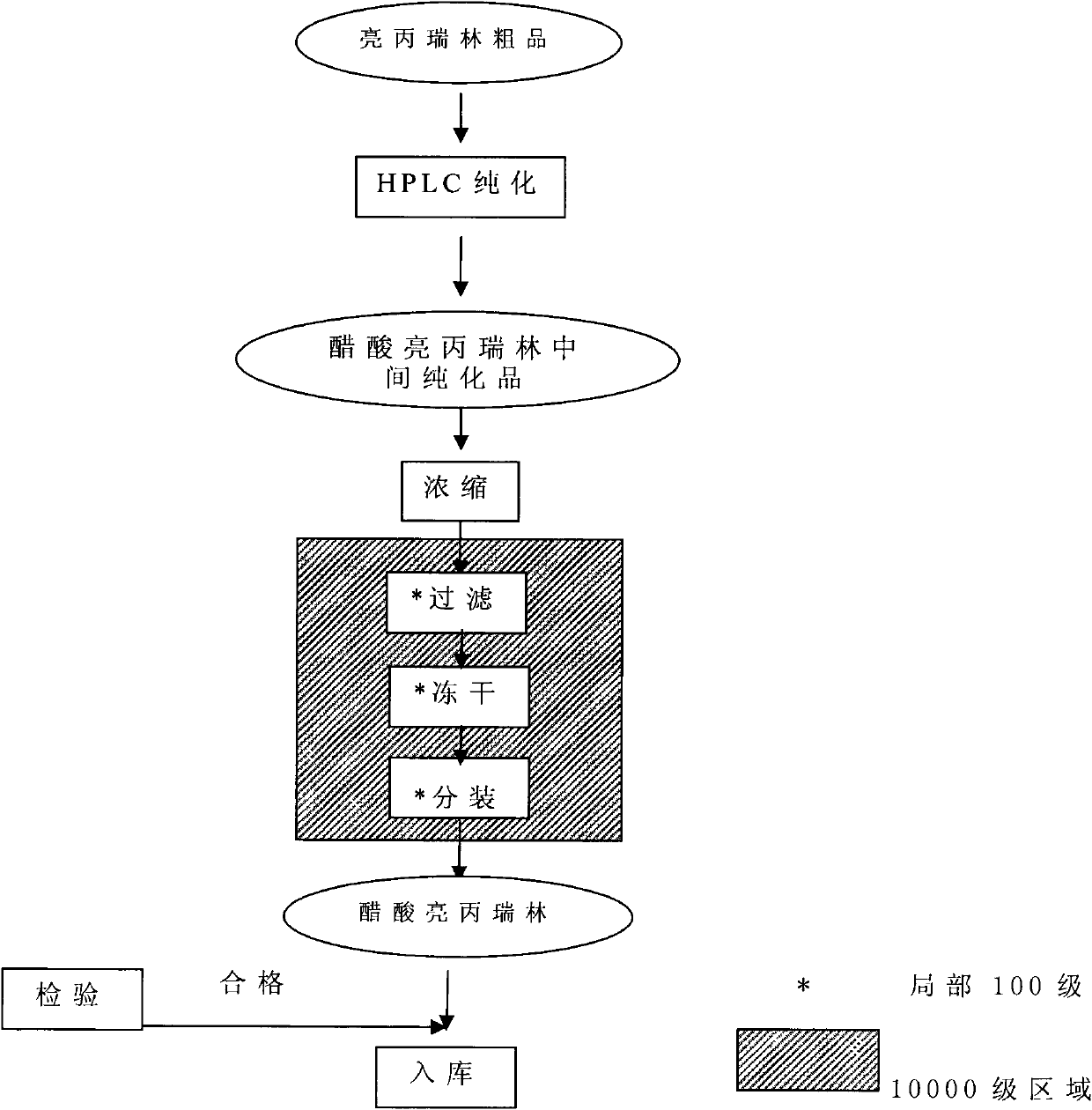
The invention provides a method for preparing leuprorelin acetate. The method comprises the following steps of: Boc-Pro-(p)preparation-solid phase peptide connection-aminolysis-HPLC (High Performance Liquid Chromatography) purification-concentration-filtering and drying, wherein in the solid-phase peptide connection step, obtaining a leuprorelin nonapeptide resin by preparing raw materials and connecting dipeptide, tripeptide, tetrapeptide, pentapeptide, hexapeptide, heptapeptide, octapeptide and nonapeptide. According to the preparation method provided by the invention, the leuprorelin acetate with higher purity and yield can be obtained, the preparation process has little pollution to environments and the safety of the product is high.
Owner:上海丽珠制药有限公司
Insoluble leuprorelin sustained-release preparation
ActiveCN105797134AHigh encapsulation efficiencyReduced burstPeptide/protein ingredientsPharmaceutical non-active ingredientsLeuprorelinEmulsion
The invention discloses an insoluble leuprorelin sustained-release preparation.Leuprorelin acetate is prepared into insoluble salt firstly, then emulsification is carried out through a single emulsion method, and leuprorelin microspheres are prepared through an in-liquid drying technology.The production process is simplified, there are few pores in the surfaces of the microspheres, the drug encapsulation rate is high, the product yield is high, and drug burst release is remarkably lowered.
Owner:ZHEJIANG SUNDOC PHARMA SCI & TECH CO LTD
Leuprorelin synthesis method
InactiveCN105330726AHigh yieldHigh purityLuteinising hormone-releasing hormonePeptide preparation methodsDipeptideLeuprorelin
The invention discloses a leuprorelin synthesis method. According to the method, a dipeptide midbody is obtained through liquid-phase synthesis and then used for solid-phase synthesis to generate full-protection nonapeptide peptide resin, full-protection nonapeptide is cut off from the resin and then inoculated with ethylamine in the liquid phase to generate full-protection leuprorelin, and a leuprorelin crude product is obtained through splitting decomposition. The method specifically comprises the steps of obtaining Fmoc-Leu-OSU fat through condensation under the action of a condensing agent; reacting with H-Arg(pbf)-OH under the action of alkali to generate Fmoc-Leu-Arg(pbf)-OH; obtaining Fmoc-Pro-CTC Resin through reaction under the action of the alkali; removing Fmoc, and conducting amino acid coupling in sequence under the action of a condensing agent to generate full-protection nonapeptide peptide resin; cutting the full-protection nonapeptide peptide resin with a cutting reagent to generate full-protection nonapeptide; generating leuprorelin full-protection peptide by means of full-protection nonapeptide and ethylamine hydrochloride under the action of a condensing agent, and obtaining the leuprorelin crude product through splitting decomposition. By the adoption of the method, the yield and purity of leuprorelin are improved remarkably, ammonolysis is not needed, and reaction is easy and controllable. The method is suitable for industrial production.
Owner:SINOPEP ALLSINO BIOPHARMACEUTICAL CO LTD
Method for synthesizing leuprorelin by solid phase and liquid phase
InactiveCN105622727AHigh yield of solid phase synthesisImprove reaction efficiencyLuteinising hormone-releasing hormonePeptide preparation methodsLeuprorelinSide chain
The invention relates to synthesis of leuprorelin, in particular to a method for synthesizing leuprorelin by a solid phase and a liquid phase, and mainly solves the technical problem that as tryptophan serine fragments in the conventional leuprorelin synthesis process has the probability of secondary conformation changes, the molecular activity is influenced to result in low yield of HPLC chromatography purification products. The method comprises the following steps: (1) enabling reaction between Fmoc-Pro-OH and 2-chlorotrityl choloride resin with the substitution degree of 0.4-0.6 mmol to produce Fmoc-Pro-resin; (2) sequentially coupling amino acids to form a solid-phase polypeptide; (3) cutting to obtain an all-protected polypeptide sequence; (4) performing liquid-phase condensation on all-protected polypeptides and ethylamine, closing the polypeptide carboxyl terminal, and performing side chain removal protection to obtain a leuprorelin crude peptide; (5) adopting efficient counter-current chromatography extraction, and then freeze-drying to obtain leuprorelin pure peptide.
Owner:WUXI ASIAPEPTIDE BIOTECH
Method for purifying Leuprorelin
ActiveCN101597325AHigh purityHigh yieldPeptide preparation methodsAntineoplastic agentsLeuprorelinPhosphate
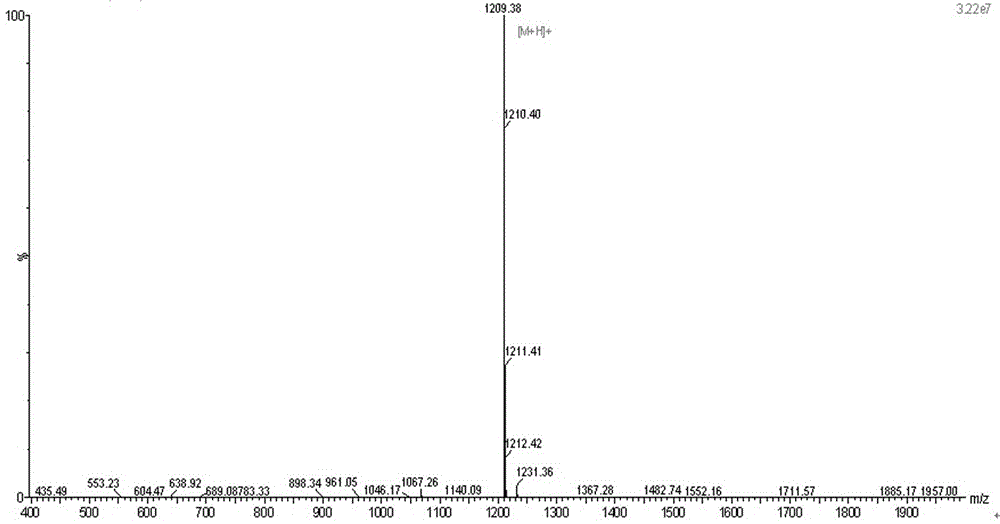
The invention discloses a method for purifying Leuprorelin, which comprises the following steps: 1) after crude peptide synthesized by a solid phase is dissolved, using reverse-phase silica gel column as the solid phase, flowing-phase phosphate buffer solution as an A phase and mixed solution of chromatographic pure acetonitrile and methanol as a B phase to carry out gradient elution and purification, and collecting peptide solution of a target peak value; and 3) exchanging phosphate into acetate by adopting negative ions. The invention provides the process method which is suitable for industrialized purification of the Leuprorelin; and the method for purifying the Leuprorelin by using reverse-phase efficient liquid chromatography has high purity and good yield, and achieves the industrialized requirement.
Owner:HYBIO PHARMA
Polymeric delivery formulations of leuprolide with improved efficacy
The present invention is directed to a flowable composition that is suitable for use as a controlled release implant. The flowable composition includes a biodegradable thermoplastic polyester that is at least substantially insoluble in aqueous medium or body fluid. The flowable composition also includes a biocompatible polar aprotic solvent. The biocompatible polar aprotic solvent is miscible to dispersible in aqueous medium or body fluid. The flowable composition also includes leuprolide acetate.
Owner:TOLMAR THERAPEUTICS
Preparation method of leuprorelin microspheres
ActiveCN106668831ACancel the cooling stepThe process steps are simplePeptide/protein ingredientsGranular deliveryLeuprorelinMicrosphere
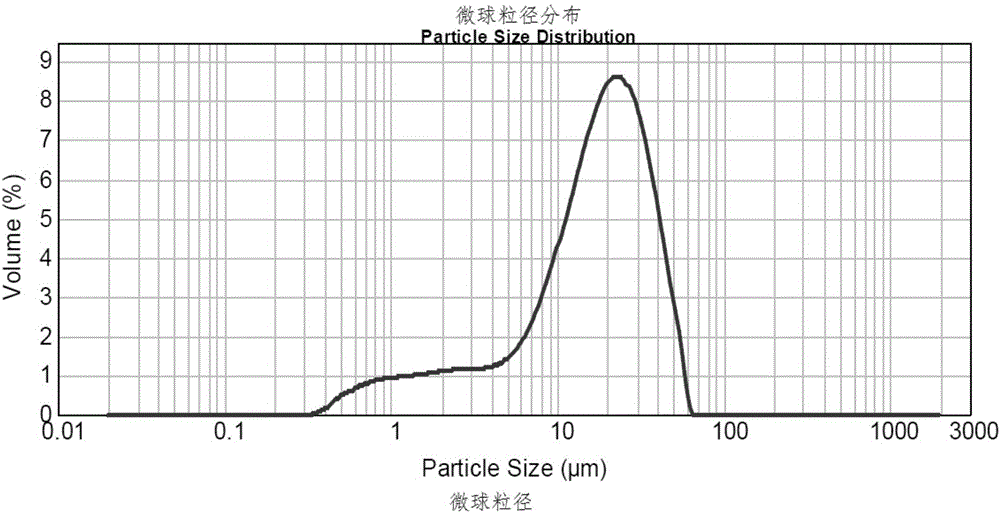
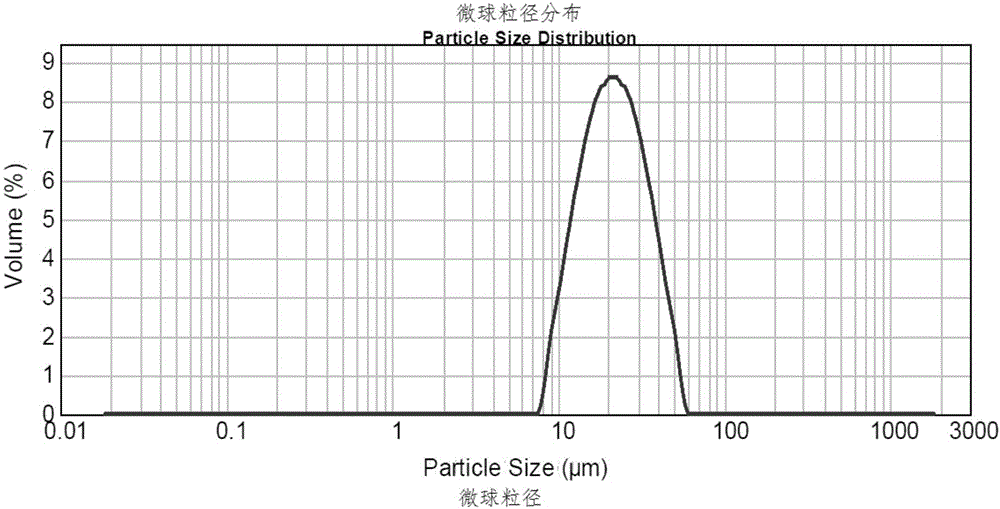
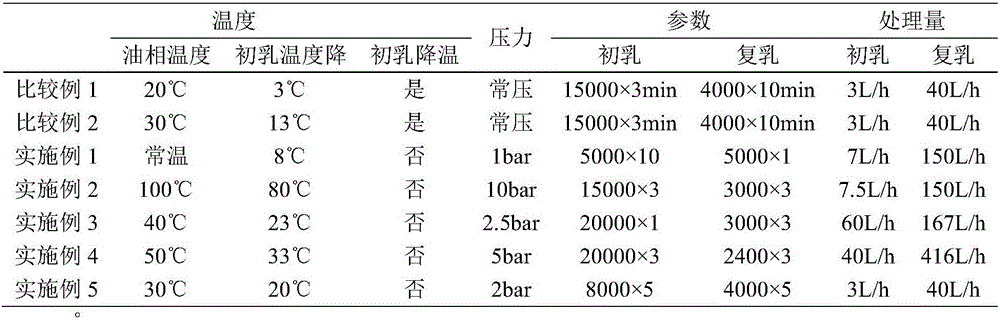
The invention discloses a preparation method of leuprorelin microspheres. The method comprises steps of preparation of an oil phase, preparation of an inner water phase, preparation of an outer water phase, preparation of a primary emulsion, preparation of a compound emulsion, drying in a liquid and the like. A total-closed online emulsification and separate tank drying mode is adopted, and the problems of high probability of evaporation and boiling of DCM in the emulsification process are effectively solved by accurately controlling temperatures and pressure; through reduction of the viscosity of the oil phase, the emulsification efficiency is improved, residues of the primary emulsion in tanks are reduced, and the overall yield of a product is increased. Besides, batch production of the leuprorelin microspheres is changed into continuous production, transition from a lab-scale test to a pilot plant test to production is smoother, and room for improvement of the productivity of a workshop is larger.
Owner:ZHEJIANG SUNDOC PHARMA SCI & TECH CO LTD
Solid-liquid synthesizing method for leuprorelin
ActiveCN101195653AAvoid the inconvenience of cuttingAvoid insecurityLuteinising hormone-releasing hormoneLeuprorelinSynthesis methods
The invention relates to a synthesis method of leuprorelin, which can resolve the problems that prior art uses solid-phase synthesis which cuts leuprorelin from resin unsafely, and has long liquid phase synthesis time. The invention comprises a, solid-phase synthesizing whole-protected peptide chain PGIuR-8, b, synthesizing material Pro-NHEt. HCI, c, condensing liquid-phase fractions to synthesize whole-protected PGIuP-9-NHEt via using DIC or BOP to react for 24-36h at 10-30DEG C, d, removing protection to obtain crude leuprorelin. The invention can synthesize polypeptide whose C end is amide.
Owner:GL BIOCHEM SHANGHAI
Preparation method of pamoic acid leuprorelin slow release preparation
ActiveCN105796501AReduce porosityHigh encapsulation efficiencyPowder deliveryPeptide/protein ingredientsLeuprorelinMicrosphere
The invention discloses a preparation method of a pamoic acid leuprorelin slow release preparation.The technology that leuprorelin acetate is prepared into indissoluble salt is adopted for the first time, and then a hot-melt extrusion technology is adopted; therefore, the production technologies are simplified, the pores on the surfaces of microspheres are few, the medicine packaging rate is high, the product yield is high, and the medicine burst release condition is significantly reduced.
Owner:ZHEJIANG SUNDOC PHARMA SCI & TECH CO LTD
Method for preparing Leuprorelin by combination of solid phase method and liquid phase method
ActiveCN101538315BReduce pollutionReduce investmentPeptide preparation methodsAntineoplastic agentsLeuprorelinSide chain
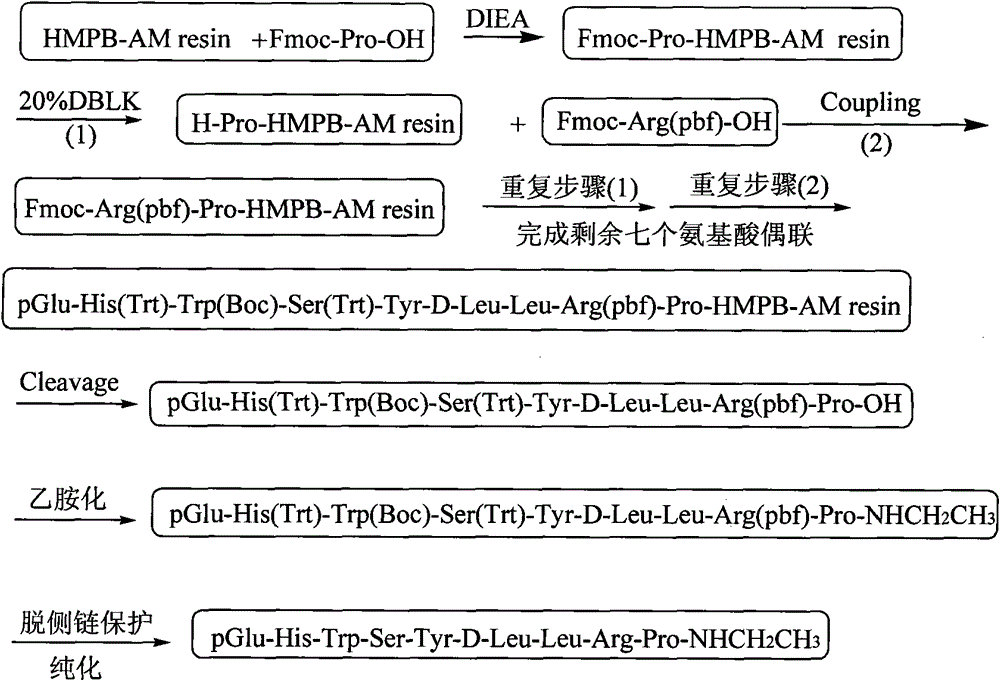
The invention discloses a new technique for synthesizing Leuprorelin by the combination of a solid phase method and a liquid phase method, which comprises the following steps of: 1) using Fmoc-Pro-OH and HMPB-AM resin as starting material, and obtaining Fmoc-Pro-HMPB-AM resin; 2) coupling in sequence and synthesizing Leuprorelin precursor peptide-HMPB-AM resin with full protective lateral chains;3) cutting the resin and obtaining Leuprorelin precursor peptide with full protective lateral chains; 4) processing the Leuprorelin precursor peptide with full protective lateral chains with methylamine, and obtaining Leuprorelin with full protective lateral chains; 5) removing the protective groups of the lateral chains of Leuprorelin with full protective lateral chains, and obtaining the crude product of Leuprorelin; and 6) conducting separation and purification and freeze drying to the crude product of Leuprorelin, and obtaining refined Leuprorelin peptide. The technology has the capability of large-scale production, easy operation, stable technique, low production cost and total yield of more than 50 percent, and has considerable economical and practical value and wide application prospect.
Owner:HYBIO PHARMA
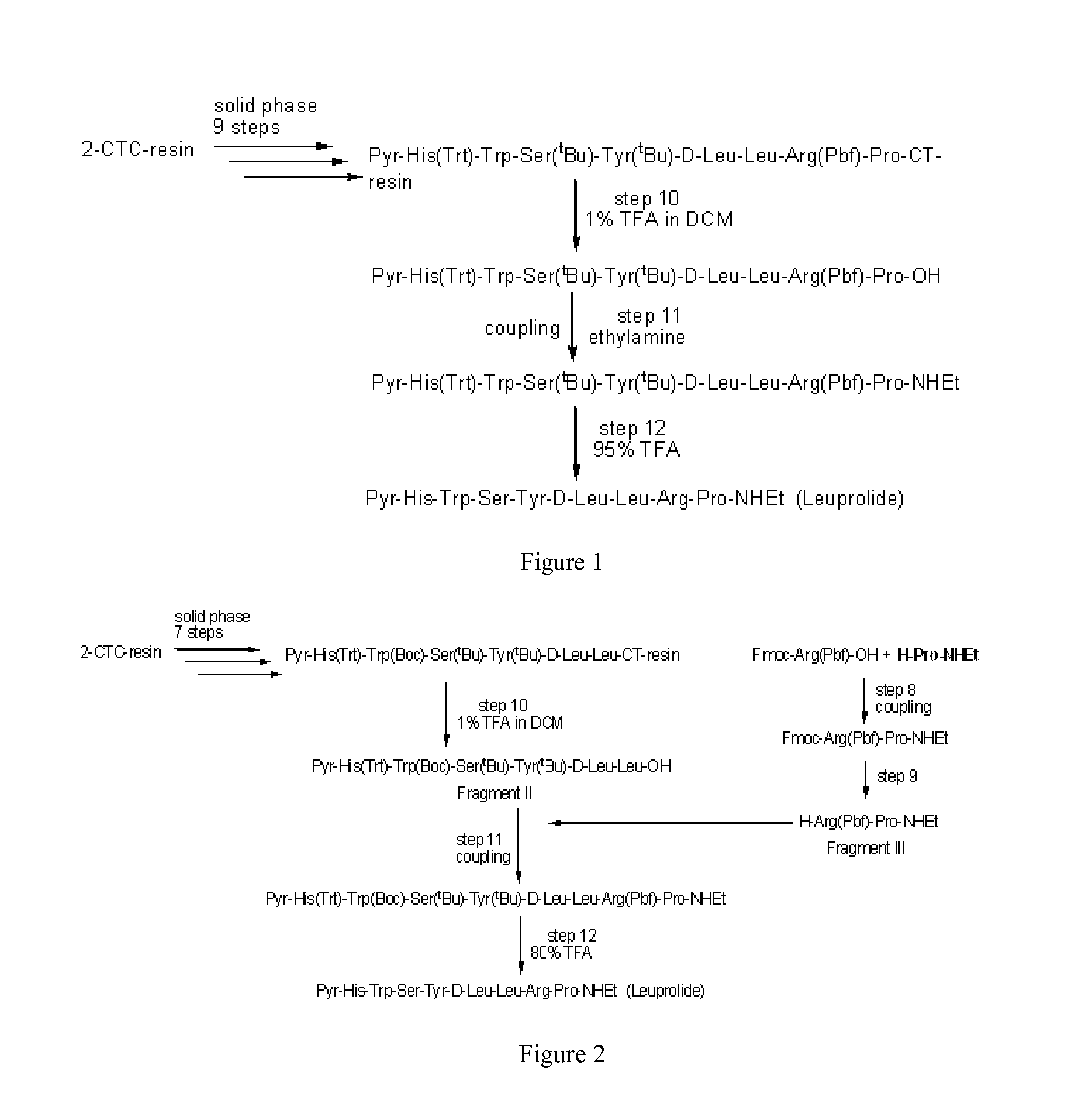
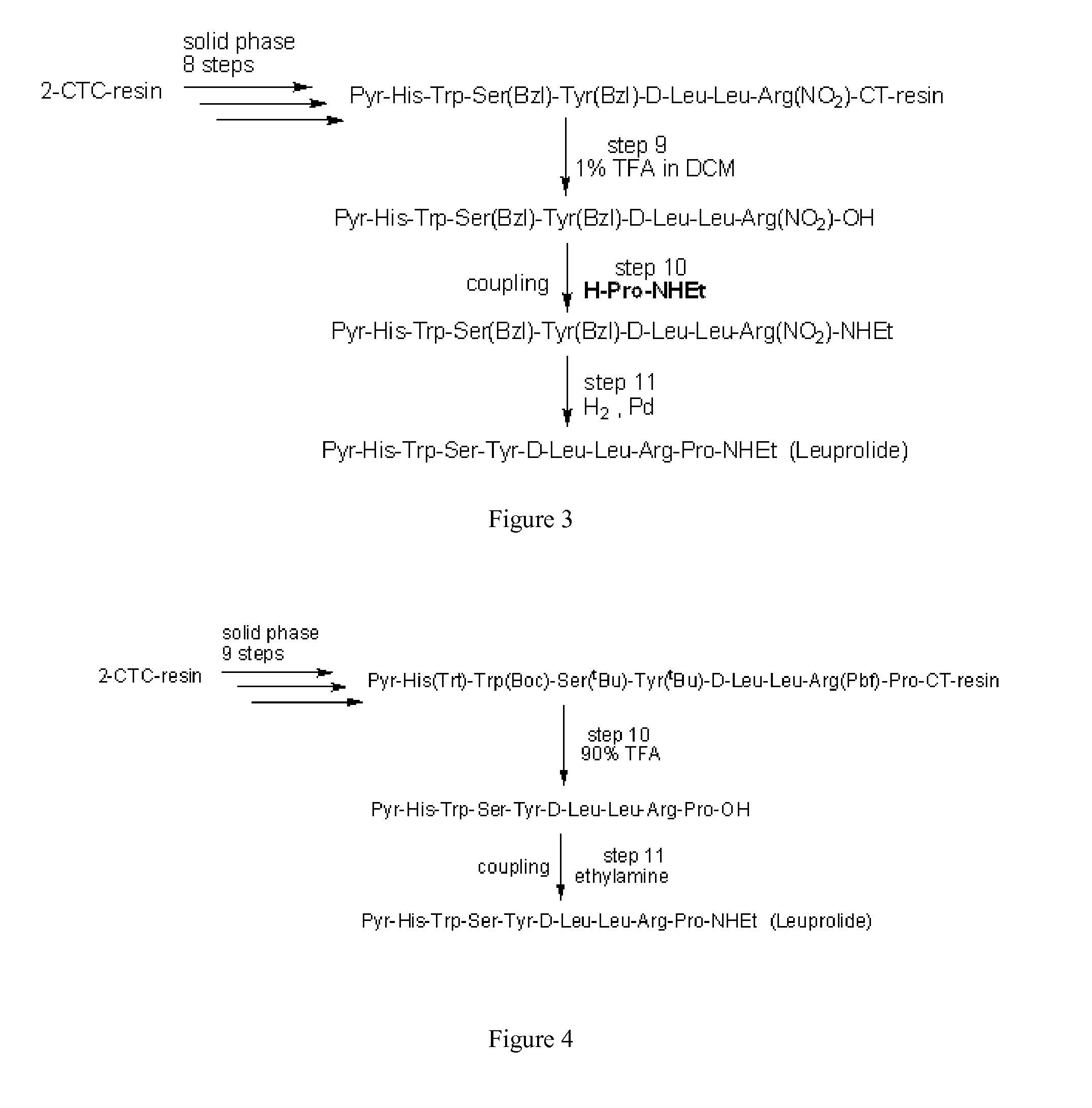
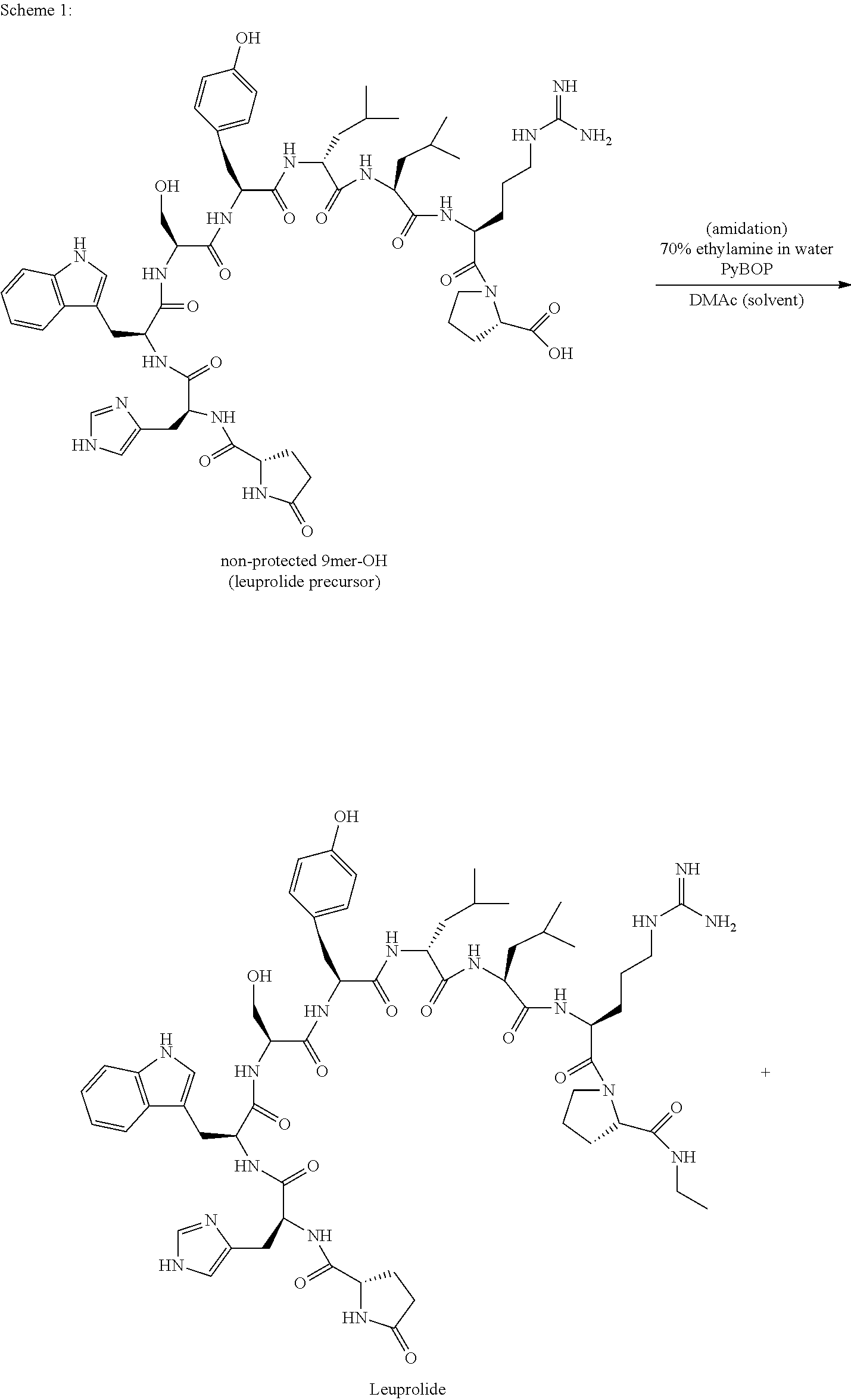
Process for the preparation of leuprolide and its pharmaceutically acceptable salts
ActiveUS20150166602A1Peptide/protein ingredientsLuteinising hormone-releasing hormoneMedicinal chemistrySolid-phase synthesis
The invention provides a process for the preparation of leuprolide or its pharmaceutical acceptable salts by a combination of solid phase synthesis and post assembly solution phase amidation. The invention also relates to applying a non-protected leuprolide precursor to prepare leuprolide or its pharmaceutically acceptable salts.
Owner:SCINOPHARM TAIWAN LTD
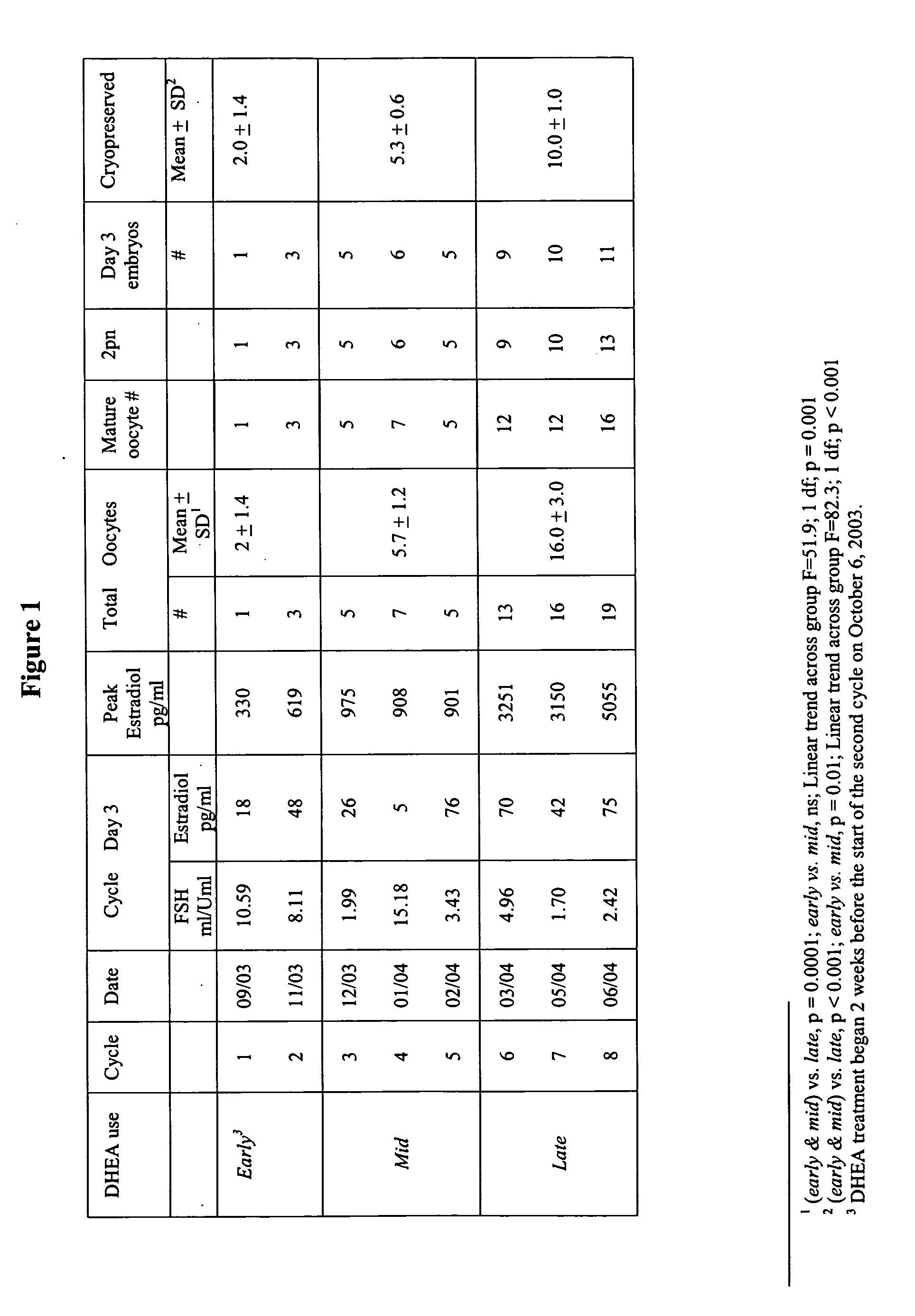
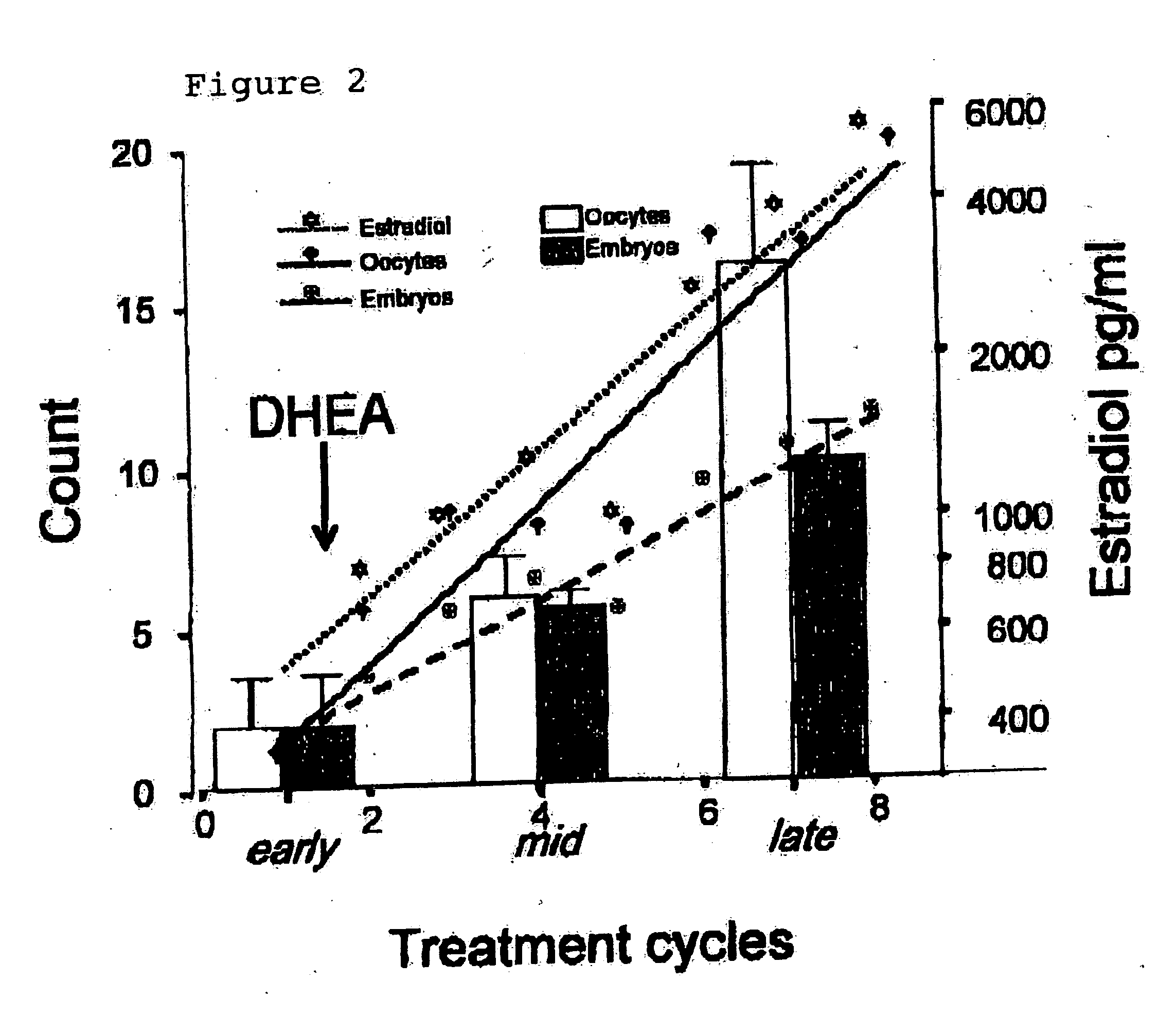
Method of improving ovulation induction using an androgen such as dehydroepiandrosterone
InactiveUS20060089308A1Maximize ovulation inductionBiocideOrganic active ingredientsLeuprorelinHuman Females
A method of preconditioning ovulation induction in a human female comprises of administering an androgen, for example, DHEA, for at least about four consecutive months. DHEA may be administered along with high dose gonadotrophins in ovulation induction treatments. Moreover, DHEA may be administered with follicle stimulating hormone, human menopausal gonadotrophin, norethindrone acetate, leuprolide acetate, and human chorionic gonadotrophin in ovulation induction treatments.
Owner:AMERICAN INFERTILITY OF NEW YORK
Leuprorelin synthesis preparation method
ActiveCN106518979AHigh yieldSimple processLuteinising hormone-releasing hormoneCorticotropinLeuprorelinN dimethylformamide
The invention discloses a leuprorelin synthesis preparation method. The method comprises the following steps: using a chloride resin as a starting resin carrier, dissolving mercaptoethylamine with alkaline solvent, mixing with the chloride resin under a homothermal condition, and reacting over night to obtain an ethylamine chloride resin; adding methanol and DIEA (diisopropylethylamine) having equal volume ratios into the ethylamine chloride resin, and performing end-capping reaction; washing the end-capped ethylamine chloride resin with DMF (N,N-dimethylformamide), sequentially putting activated Fmoc protective amino acid into a reaction vessel, and performing continuous peptide chain condensation reaction; treating the reactive chloride resin by means of a microwave technique, and washing the peptide chain-chloride resin obtained after the reaction with a DMF solution; and performing cleavage reaction on the fully-protected leuprorelin-chloride resin by means of a cleavage reagent to obtain a leuprorelin crude peptide, and then purifying through reversed-phase high-performance liquid chromatography. The method disclosed by the invention greatly simplifies the process flow, shortens the production time, lowers the production cost, and improves the reaction condensation efficiency and pure product yield, thereby having considerable market competitiveness and application prospects.
Owner:合肥国肽生物科技有限公司
Leuprorelin slow-controlled drug release stick and preparation method thereof
ActiveCN103446041AGood curative effectLittle side effectsPeptide/protein ingredientsPharmaceutical delivery mechanismDrug release rateSide effect
The invention provides a leuprorelin slow-controlled drug release stick and a preparation method thereof. The preparation method comprises the following steps of mixing raw leuprorelin, high molecular weight type resin and low molecular weight type resin to obtain a solution or suspension; drying the solution in a vacuum manner; evaporating to remove a solvent to obtain a solid; pressing the solid to obtain a drug stick. The preparation method provided by the invention is simple in process and preparation is easy to carry out; the prepared leuprorelin stick is innocuous and unpoisonous to a human body, has no side effect, and is high in curative effect and is small in dose.
Owner:薛飞
Anticancer sustained-release preparation loaded with anti-cancer medicine and synergist thereof
InactiveCN101380308AGood treatment effectLow toxicitySolution deliveryEmulsion deliveryGoserelinSuspending Agents
An anticarcinogenic slow release formulation carrying an anticancer drug and a synergist is a slow release injection or a slow release implant, and the slow release injection is made from slow release microspheres and a dissolvant. The slow release microspheres comprise anticancer active components and a slow adjuvant, and the dissolvant is a special dissolvant containing a suspending agent which is selected from sodium carboxymethyl cellulose and the like, and the viscosity of the suspending agent is 80cp-3,000cp (at room temperature). The anticancer active components are alkylating agents such as melphalan, ifosfamide and the like, purine analogues such as O6-BG and the like, and / or hormones anticancer drugs selected from triptorelin, goserelin, leuprorelin and a composition selected from epothilone (A-F) and derivatives thereof; the slow release adjuvant is chosen from one of or the copolymer or the mixture of polylactic acid and a copolymer thereof, polifeprosan and the copolymer or the mixture of polylactic acid and sebacic acid copolymer; the slow release implant and the slow release injection are injected or put in tumors or around the tumors, which is beneficial to diffusing the drug in the solid tumors, maintaining high concentration, reducing drug tolerance, being capable of mutual synergy and enhancing curative effects of chemotherapy and / or radiotherapy.
Owner:JINAN SHUAIHUA PHARMA TECH
Temperature controlled sustained-release injection containing steroids anti-cancer drugs
InactiveCN101273963APharmaceutical delivery mechanismPharmaceutical non-active ingredientsGoserelinTherapeutic effect
The invention relates to a temperature-controlled sustained-release injection containing a hormone anti-cancer drug, which comprises the anti-cancer drug, an amphiphilic block copolymer, a solvent and a certain amount of drug release regulator, wherein, the mixture of the amphiphilic block copolymer and a solvent without organic solvent has the temperature-sensitive gelatinization feature, which is flowable liquid in the environment that is lower than the body temperature and can be automatically converted to the water-insoluble gel that can not flow and be biodegradable for absorption in an endotherm, and the water-insoluble gel can allow the contained hormone anti-cancer drug to have the local sustained release in a tumor and maintain the effective drug concentration for a plurality of weeks to a plurality of months; the viscosity of the temperature-controlled sustained-release injection is 10cp to 3000cp ( at 5 DEG C to 30 DEG C ), and the gelatinization temperature is 35 DEG C to 37 DEG C. The sustained-release injection can be injected in the tumor or the tumor periphery or be arranged in the postoperative tumor cavity, thus significantly reducing the systemic reaction of the drug, selectively strengthening the treatment effects of chemotherapy, radiotherapy and other non-surgical therapies, and being used for the treatment of the tumors in different stages. The anti-cancer drug can be triptorelin, goserelin, leuprorelin, anastrozole, idoxifene, tamoxifen and other hormone anti-cancer drugs.
Owner:SHANDONG LANJIN PHARMA +1
Leuprorelin medicinal composition injection type hypodermic implantation agent
InactiveCN105535933AHydrocarbon active ingredientsPeptide/protein ingredientsDiseaseControlled release
The invention relates to a leuprorelin medicinal composition injection type hypodermic implantation agent. The leuprorelin medicinal composition injection type hypodermic implantation agent is characterized in that medicine active ingredients are leuprorelin, plant extract and hormone or resistance hormone active medicine cooperating with leuprorelin; in addition, an implantation agent is injected subcutaneously in a liquid mode, under the effect of the body temperature, the solution is changed into gel, and then the effect of controlled release of multiple sex hormone dependent diseases is exerted. Through the implantation agent prepared through the method, on the one hand, the use amount is reduced while the medicine effect is kept, toxic and side effects are reduced, and medicine use safety and compliance are improved; on the other hand, the medicine is slowly released in the body, and the long-acting effect of treating the sex hormone dependent diseases is achieved.
Owner:SHENZHEN JYMED TECH
Industrial preparation method of leuprolide acetate
InactiveCN106146622AReduce dosageInhibitionLuteinising hormone-releasing hormonePeptide preparation methodsSide chainFreeze-drying
The invention discloses a method suitable for preparing leuprolide industrially on a large scale. According to the method, PAM resin is adopted as a solid-phase carrier, amino acid is sequentially coupled through a Boc and Fmoc combined solid-phase synthesis method, and fully-protected leuprolide precursor peptide resin is obtained. A low-temperature connecting method and side chain protection are adopted in the amino acid coupling process, and racemization and side reactions on a side chain are reduced. Through an ethylamine reaction on the peptide resin, fully-protected leuprolide crude peptide is directly obtained, a side chain protecting group is removed with a trifluoroacetic acid solution, and leuprolide crude peptide is obtained. Leuprolide acetate is obtained after purification through reversion phase chromatography, salt change and freeze drying. The method is easy and convenient to operate and high in synthesis efficiency, the total yield is 36%, the product purity reaches 99% or above, pollution to the environment is reduced, and the safety of the preparation process is improved.
Owner:CHINESE PEPTIDE CO
Purification method for leuprorelin acetate crude product
InactiveCN107163106AHigh purityAct as a buffer saltLuteinising hormone-releasing hormonePeptide preparation methodsLeuprorelinPurification methods
The invention provides a purification method for a leuprorelin acetate crude product. The purification method comprises performing first elution, namely performing first elution on the leuprorelin acetate crude product by employing a reversed phase chromatographic column, so as to obtain a first elution product, wherein the elution conditions for the first elution comprise that a first mobile phase is an aqueous solution of trifluoroacetic acid, and a second mobile phase is an acetonitrile solution of trifluoroacetic acid; successively performing salt conversion and salt removal on the first elution product, so as to obtain a leuprorelin acetate product. By employing trifluoroacetic acid as the mobile phases, the product purity is improved, chromatographic peak shape tailing is reduced and the peak shape is improved, and then the leuprorelin acetate product is obtained through the processes of salt conversion and salt removal. Additionally, the purification method provided by the invention does not introduce new counter ions, and has the advantages of high product purity, controllable quality, low cost, operation convenience and the like.
Owner:ASYMCHEM LAB TIANJIN
Solid phase polypeptide synthesis preparation method for leuprorelin
ActiveCN1865280BConvenient sourceReduce usagePeptide preparation methodsBulk chemical productionHydrogen fluorideLeuprorelin
The invention discloses a bright-ala-ruilin preparing method of solid-phase polypeptide, which comprises the following steps: adopting Wang resin or CTC resin as original material to connect amino with protective group to produce protective nonapeptide resin; removing Fmoc-protective group sequently; proceeding side-chain protective group synchronizingly and cutting peptide; connecting ethylaminethrough ethylamine-to-HOBT to produce crude product; proceeding separation and purifying through C18 (or C8) pillar to produce fine bright-ala-ruilin. The invention avoids the utility of poisonous agent, which improves the purifying, peptide connecting and obtaining rate.
Owner:SHANGHAI SOHO YIMING PHARMA
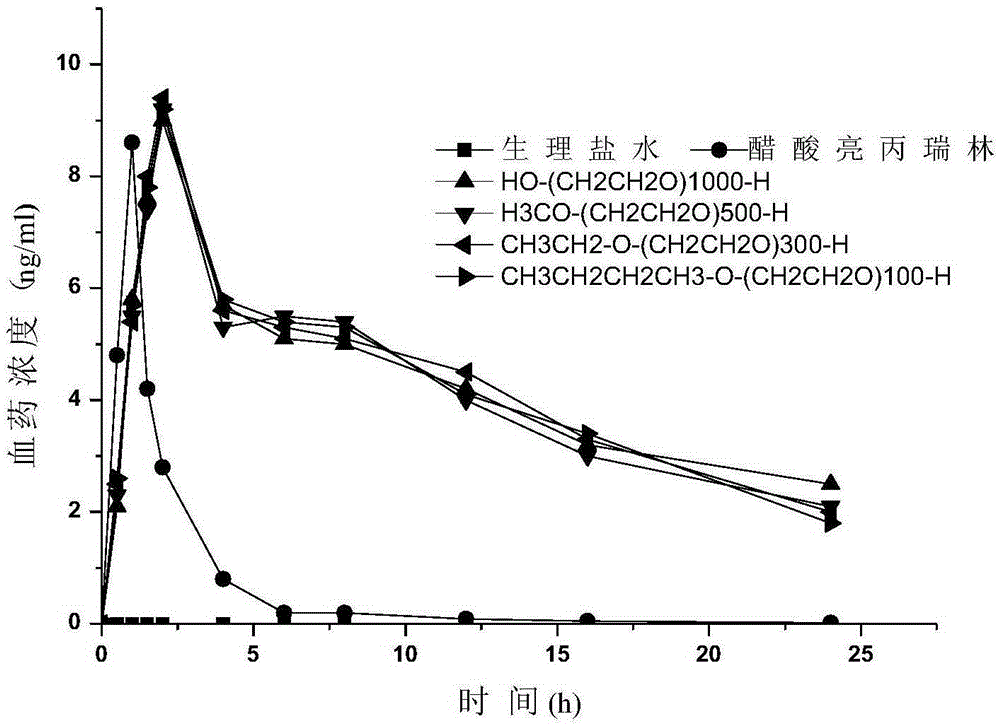
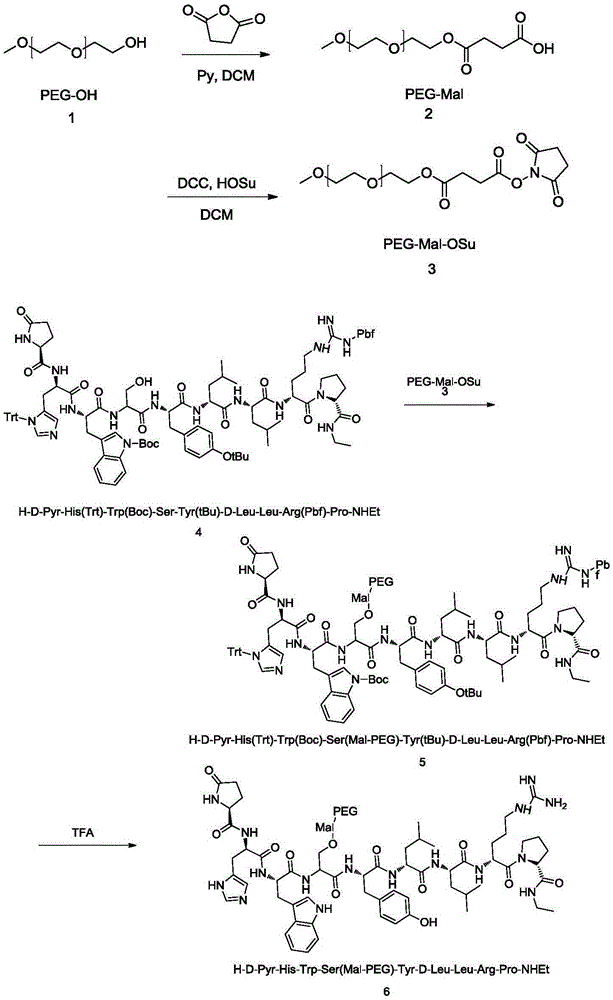
Pegylated leuprorelin
ActiveCN105237762APeptide/protein ingredientsPharmaceutical non-active ingredientsPolymer scienceLeuprorelin
The invention provides a PEG-leuprorelin conjugate and a preparation method thereof. The preparation method is characterized in that: firstly PEG is connected with maleic anhydride for forming an active ester, the PEG modification agent is connected to leuprorelin with protection by Mal, and then a de-protection is carried out to obtain a PEG-leuprorelin conjugate with a single site modification. The compound has the original activity of leuprorelin, and simultaneously has a longer half life and a longer average peak time.
Owner:SHENZHEN XINGYIN PHARML
Anticancer medicament and synergist simultaneously carrying anticancer sustained release agent
InactiveCN1969821AGood treatment effectLow toxicityOrganic active ingredientsPharmaceutical delivery mechanismAdjuvantTreatment effect
Disclosed is an anti-cancer slow release agent in the form of slow release injection or slow release implantation agent carrying both anti-cancer drugs and synergistic agent, the slow release injection comprises slow release microspheres and dissolvent, wherein the slow release microballoons comprise anti-cancer active constituents and slow release auxiliary materials, the dissolvent being specific dissolvent containing suspension adjuvant. The suspending agent is selected from carboxymethylcellulose, the viscosity of the suspension adjuvant is 80-3000cp (at room temperature). The anticancer active constituent being the combination of alkylating agent such as Melphalan and isoendoxan, purine analogues such as 06-BG and / or hormone group anti-cancer drugs selected from Triptorelin, Goserelin Leuprorelin and Epothilone and its derivatives (Epothilone A-F), the slow release auxiliary materials are selected from polylactic acid and its copolymer, Polifeprosan, polylactic acid copolymer or mixture, sebacylic acid copolymer or mixture. The slow release injection and slow release implanting agent can be used independently for effectively suppressing tumor accretion, or used in combination with non-operative methods such as chemotherapy and / or radiotheraphy with the function of improving their treatment effects.
Owner:JINAN SHUAIHUA PHARMA TECH
Method for synthesizing leuprorelin from polypeptide solid-liquid fragment
InactiveCN112279893ALow costReduce manufacturing costLuteinising hormone-releasing hormonePeptide preparation methodsLeuprorelinBiochemical engineering
The invention provides a method for synthesizing leuprorelin by using a polypeptide solid-liquid fragment. The method comprises the following steps: 1) synthesizing a compound 1: Boc-Pglu-His(Boc)-Trp(Boc)-Ser(tBu)-Tyr(tBu)-D-Leu-Leu-OH by a polypeptide solid phase synthesis method; 2) synthesizing a compound 2: H-Arg(pbf)-Pro-NHEt by using a polypeptide liquid phase synthesis method; (3) synthesizing a compound 3: Boc-Plu-His(Boc)-Trp(Boc)-Ser(tBu)-Tyr(tBu)-D-Leu-Leu-Arg(pbf)-Pro-NHEt in a liquid phase; 4) synthesizing a leuprorelin crude product, namely Pglu-His-Trp-Ser-Tyr-D-Leu-Leu-Arg-Pro-NHEt. Piperazine is used as an uncapping reagent, CTC resin is used for synthesizing a fragment, a liquid phase method is combined for synthesizing leuprorelin, and the CTC resin synthesized fragmentcan be conveniently and automatically produced; CTC resin can be recycled, piperazine is not a precursor reagent, the cost of piperazine is lower than that of a precursor reagent piperidine used in aclassical synthesis method, and piperazine is more convenient to transport and store; the method is suitable for large-scale industrial production, does not have violent chemical reaction, and lowersthe production cost.
Owner:湖南津安生物科技有限公司
Pharmaceutical Compositions having a Selected Release Duration
ActiveUS20200390849A1Improve stabilitySatisfied with stabilityPeptide/protein ingredientsPharmaceutical delivery mechanismLeuprorelinLactide
The present invention provides for a stabilized biodegradable polymeric composition useful as a controlled release delivery system for peptide agents. The compositions of the present invention comprise a) a strong acid salt of a LHRH agonist or antagonist; b) a biodegradable polymer of poly(lactide-co-glycolide), wherein the ratio of lactide:glycolide of the copolymer is from 50:50 to about 100:0; and c) N-methyl-2-pyrrolidone (NMP), wherein the composition does not contain excess strong acid in addition to the strong acid used to form the salt of the LHRH agonist or antagonist. The composition, when injected, can provide a controlled release of leuprolide for a period of up to 6 months.
Owner:FORESEE PHARMA CO LTD
Leuprorelin synthesis process
InactiveCN105646670AOvercome the problems of many processes and heavy workloadIncrease conversion rate per passLuteinising hormone-releasing hormonePeptide preparation methodsLeuprorelinFreeze-drying
The present invention discloses a leuprorelin synthesis process, which comprises: 1) adopting Fmoc-Pro-OH and a HMPB-AM resin as starting raw materials to obtain a Fmoc-Pro-HMPB-AM resin; 2) carrying out sequential coupling synthesis of a side chain full protection leuprorelin precursor peptide-HMPB-AM resin; 3) cutting to obtain a side chain full protection leuprorelin precursor peptide; 4) carrying out ethylamine treatment on the side chain full protection leuprorelin precursor peptide to obtain side chain full protection leuprorelin; 5) carrying out a side chain protection group removing reaction on the side chain full protection leuprorelin to obtain a leuprorelin crude product; and 6) separating and purifying the leuprorelin crude product, and carrying out freeze drying to obtain the leuprorelin fine peptide. According to the present invention, the leuprorelin synthesis process has characteristics of large-scale production capacity, easy operation, stable process, low production cost, considerable economic and practical value, and broad application prospects, wherein the total yield exceeds 50%.
Owner:吕艳
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com