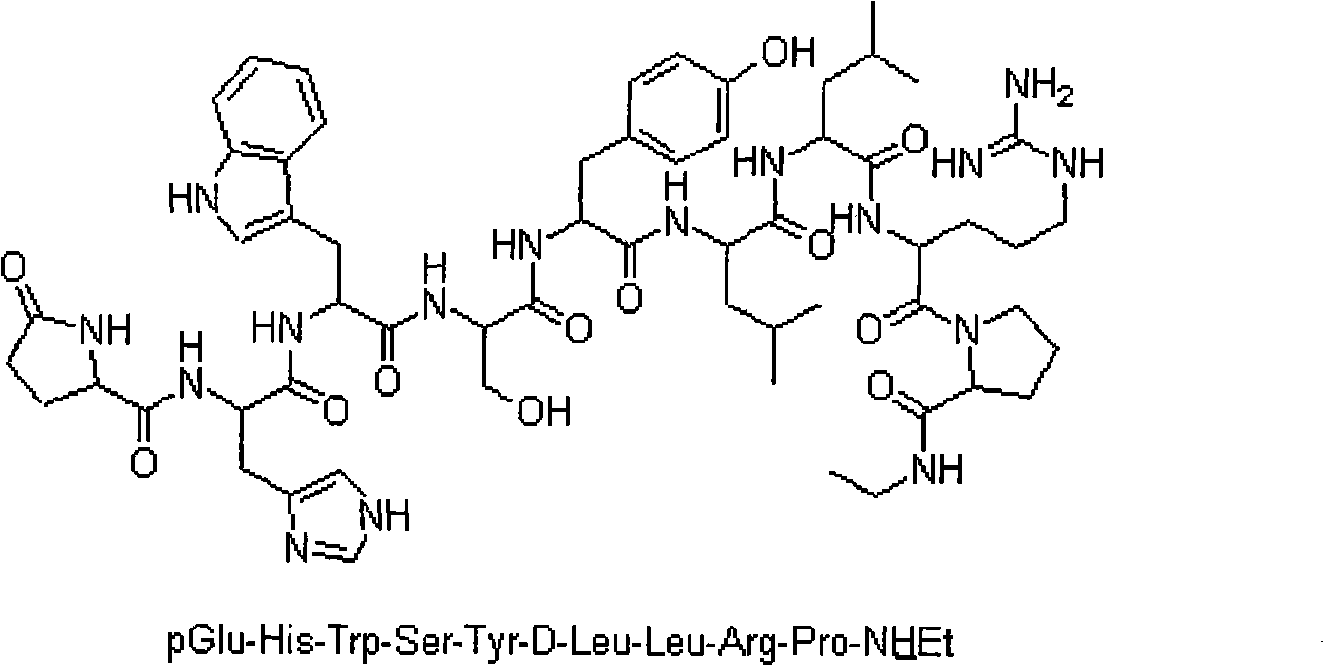
Method for purifying Leuprorelin
A technology for leuprolide and purpose, applied in the field of HPLC, can solve problems such as no large-scale production, and achieve the effect of good yield and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] 1. Sample treatment: Dissolve each gram of crude peptide with a mixture of ultrapure water, acetonitrile and acetic acid (V water: V acetonitrile: V acetic acid = 85:10:5), and filter the sample after it is completely dissolved, and collect the filtrate spare.
[0024] 2. The first step of purification: purification conditions: chromatographic column: a chromatographic column with octadecylsilane bonded silica gel as the stationary phase, and the diameter and length of the column are: 5cm×25cm. Mobile phase: A phase: 0.1%-0.3% phosphoric acid aqueous solution with triethylamine to adjust the pH to 2.0-3.0; B phase: acetonitrile and methanol (V acetonitrile: V methanol = 4:1) mixture. Flow rate: 70-80ml / min. Detection wavelength: 280nm. Gradient: B%: 12% to 43% (55min). The injection volume is 1.3-1.5g.
[0025] Purification process: equilibrate the chromatographic column with mobile phase A and load the sample, the sample volume is 13-15ml of sample solution. After...
Embodiment 2
[0028] 1. Sample treatment: Dissolve each gram of crude peptide with a mixture of ultrapure water, acetonitrile and acetic acid (V water: V acetonitrile: V acetic acid = 85:10:5), and filter the sample after it is completely dissolved, and collect the filtrate spare.
[0029] 2. The first step of purification: purification conditions: chromatographic column: a chromatographic column with octaalkylsilane bonded silica gel as the stationary phase, and the diameter and length of the column are: 15cm×25cm. Mobile phase: A phase: 0.1%-0.3% phosphoric acid aqueous solution with triethylamine to adjust the pH to 2.0-3.0; B phase: acetonitrile and methanol (V acetonitrile: V methanol = 4:1) mixture. Flow rate: 450-550ml / min. Detection wavelength: 280nm. Gradient: B%: 18% to 33% (45min). The injection volume is 13-15g.
[0030] Purification process: equilibrate the chromatographic column with mobile phase A and load the sample, the sample volume is 130-150ml of sample solution. Af...
Embodiment 3
[0033] 1. Sample treatment: Dissolve each gram of crude peptide with a mixture of ultrapure water, acetonitrile and acetic acid (V water: V acetonitrile: V acetic acid = 85:10:5), and filter the sample after it is completely dissolved, and collect the filtrate spare.
[0034] 2. The first step of purification: purification conditions: chromatographic column: a chromatographic column with octadecylsilane bonded silica gel as the stationary phase, and the diameter and length of the column are: 30cm×25cm. Mobile phase: A phase: 0.1%-0.3% phosphoric acid aqueous solution with triethylamine to adjust the pH to 2.0-3.0; B phase: acetonitrile and methanol (V acetonitrile: V methanol = 4:1) mixture. Flow rate: 1900-2200ml / min. Detection wavelength: 280nm. Gradient: B%: 12% to 53% (65min). The injection volume is 55-75g.
[0035] Purification process: equilibrate the chromatographic column with mobile phase A and load the sample, the sample volume is 550-750ml of sample solution. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com