Leuprorelin medicinal composition injection type hypodermic implantation agent
A technology of leuprolide and implants, applied in the directions of drug combination, drug delivery, hydrocarbon compound active ingredients, etc., can solve problems such as body damage, poor patient compliance, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
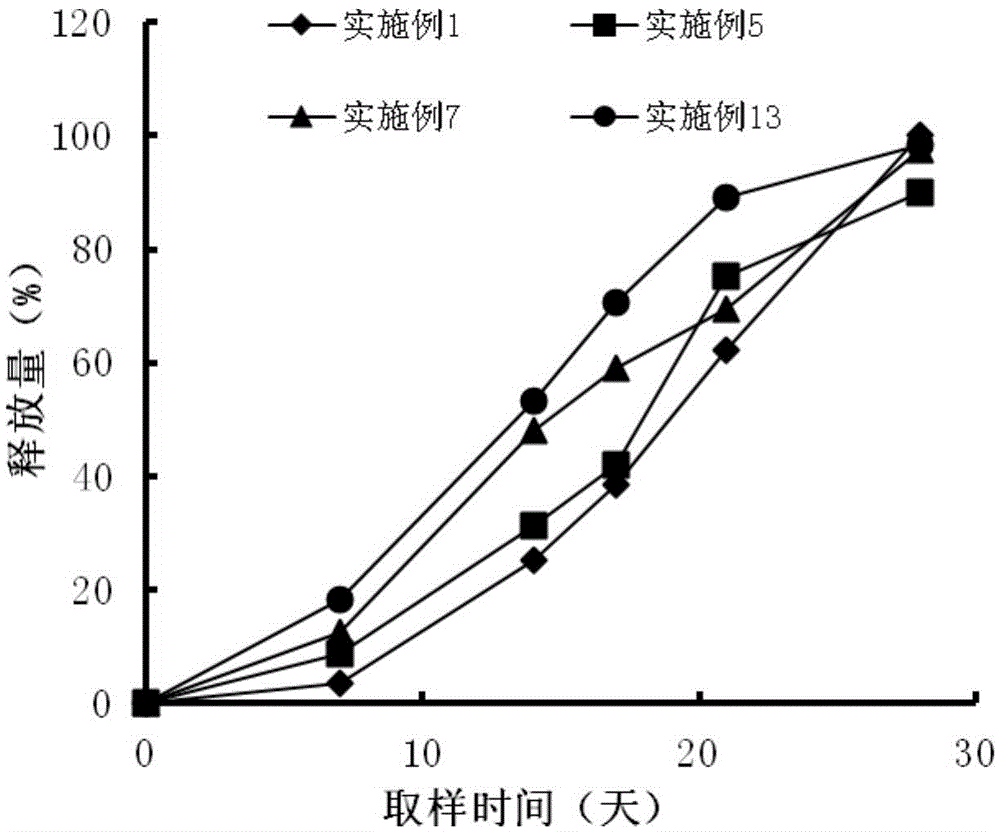
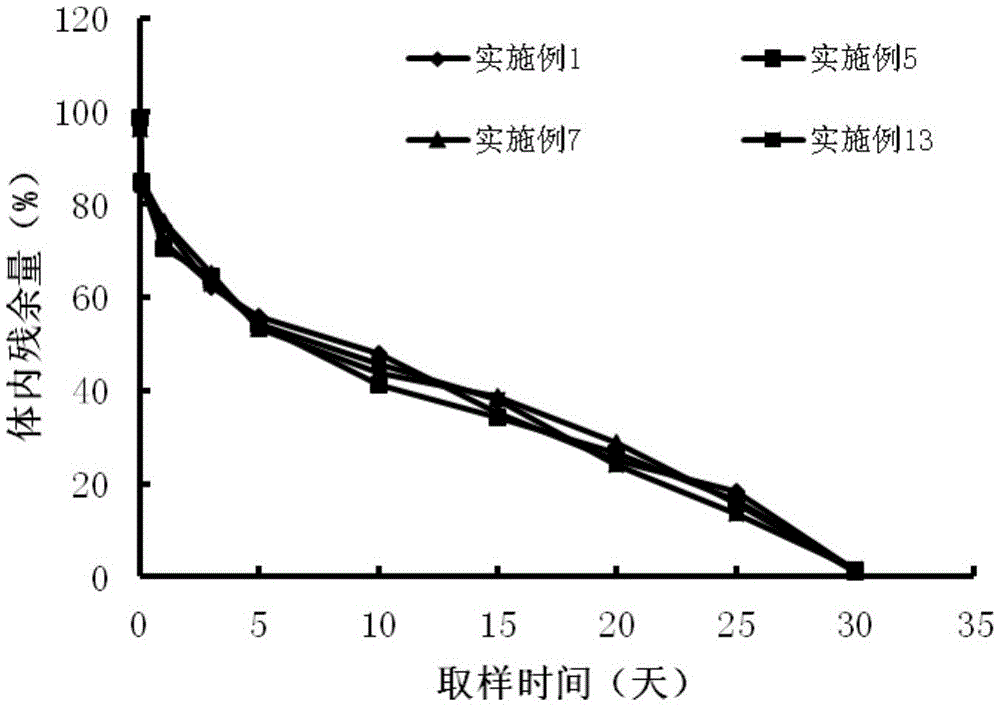
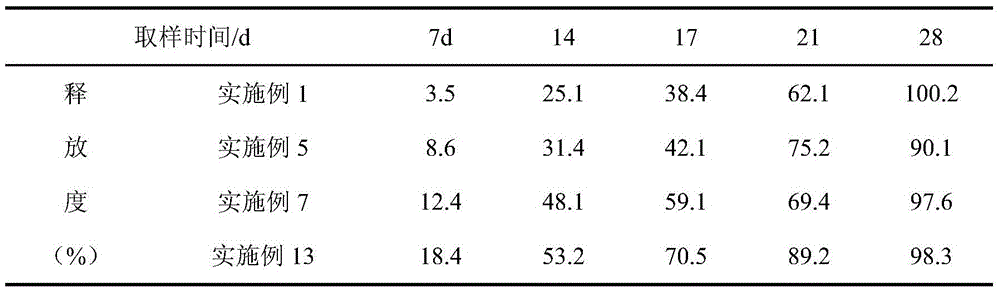
[0031] Get 0.7g PLGA (20000 Daltons), join in appropriate amount of dichloromethane, stir and make it dissolve completely, make 2% PLGA organic solution, with 0.08g pharmaceutical composition (leuprolide: ginsenoside rh2=1 : 1) Add to the organic solution, spray dry at 25°C, 0.3MPa to prepare microcapsules; dissolve Poloxamer 407 (30,000 Daltons) in water at a low temperature of 5°C to fully swell to a solution state to make a hydrogel solution; adding microcapsule powder into the hydrogel solution, mixing evenly, adding trehalose, and freeze-drying to prepare a leuprolide pharmaceutical composition injection-type subcutaneous implant.
Embodiment 2
[0033] Take 1g PLA (30000 Daltons), add it to an appropriate amount of ethyl acetate, stir to make it completely dissolved, and make a 5% PLGA organic solution, mix 0.1g of the pharmaceutical composition (leuprolide: lycopene=4: 1) Add to the organic solution, spray dry at 25°C and 0.3MPa to prepare microcapsules; under the low temperature condition of 5°C, dissolve PLA-PEG-PLA (30,000 Daltons) with water to fully swell to a solution state, Prepare a hydrogel solution; add microcapsule powder into the hydrogel solution, mix evenly, add sorbitol, and freeze-dry to prepare a leuprolide pharmaceutical composition injection-type subcutaneous implant.
Embodiment 3
[0035] Get 4g PLGA (30000 Daltons), join in appropriate amount of dichloromethane, stir to make it dissolve completely, make 10% PLGA organic solution, 0.1g pharmaceutical composition (leuprolide: coix seed oil=2: 1) adding to the organic solution, spraying and drying at 25°C and 0.3MPa to prepare microcapsules; at a low temperature of 10°C, dissolving Poloxamer 407 (50,000 Daltons) in water to fully swell to a solution state, Prepare a hydrogel solution; add microcapsule powder into the hydrogel solution, mix evenly, add trehalose, and freeze-dry to prepare a leuprolide pharmaceutical composition injection-type subcutaneous implant.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com