Medical device with drug
a technology of stent and drug, which is applied in the field of expandable stent prosthesis, can solve the problems of reducing the cross-sectional area available for flow through the stent, reducing the relative amount of open space in the structure, and reducing the ability to load and deliver polymeric stents using vascular catheter delivery systems, so as to reduce the burst effect, increase the release time, and increase the density
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
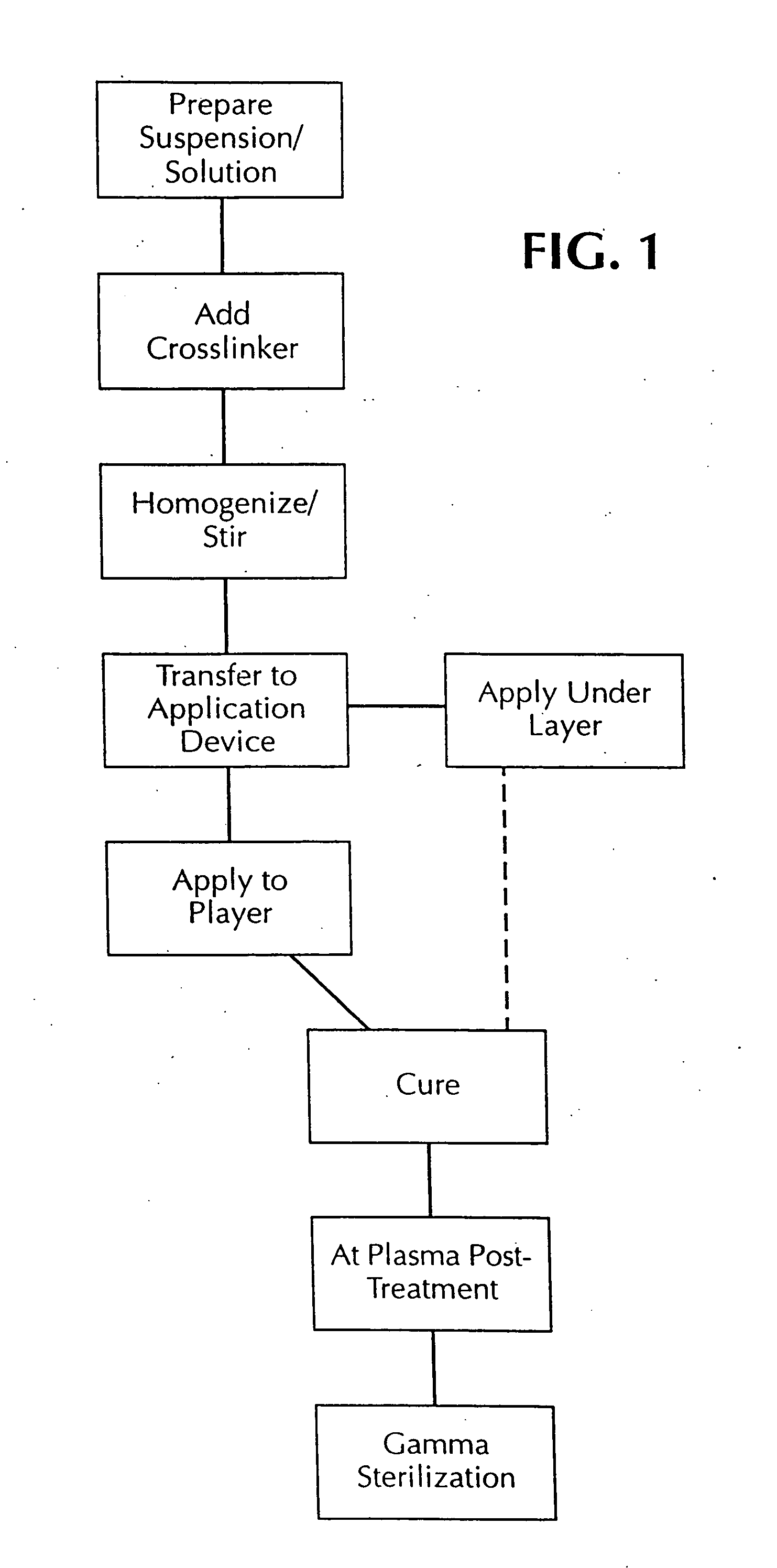
[0042] According to the present invention, the stent coatings incorporating biologically active materials for timed delivery in situ in a body lumen of interest are preferably sprayed in many thin layers from prepared coating solutions or suspensions. The steps of the process are illustrated generally in FIG. 1. The coating solutions or suspensions are prepared at 10 as will be described later. The desired amount of crosslinking agent is added to the suspension / solution as at 12 and material is then agitated or stirred to produce a homogenous coating composition at 14 which is thereafter transferred to an application container or device which may be a container for spray painting at 16. Typical exemplary preparations of coating solutions that were used for heparin and dexamethasone appear next.
General Preparation of Heparin Coating Composition
[0043] Silicone was obtained as a polymer precursor in solvent (xylene) mixture. For example, a 35% solid silicone weight content in xylene...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| weight percent | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com