Preparation method of pamoic acid leuprorelin slow release preparation
A technology of leuprolide acetate and leuprolide, which is applied in directions such as pharmaceutical formulations, medical preparations without active ingredients, and medical preparations containing active ingredients, etc., can solve the problem of restricting the development of leuprolide microsphere industry, The problems of low product yield and long production cycle can achieve the effect of less pores, high product yield and simplified production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
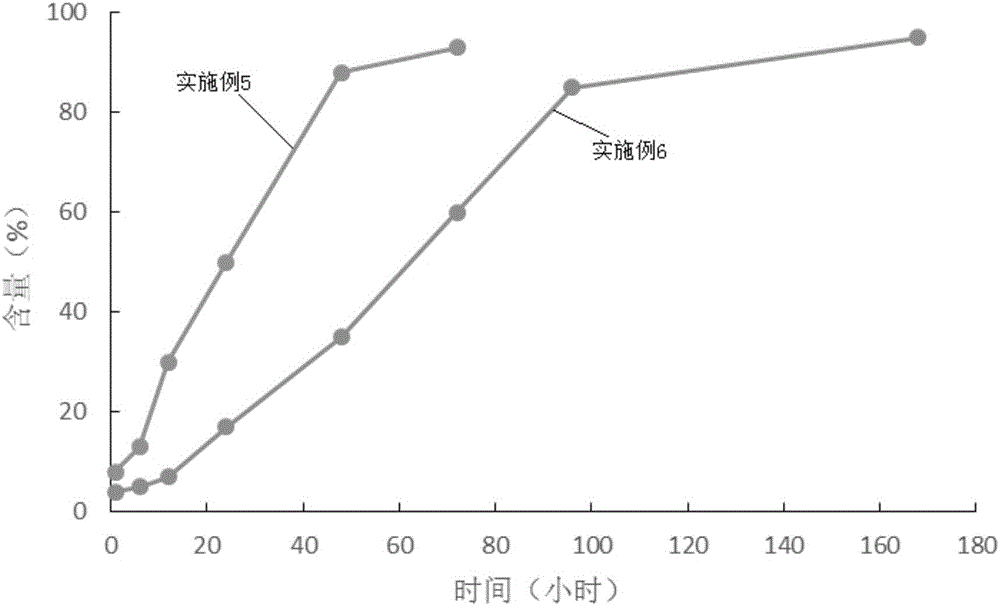
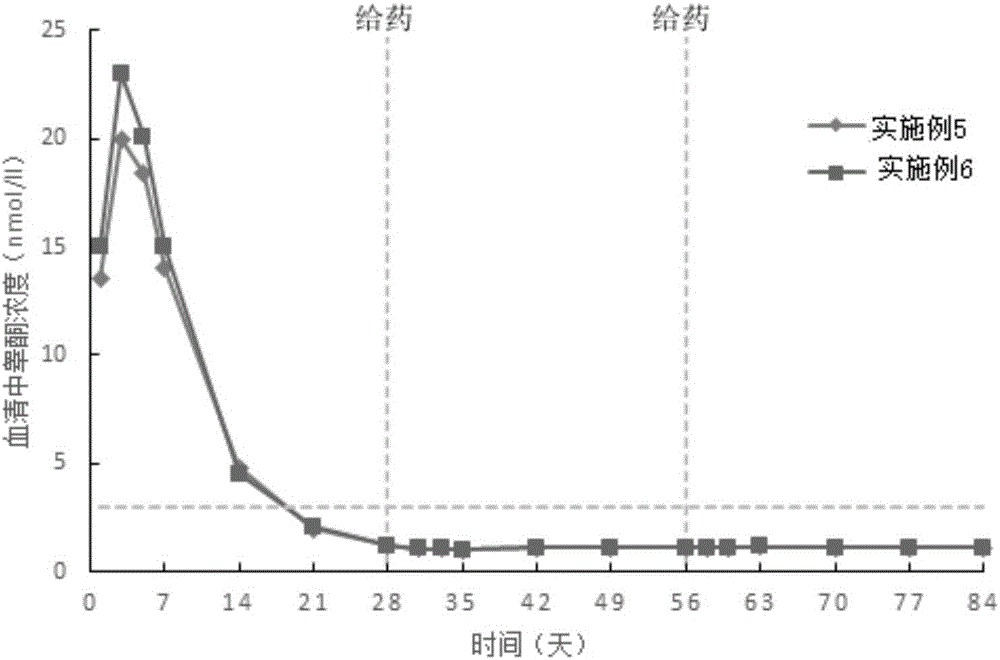
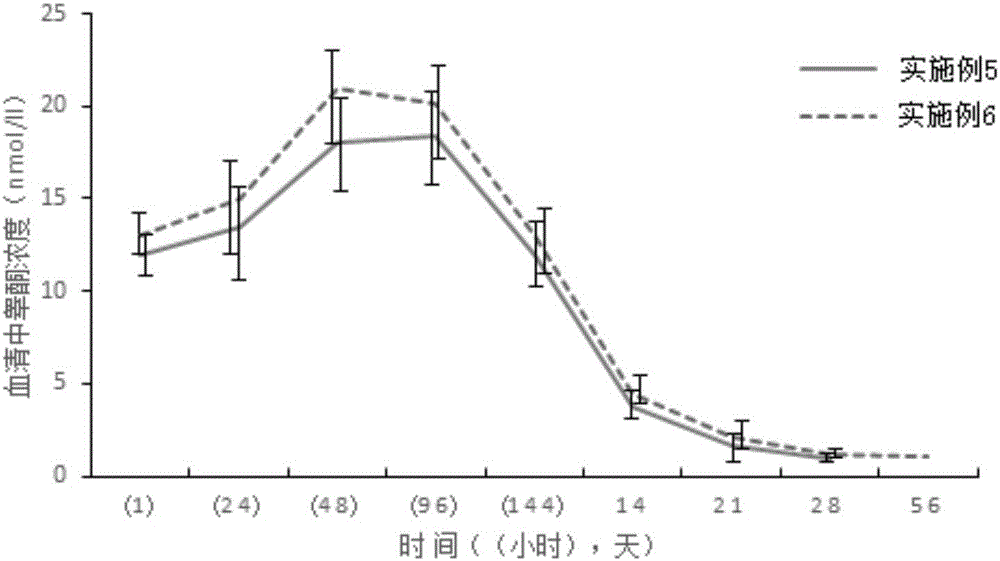
Examples
Embodiment 1
[0062] A preparation method of leuprolide pamoate sustained-release preparation, comprising the following steps:
[0063] (1) Salt formation and purification: Leuprolide acetate and pamoic acid disodium salt (4,4'-methylenebis(3-hydroxy-2-naphthoic acid) disodium salt, CAS: 6640-22 -8, commercially available) is the synthetic leuprolide pamoate of raw material, then leuprolide pamoate is purified;
[0064] The specific process steps of salt formation are:
[0065] a. Filtration to remove impurities: Leuprolide acetate crude product is dissolved in ultrapure water, prepared into a solution with a mass concentration of 5%, filtered through a 1 μm membrane stack to remove water-insoluble impurities to obtain a leuprolide acetate solution; pamoate disodium salt Dissolve in ultrapure water, prepare a solution with a mass concentration of 5%, and filter through a 1 μm membrane stack to remove water-insoluble impurities to obtain a pamoic acid disodium salt solution;
[0066] b. St...
Embodiment 2
[0083] A preparation method of leuprolide pamoate sustained-release preparation, comprising the following steps:
[0084] (1) Salt formation and purification: Leuprolide acetate and pamoic acid disodium salt (4,4'-methylenebis(3-hydroxy-2-naphthoic acid) disodium salt, CAS: 6640-22 -8, commercially available) is the synthetic leuprolide pamoate of raw material, then leuprolide pamoate is purified;
[0085] The specific process steps of salt formation are:
[0086] a. Filtration to remove impurities: Leuprolide acetate crude product is dissolved in ultrapure water, prepared into a solution with a mass concentration of 90%, filtered through a 1 μm membrane stack to remove water-insoluble impurities to obtain a leuprolide acetate solution; pamoate disodium salt Dissolve in ultrapure water, prepare a solution with a mass concentration of 90%, and filter through a 1 μm membrane stack to remove water-insoluble impurities to obtain a pamoic acid disodium salt solution;
[0087] b. ...
Embodiment 3
[0104] A preparation method of leuprolide pamoate sustained-release preparation, comprising the following steps:
[0105] (1) Salt formation and purification: Leuprolide acetate and pamoic acid disodium salt (4,4'-methylenebis(3-hydroxy-2-naphthoic acid) disodium salt, CAS: 6640-22 -8, commercially available) is the synthetic leuprolide pamoate of raw material, then leuprolide pamoate is purified;
[0106] The specific process steps of salt formation are:
[0107] a. Filtration to remove impurities: Leuprolide acetate crude product is dissolved in ultrapure water, prepared into a solution with a mass concentration of 50%, filtered through a 1 μm membrane stack to remove water-insoluble impurities to obtain a leuprolide acetate solution; pamoate disodium salt Dissolve in ultrapure water, prepare a solution with a mass concentration of 50%, and filter through a 1 μm membrane stack to remove water-insoluble impurities to obtain a pamoic acid disodium salt solution;
[0108] b. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Intrinsic viscosity | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com