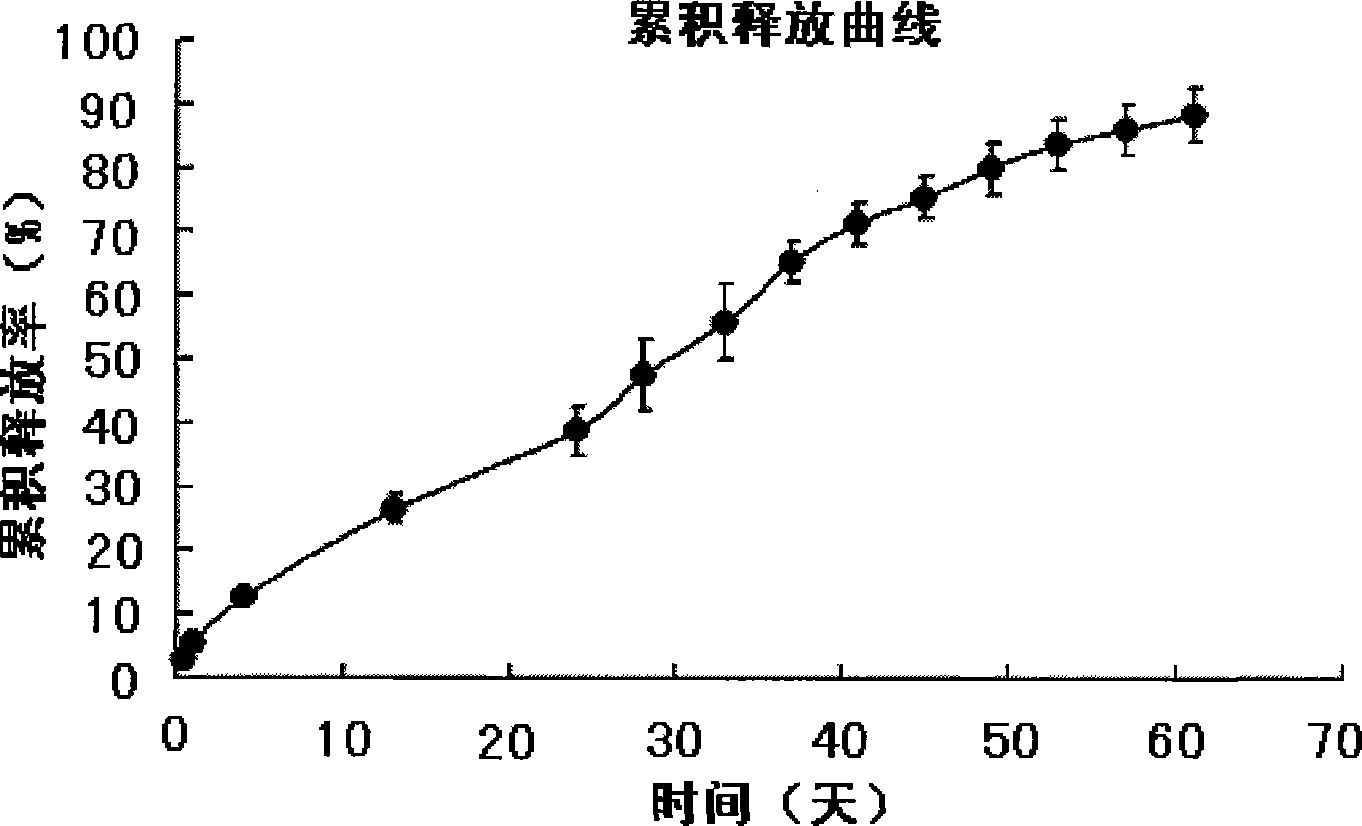
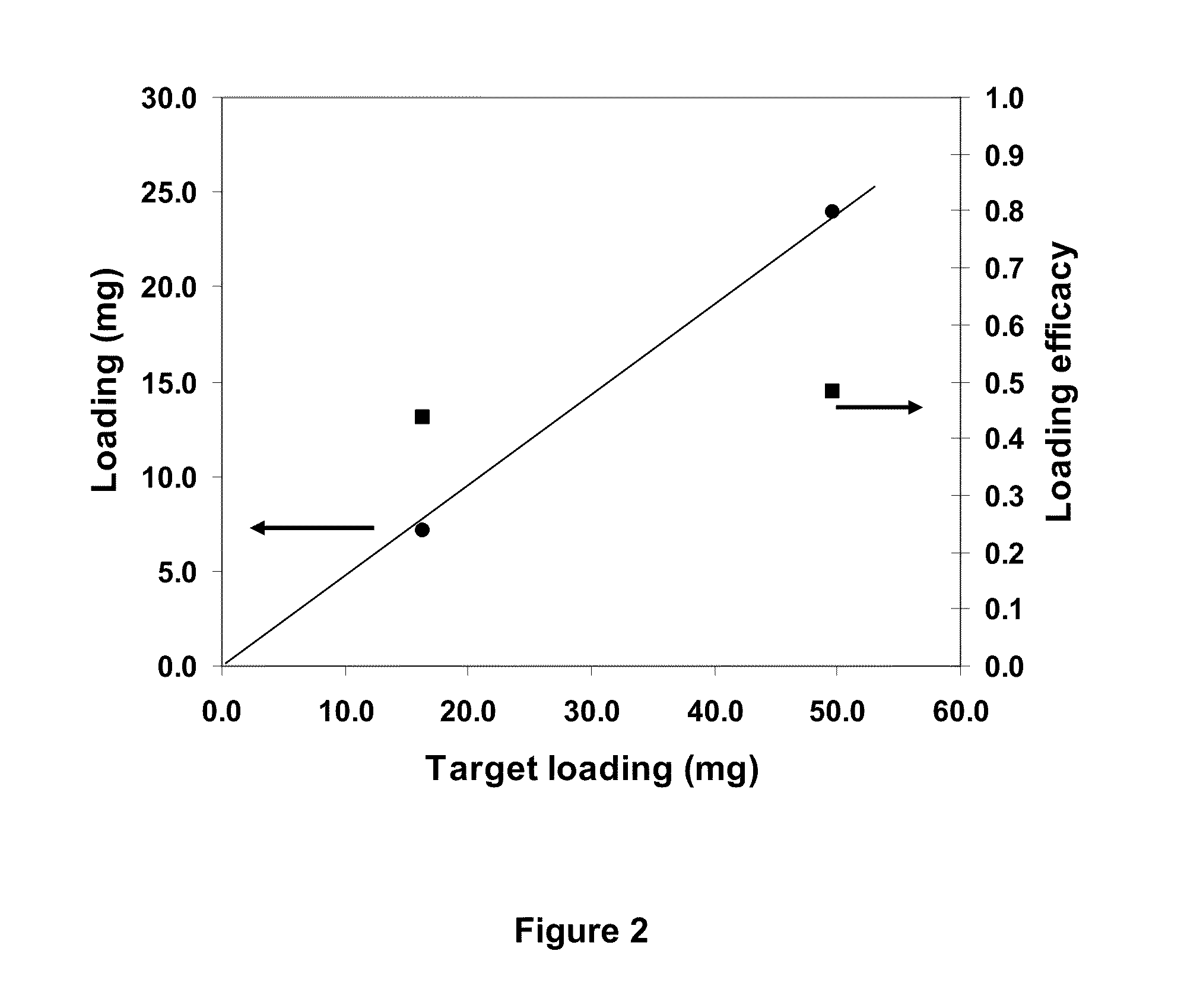
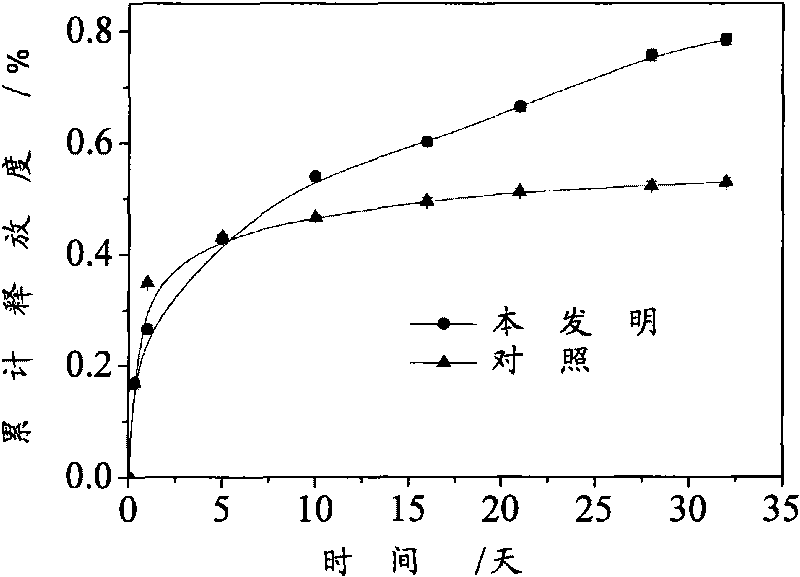
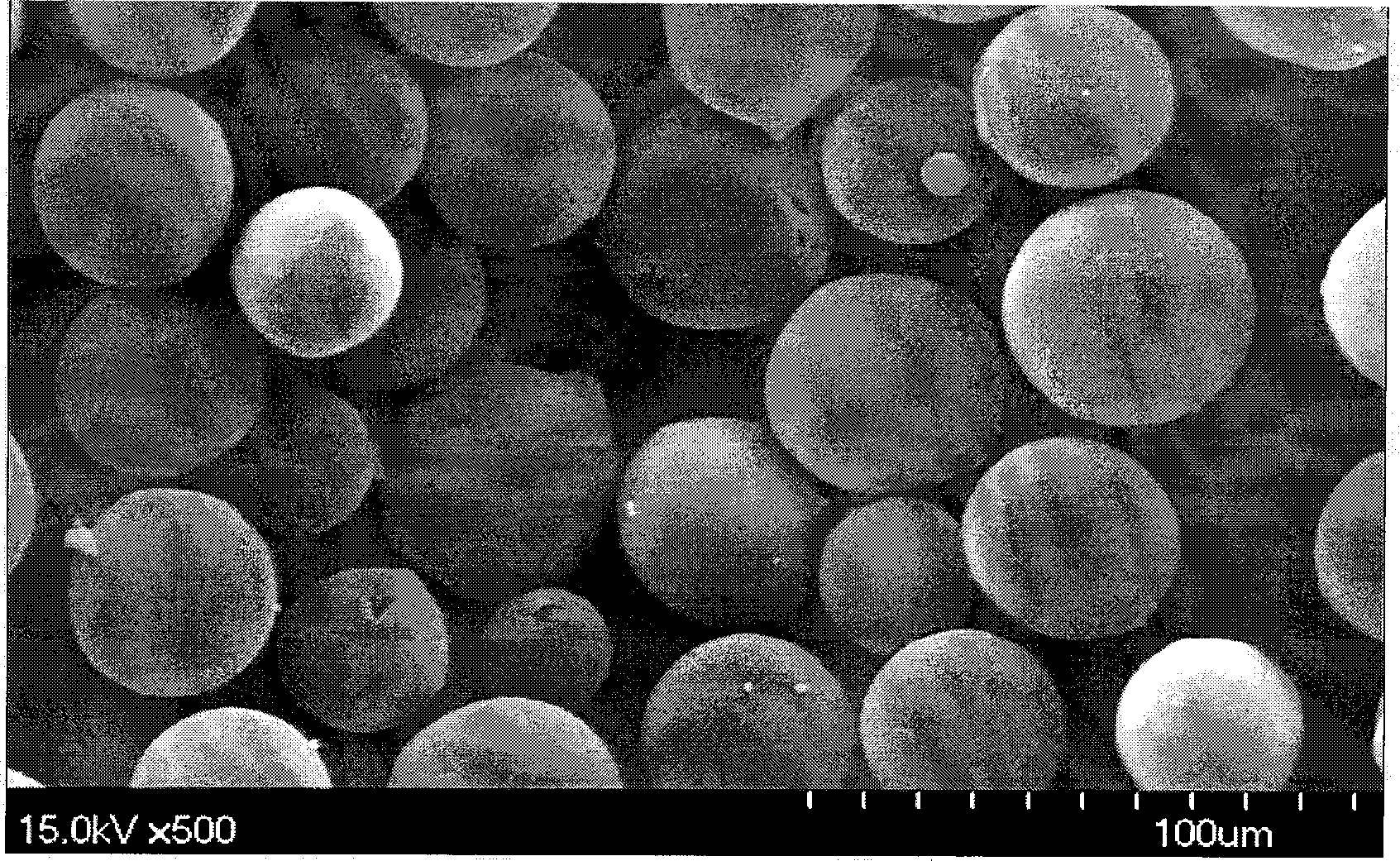Patents
Literature
69 results about "Burst effect" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
"Burst, Emanation, or Spread: Most spells that affect an area function as a burst, an emanation, or a spread. In each case, you select the spell's point of origin and measure its effect from that point.".
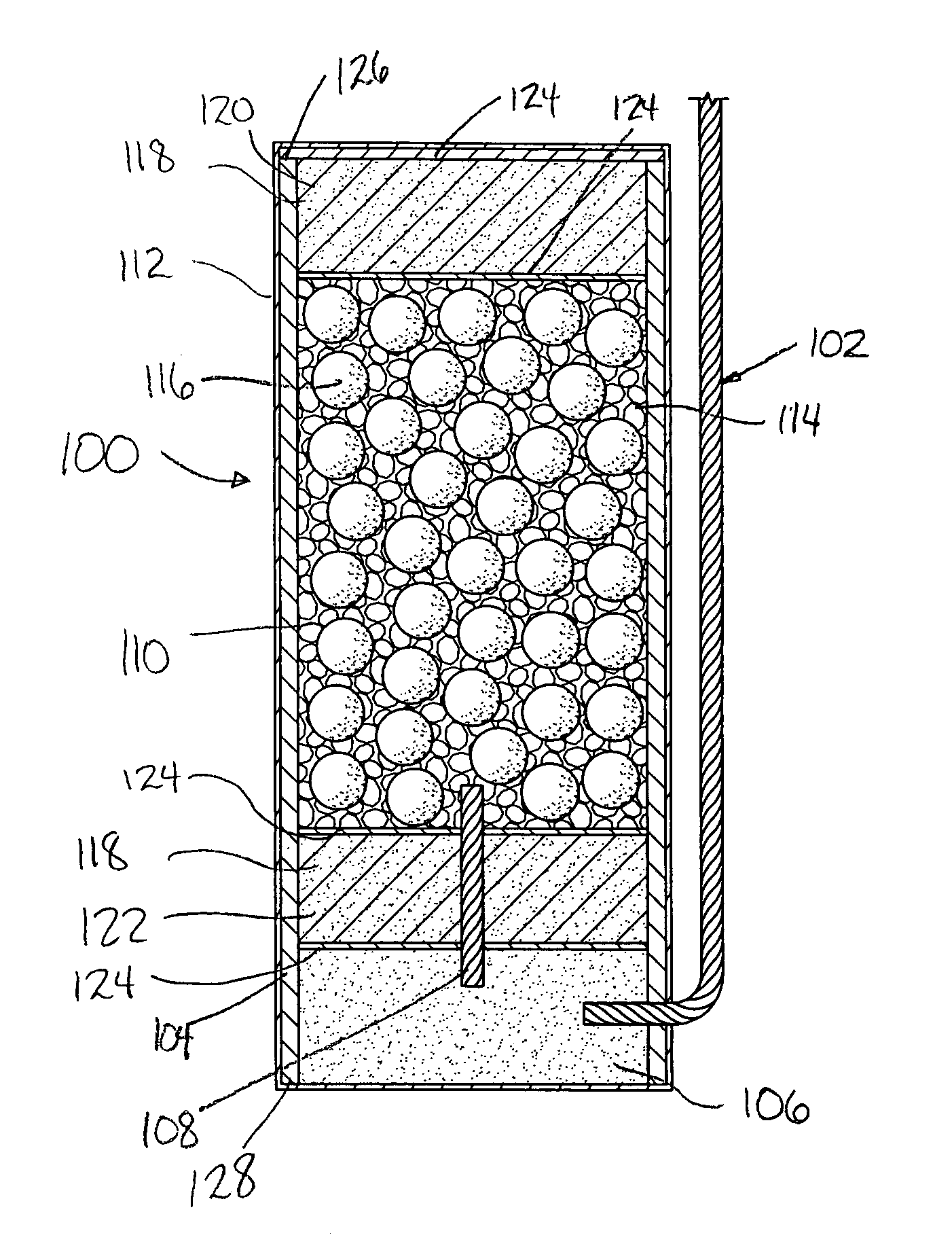
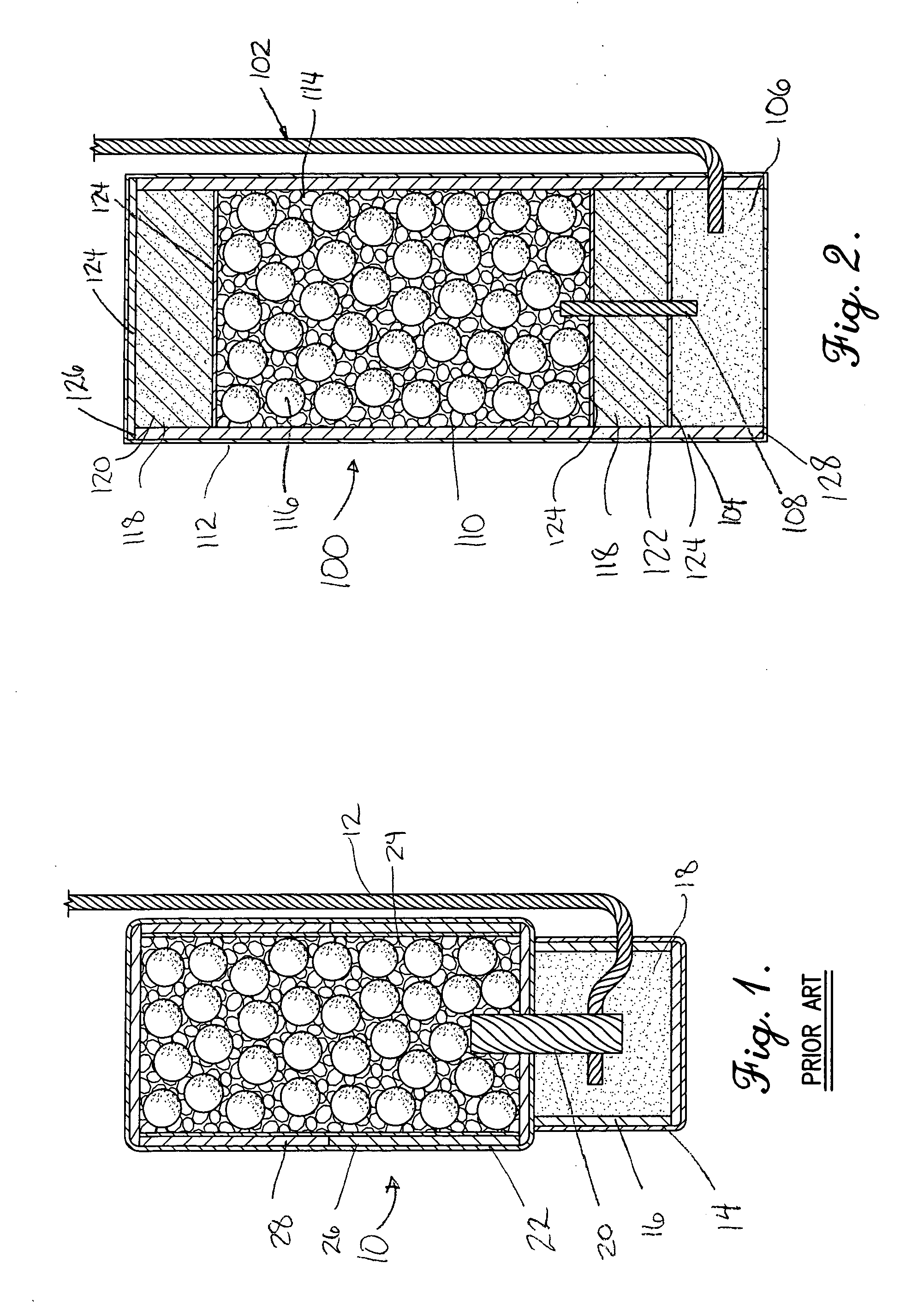
Controlled drug delivery system using the conjugation of drug to biodegradable polyester
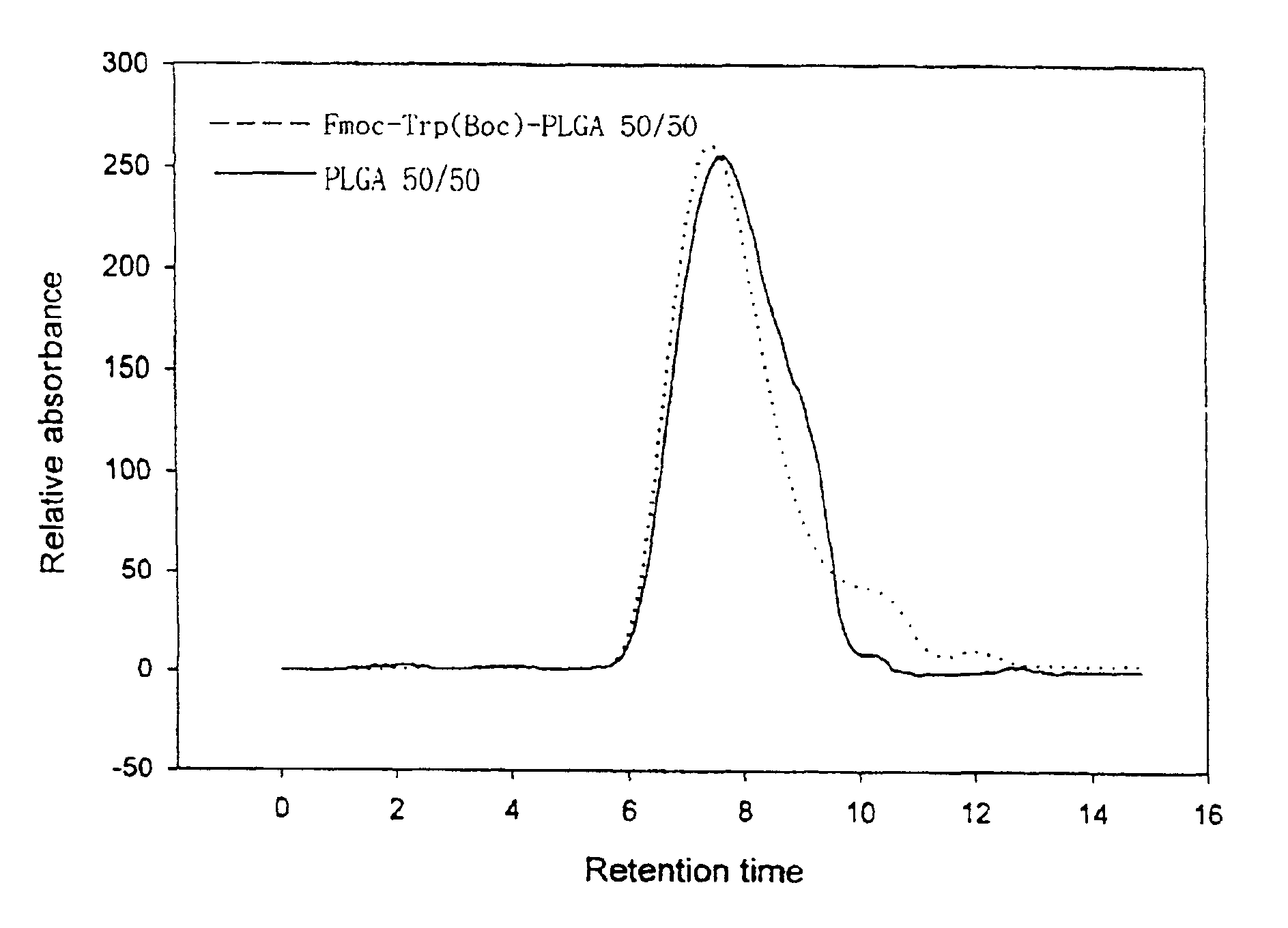
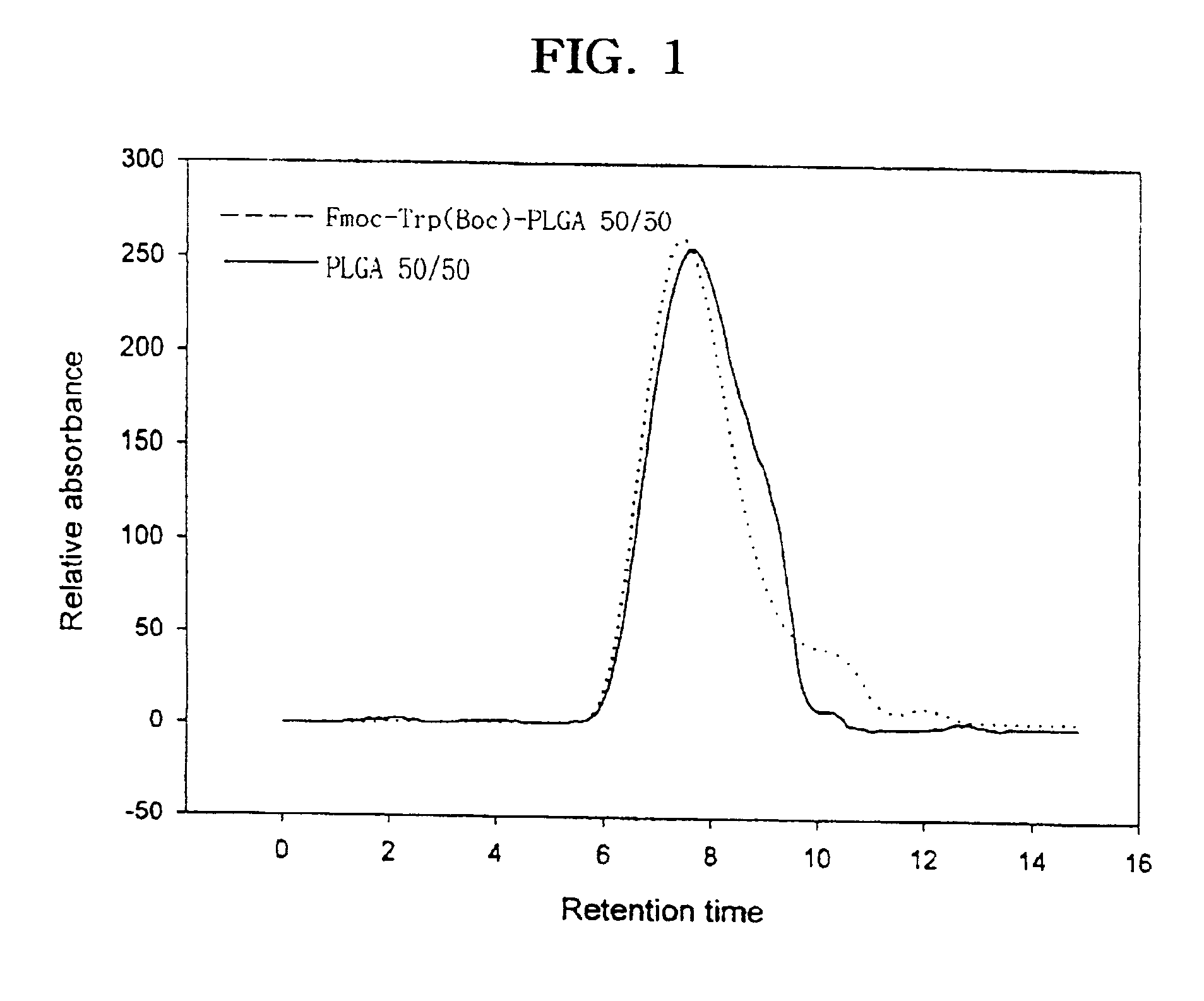
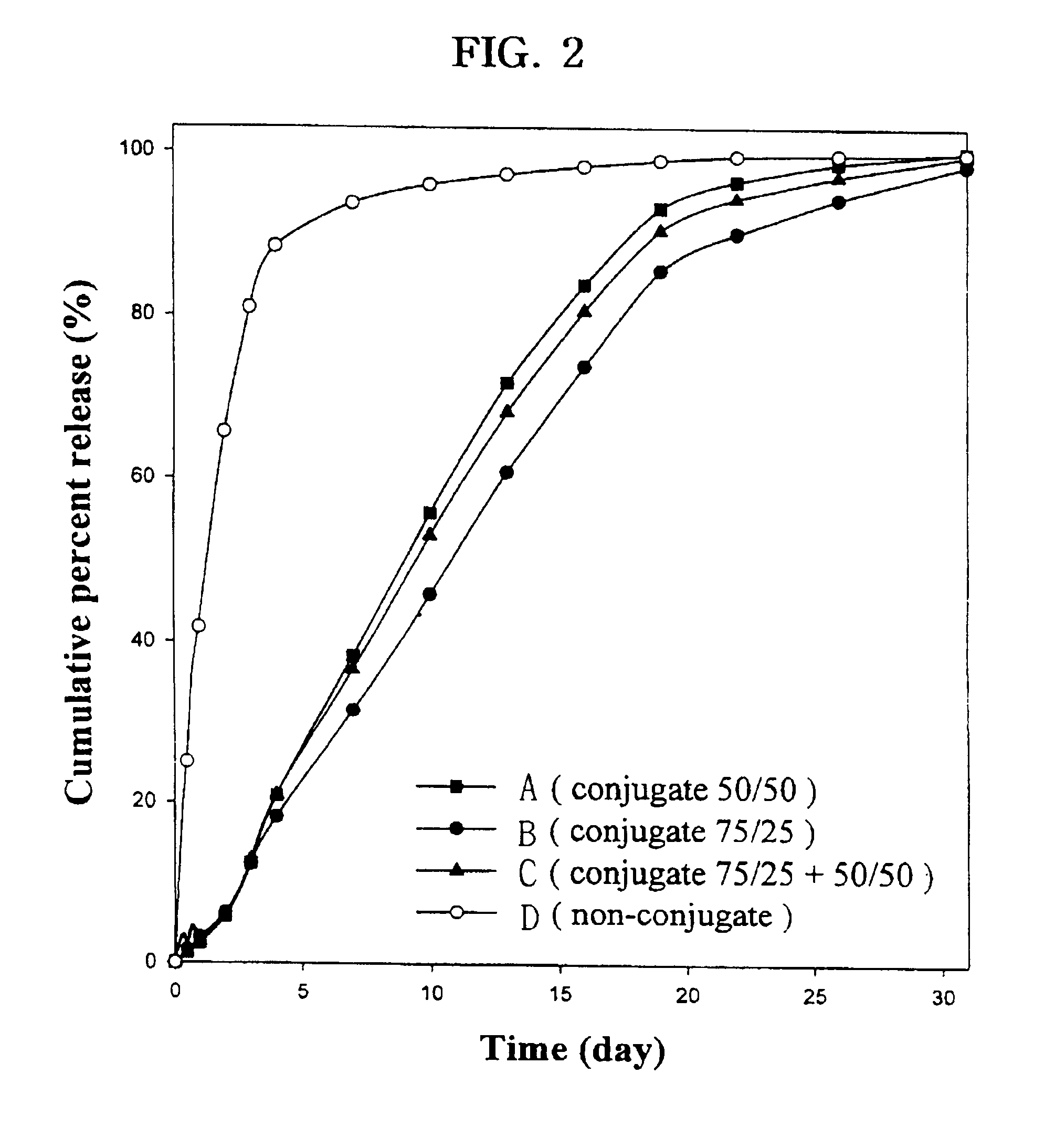
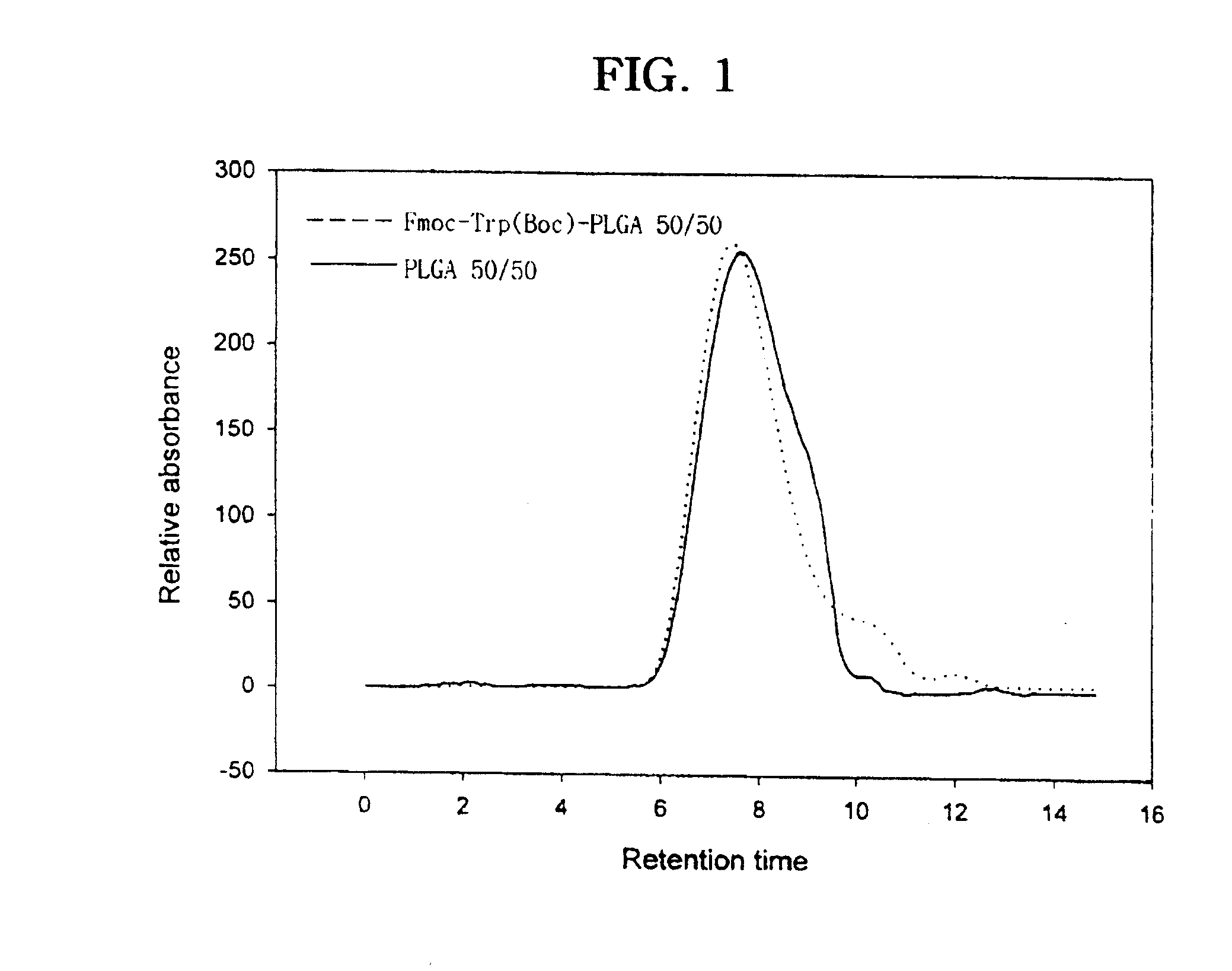
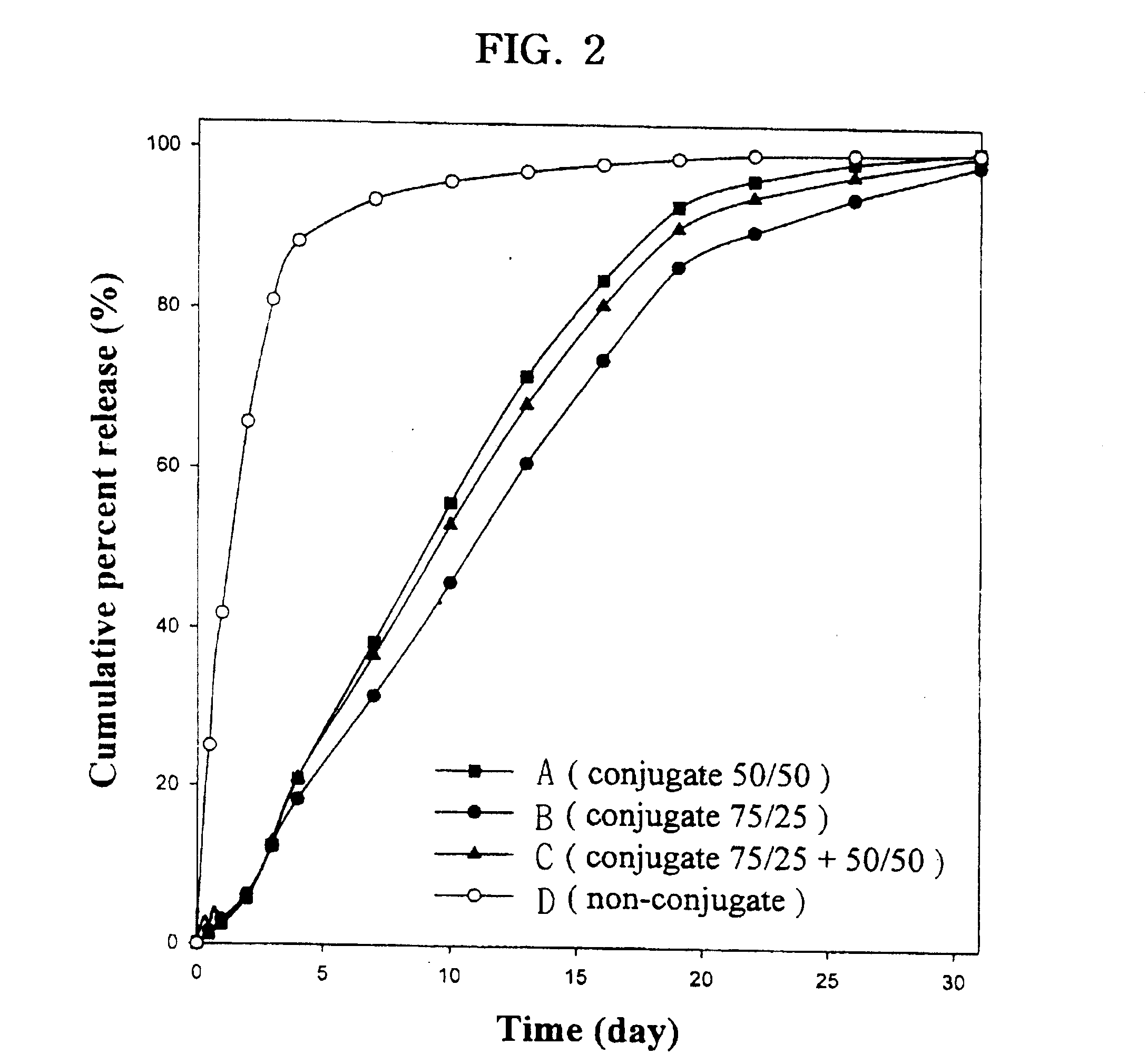
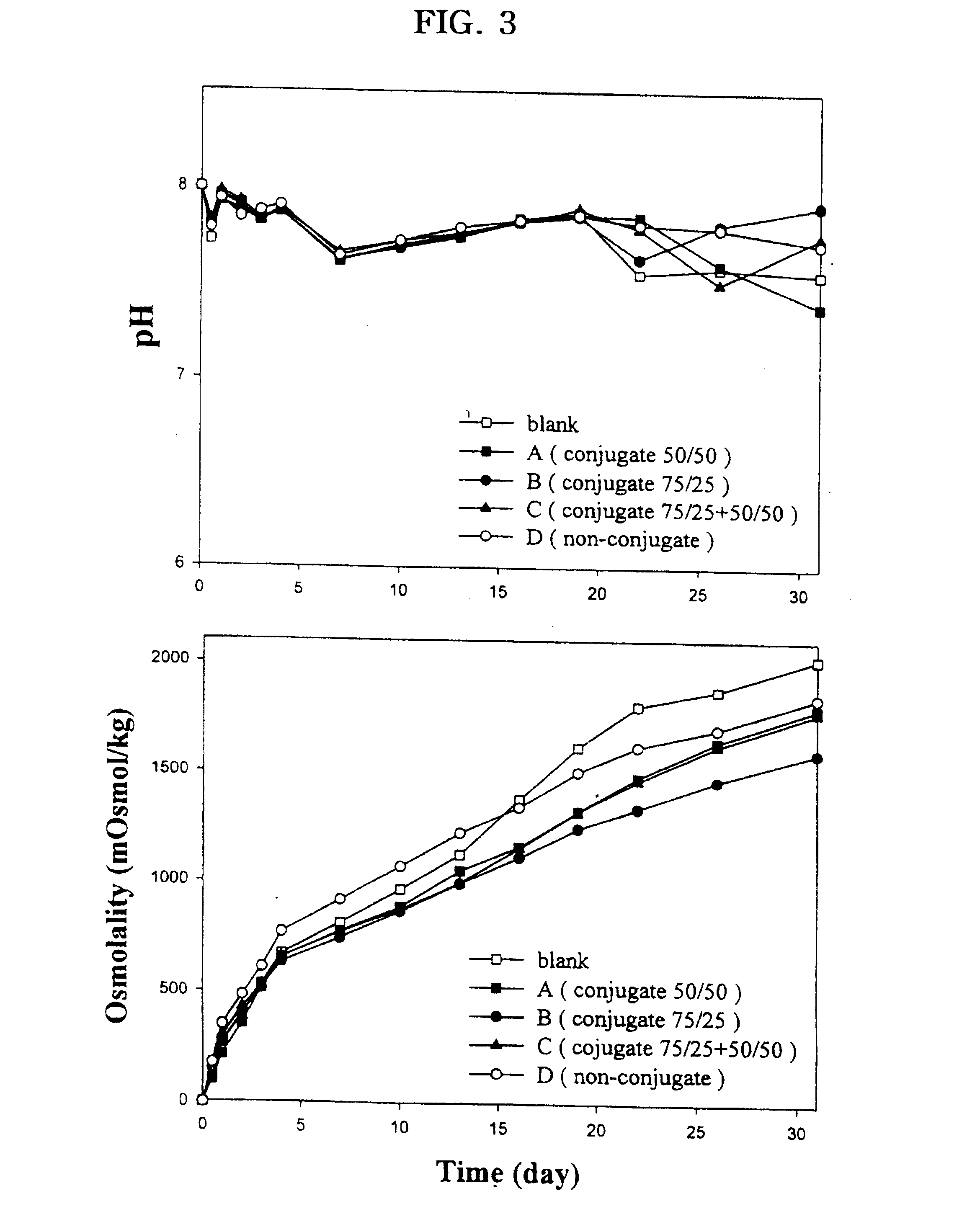
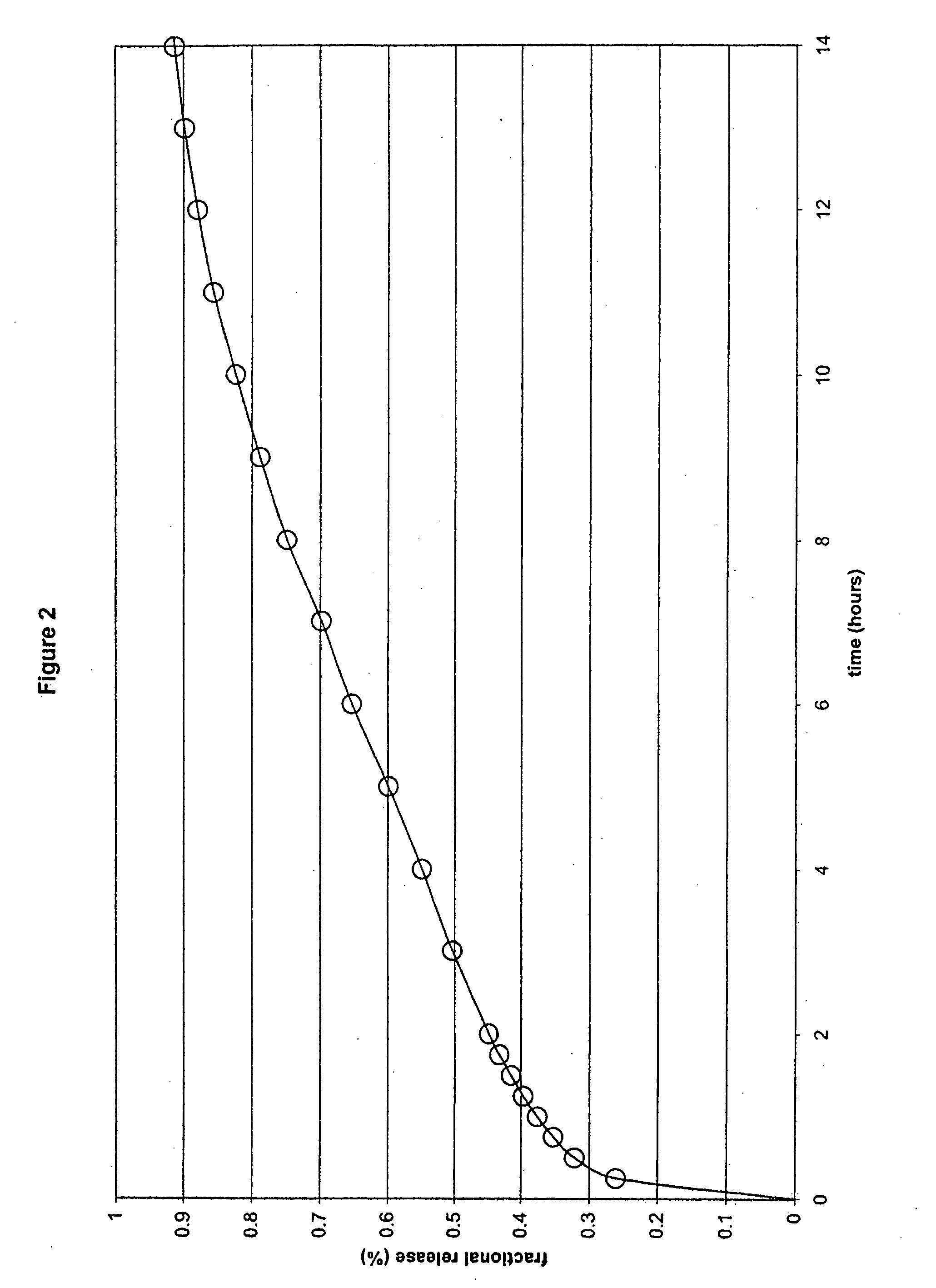
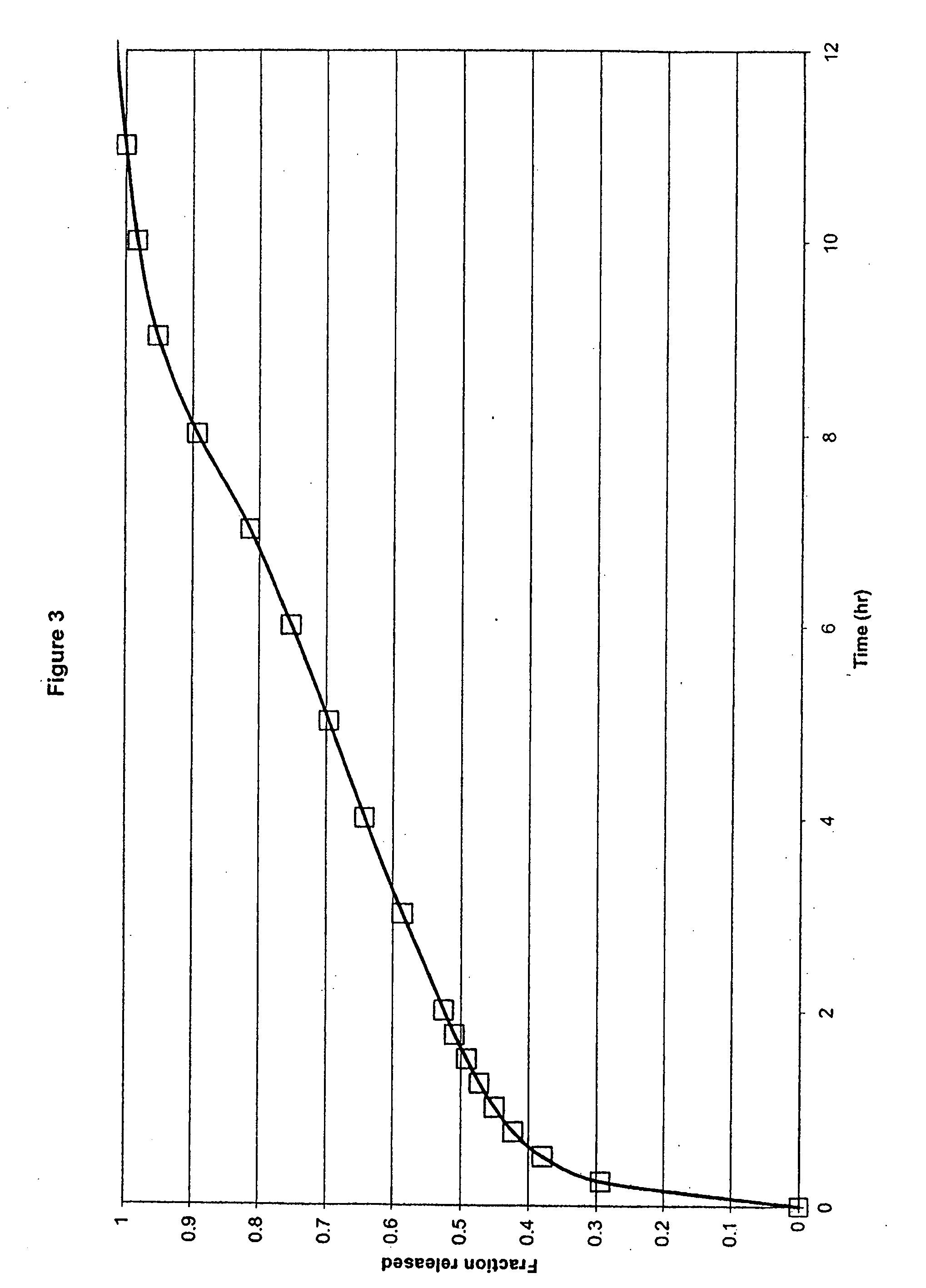
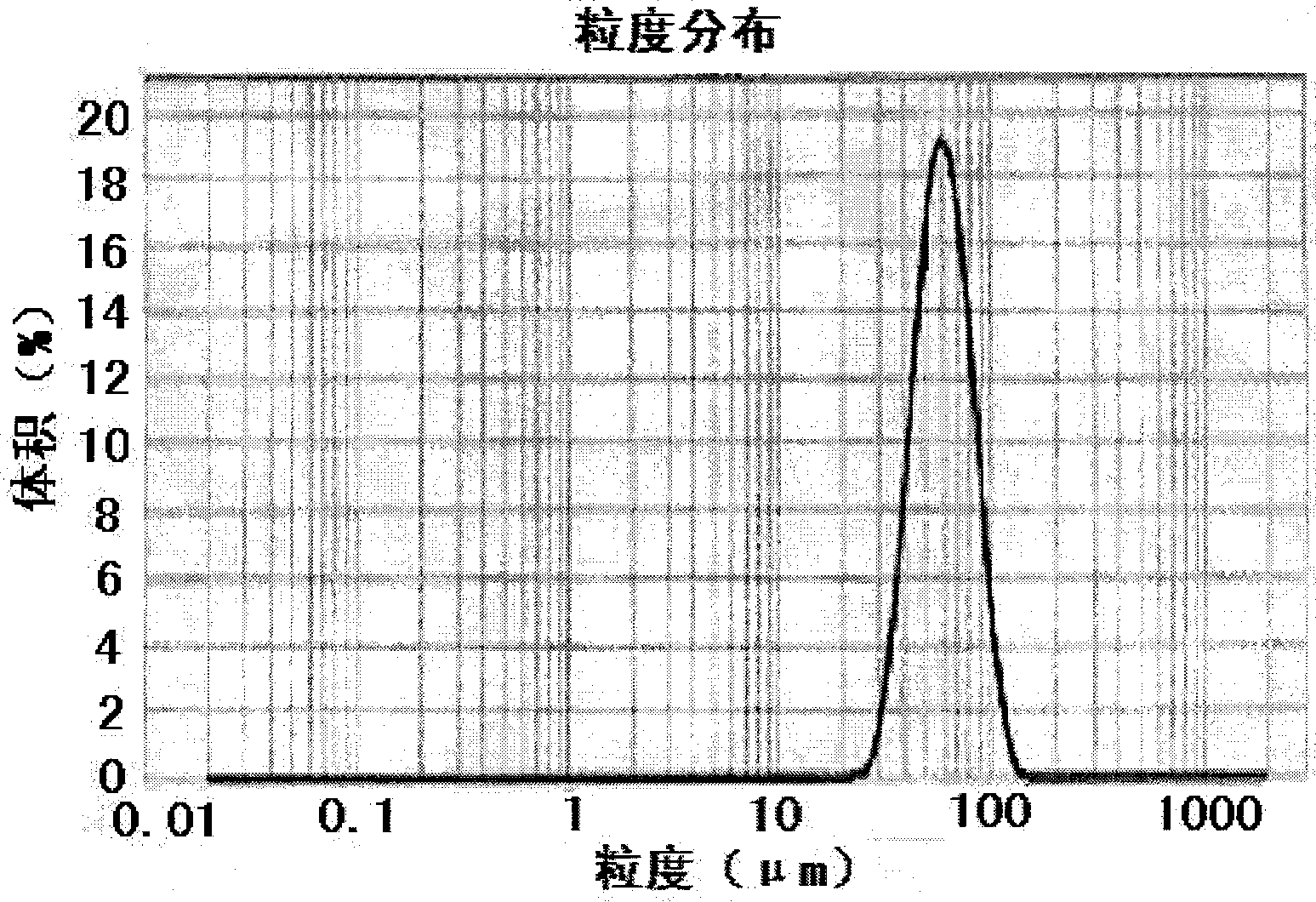
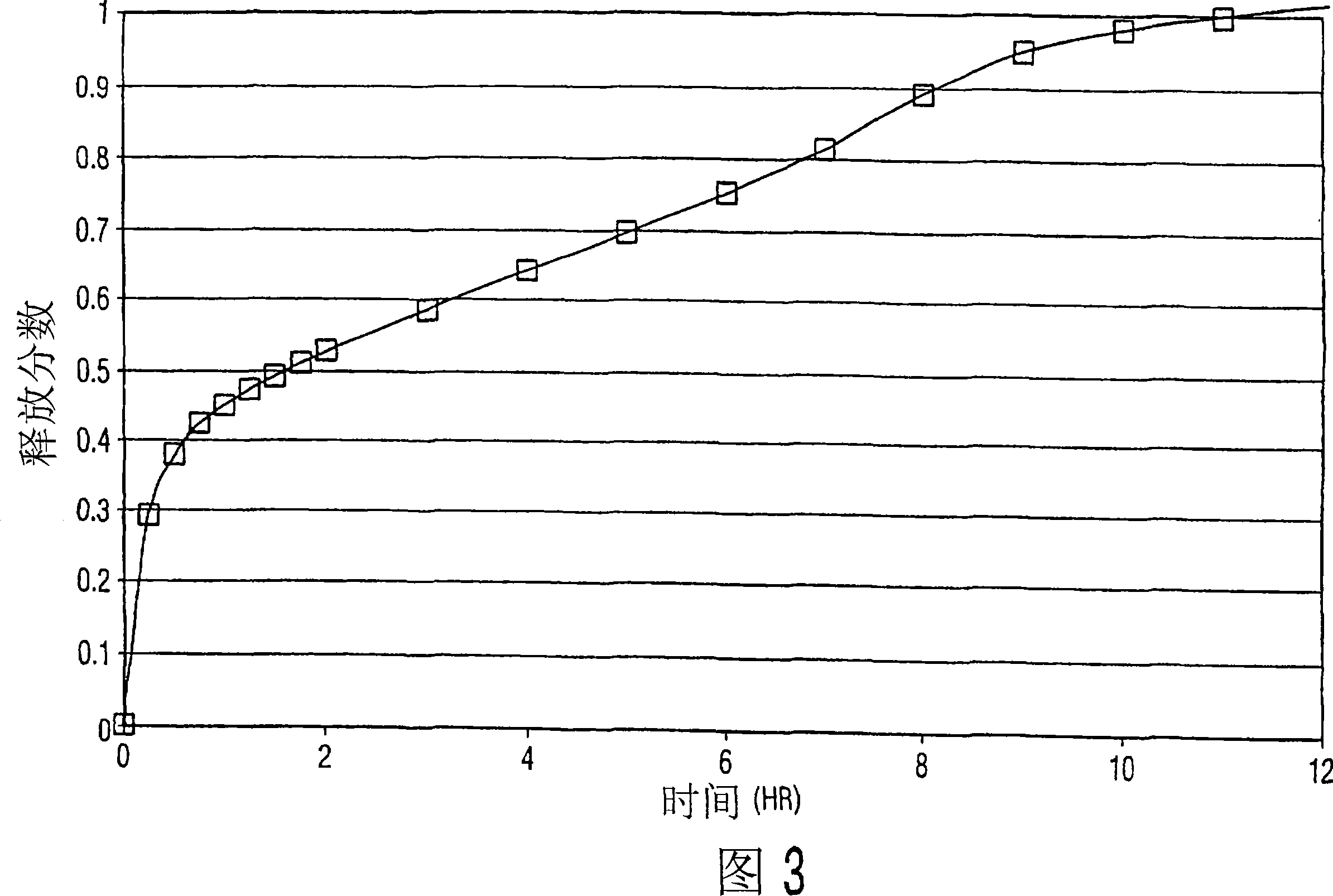
The present invention relates to a molecular sustained controlled release system constructed by the conjugation of molecules to be released with biodegradable polyester polymer via covalent bond and method for preparation thereof. In accordance with the present invention, the system may be formulated into microspheres, nanoparticles, or films. The molecular release rate from the above system can be regulated to be proportional to the chemical degradation rate of the biodegradable polyester polymers, resulting in near zero order kinetics profile of release without showing a burst effect, Moreover, a high loading efficiency of hydrophilic drugs can be achieved.
Owner:MOGAM BIOTECH RES INST +1
Controlled drug delivery system using the conjugation of drug to biodegradable polyester
The present invention relates to the molecular sustained controlled release system constructed by the conjugation of molecules to be released with biodegradable polyester polymer via covalent bond and method for preparation thereof. In accordance with the present invention, the system may be formulated into microspheres, nanoparticles, or films. The molecular release rate from the above system can be regulated to be proportional to the chemical degradation rate of the biodegradable polyester polymers, resulting in near zero order kinetics profile of release without showing a burst effect. Moreover, the high loading efficiency of hydrophilic drugs can be achieved.
Owner:OH JONG EUN +3
Adsorption method for preparing hydroxyapatite pesticide and slow-release fertilizer compound
InactiveCN101589709AHas biodegradable propertiesQuality improvementBiocideAnimal repellantsBurst effectPhosphate
The invention discloses an adsorption method for preparing hydroxyapatite pesticide and slow-release fertilizer compound, which comprises the following steps: soaking a nano hydroxyapatite carrier in phosphate buffer solution, then adding a starting material into the buffer solution and continuously stirring the mixture to form compound solution, finally centrifuging the compound solution to obtain sediment, and drying the sediment to obtain the compound. By using a porous structure of the nano hydroxyapatite self to embed medicaments into an internal grid of the carrier, the burst effect of the medicaments is avoided, the medicinal effect is long, the medicament residue is greatly reduced, and the medicaments are safer. A product obtained by using the nano hydroxyapatite as a pesticide and fertilizer carrier has the advantages of reliable quality, strong stability, safe use and low cost, greatly improves the medicament loading amount and adsorption amount, is easy for continuous production, and has good application prospect.
Owner:JILIN UNIV
Macromer-melt formulations
InactiveUS20070053954A1Low burst effectIncrease investmentPowder deliverySpray deliveryMedicineBurst effect
The invention provides methods and articles for the administration of a biologically active substance (BAS). These methods and articles provide for the controlled and sustained delivery of relatively large quantities of these substances with a low burst effect. The articles made using the method of the invention have increased percentages (w / w) of macromer, increased crosslinking density, and reduced pore size in comparison to articles made using solution methods.
Owner:ROWE STEPHEN C +1
Absorbable magnesium alloy stent of anticorrosion and drug release composite coating and preparation method thereof
InactiveCN101721266AExtension of timeSolve the problem of excessive release concentrationStentsMedical devicesControlled releasePlasma electrolytic oxidation
The invention relates to an absorbable magnesium alloy stent of anticorrosion and drug release composite coating and a preparation method thereof. The absorbable magnesium alloy stent comprises a magnesium alloy stent skeleton and composite coating, wherein the surface layer of the magnesium alloy stent skeleton is an inorganic anti-corrosion coating, the outside of the inorganic anti-corrosion coating is provided with organic sealing and drug release composite coating; and the inorganic anti-corrosion coating is dense coating with metallurgical structure composed of MgSiO3, MgO and SiO2 with zeolite structure. The inorganic anti-corrosion coating is prepared on the surface of a matrix through microarc oxidation, and the sealing and drug release composite coating composed of crosslinked gelatin / PLGA medicine-carried nanospheres blend film is prepared outside the anti-corrosion coating. The absorbable magnesium alloy stent of the invention effectively controls the corrosivity of the magnesium alloy stent, the organic coating has double functions, the use of the sealing inorganic coating increases the corrosion resistance, controls the drug to release, and reduces the burst effect of the drug to ensure that the drug is released in a certain concentration continuously and slowly. Meanwhile, each coating can be biodegraded and the degradation product is non-toxic, thus effectively improving the biocompatibility and blood compatibility of the magnesium alloy surface.
Owner:TIANJIN UNIV
Preparation method of pH-sensitive coaxial drug-loading nanometer fiber membrane
InactiveCN104337755ASimple processLow costOrganic active ingredientsFilament/thread formingFiberPolymer science
The invention relates to a preparation method of a pH-sensitive coaxial drug-loading nanometer fiber membrane. The method comprises the following steps: dissolving a high polymer material with pH-sensitivity into ethanol to obtain a transparent and clear high polymer solution serving as a shell material; and in addition, dissolving a medicine and a high polymer material into ethanol or a water solution, and mixing evenly to obtain a core liquid; and pouring the obtained spinning solution into an injection propeller, and spinning by using a coaxial electrostatic spinning method to obtain the drug-loading nanometer fiber membrane. The method is simple in process, and low in cost; a pH-sensitive material is applied to a shell layer in coaxial fibers; and the material with good drug compatibility is applied to a drug-loading core layer, so that the drug burst effect can be effectively avoided; and the prepared nanometer fiber membrane can achieve pH-response drug release, and has a relatively good application prospect in targeted drug delivery of gastrointestinal tracts.
Owner:DONGHUA UNIV
Micro-embedded medicament carrier and preparation method thereof
ActiveCN101947212AGuaranteed Space AllocationPromote absorptionPharmaceutical non-active ingredientsMicrocapsulesNanoparticleBurst effect
The invention discloses a micro-embedded medicament carrier and a preparation method for the carrier. A nano-carrier is embedded in a microcapsule so as to form a medicament carrier of a micro-embedded system, and medicaments are respectively carried in nanoparticles or among different nanoparticles in the microcapsule. The micro-embedded medicament carrier solves the problems of medicament burst effect, single means of adjusting a medicament dissolution rate, insufficient protection of medicament activity and possible medicament cross contamination in medicament combination sustained-release treatment of the conventional medicament sustained-release system, and has the characteristics of simple process, convenient operation, high practicality and wide application prospect.
Owner:HUAQIAO UNIVERSITY
Oral slow/controlled-release preparation containing febuxostat and preparation method thereof
ActiveCN101773498AImprove securityEffective plasma concentrationOrganic active ingredientsSkeletal disorderBlood concentrationBurst effect
The invention provides an oral slow / controlled-release preparation containing febuxostat and a preparation method thereof, which prepare the febuxostat into the long-acting oral slow / controlled-release preparation and can solve the problem that the incidence of an adverse reaction is increased because of the quicker dissolving-out and the burst effect of a common preparation existing in the prior art. The invention has the technical scheme that the oral slow / controlled-release preparation containing the febuxostat comprises the following components by weight percent: 5 to 60 percent of febuxostat, 10 to 50 percent of slow / controlled-release material, 20 to 80 percent of filling auxiliary material, 0.3 to 20 percent of adhesive and 0.1 to 7 percent of lubricant or glidant. Compared with a common quick-release preparation, the slow / controlled-release preparation can keep the effective and stable blood concentration for a longer time, avoids the burst effect of the quick-release preparation, lowers the incidence of the adverse reaction and enhances the application safety.
Owner:QINGDAO HUANGHAI PHARM CO LTD
Modified release ibuprofen dosage form
The present invention is a solid dosage form for oral administration of ibuprofen comprising a modified release formulation of ibuprofen which provides an immediate burst effect and thereafter a sustained release of sufficient ibuprofen to maintain blood levels at least 6.4 μg / ml over an extended period of at least 8 hours following administering of a single dose.
Owner:SCOLR PHARMA
Asiatic acid injectable sustained-release microballoons and preparation method thereof
InactiveCN101474157ABiodegradableStable release rateOrganic active ingredientsPharmaceutical non-active ingredientsCross-linkBurst effect
The invention relates to the technical field of pharmaceutical preparation, in particular to an asiatic acid sustained-release microsphere used for injection and a preparation method thereof. A biodegradable chitosan is taken as a carrier, an asiatic acid is taken as principal agent, and the asiatic acid sustained-release microsphere used for injection is obtained by the technology of cross-linked emulsion. Due to the obtained medicament carried microspheres, the surfaces are relatively smooth; the particle diameters range from 20mum to 110mum; and the medicament loading rate of the microspheres ranges from 4.8% to 15.6%. The asiatic acid microsphere has the characteristics that the burst effect is modest (the burst size on the first day is smaller than 15%); the release rate is stable; the sustained release time is long (4-10 weeks); and the asiatic acid microsphere is biodegradable. The implantation and the removal by operation before and after the medicament is used are avoided.
Owner:ZHEJIANG ACAD OF MEDICAL SCI
Dual drug-loading composite microsphere and preparation method thereof
InactiveCN101658497AGood compatibilityImprove hydrophilicityPeptide/protein ingredientsPharmaceutical non-active ingredientsBurst effectMicrosphere
The invention discloses a dual drug-loading composite microsphere and a preparation method thereof, belonging to the technical field of biomedical materials. The composite microsphere has a microsphere-in-microsphere structure, a matrix consists of polylactic-co-glycolic acid microspheres in the interior and a chitosan shell at the outer layer, and drugs are respectively embedded in the interior microspheres and the shell, wherein the total drug-loading rate is 1 to 10 percent, the average grain diameter of the interior microspheres is 100-1000nm, and the average grain diameter of the composite microspheres is 10-100 mum. The invention also discloses the preparation method of the composite microsphere. The composite microsphere has good biocompatibility and biodegradability, and can control drug adding amount of the two times or the drug concentration or the drug species, thus reducing burst effect and alleviating the acidity caused by PLGA degradation.
Owner:TSINGHUA UNIV
Doxycycline-hyclate-carried GTR/GBR composite membrane and preparation method thereof
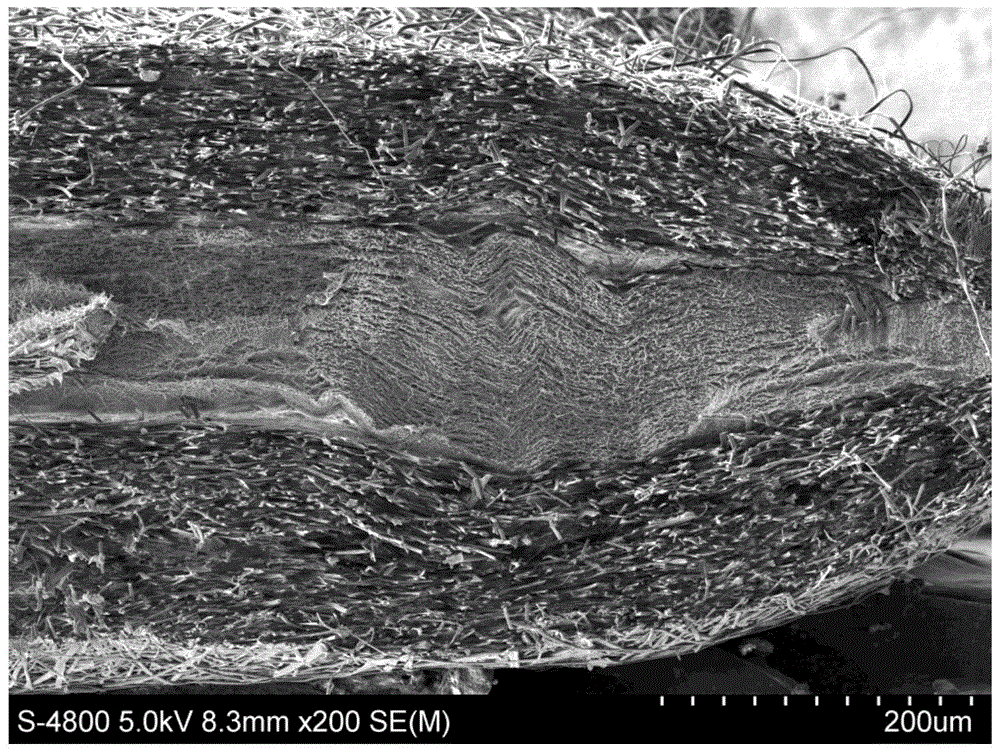
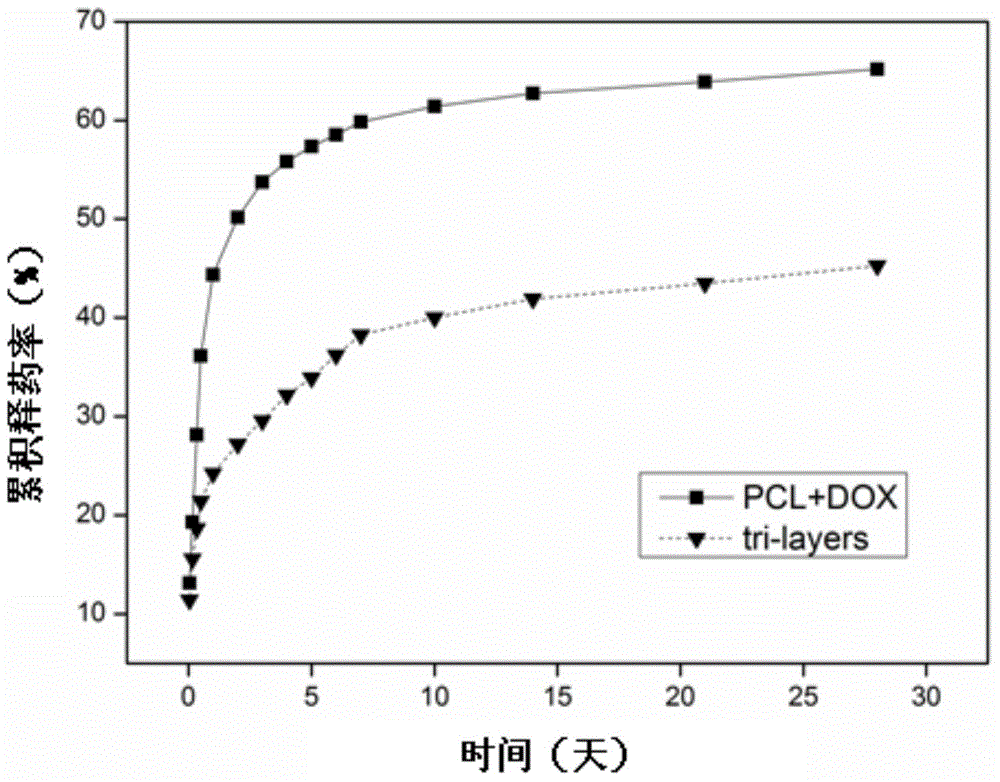
The invention discloses a doxycycline-hyclate-carried GTR (Guide Tissue Regeneration) / GBR (Guide Bone Regeneration) composite membrane and a preparation method thereof and belongs to the technical field of medicines. The GTR / GBR composite membrane adopts a three-layer structure, wherein the upper surface layer, the lower surface layer and the sandwich layer are all manufactured through sequence electrospinning; the upper surface layer and the lower surface layer are prepared from a natural polymer material and a mixed solution through which the polymer material is synthesized; the sandwich layer is a membrane layer prepared from a doxycycline-hyclate-carried solution through which the polymer material is synthesized. The uniaxial sequence electrospinning method is adopted to prepare the composite membrane adopting the three-layer structure, so that the medicine entrapment efficiency of fibers is improved, the preliminary burst effect of a medicine is reduced / eliminated, and continuously slow release of the medicine is realized. The two surface layers of the three-layer medicine-carried composite membrane can not only serve as porous barriers to control the medicine release rate so as to realize continuously slow release of the medicine but also prevent direct contact between the medicine and tissues to improve the compatibility between the tissues and the membrane so as to be more conducive to tissue repair and regeneration.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Modified release ibuprofen dosage form
The present invention is a solid dosage form for oral administration of ibuprofen comprising a modified release formulation of ibuprofen which provides an immediate burst effect and thereafter a sustained release of sufficient ibuprofen to maintain blood levels at least 6.4 μg / ml over an extended period of at least 8 hours following administration of a single dose. The dosage form releases ibuprofen at a rate sufficient to initially deliver a effective amount of ibuprofen within about 2.0 hours following administration. The dosage form then subsequently delivers the remaining amount of ibuprofen at a relatively constant rate sufficient to maintain a level of ibuprofen over a predetermined delivery period of for at least 8 hours.
Owner:SCOLR PHARMA
Fireworks artillery shell
InactiveUS6912958B2Increases burst presentationDriven more effectivelyFirework flares/torchesAerial display rocketsBurst effectFireworks
A fireworks artillery shell for use as a consumer firework which may be propelled by the use of a mortar is provided which includes a casing, a lift charge, an effects charge, a timing fuse and an ignition fuse, and seals. The seals are provided within the casing above and below the effects charge to increase the burst effect of the effects charge. The lift charge is positioned within the casing and below the lower seal, and upon ignition, lifts the fireworks artillery shell into the air. The seals promote a harder break and more explosive effect from the effects charge without interfering with the lifting charge.
Owner:JAKES FIREWORKS
Antimicrobial material and method for making the same
InactiveUS7998498B2Reduce infectionSubstantial unmet needAntibacterial agentsBiocidePolyethylene vinyl acetateBurst effect
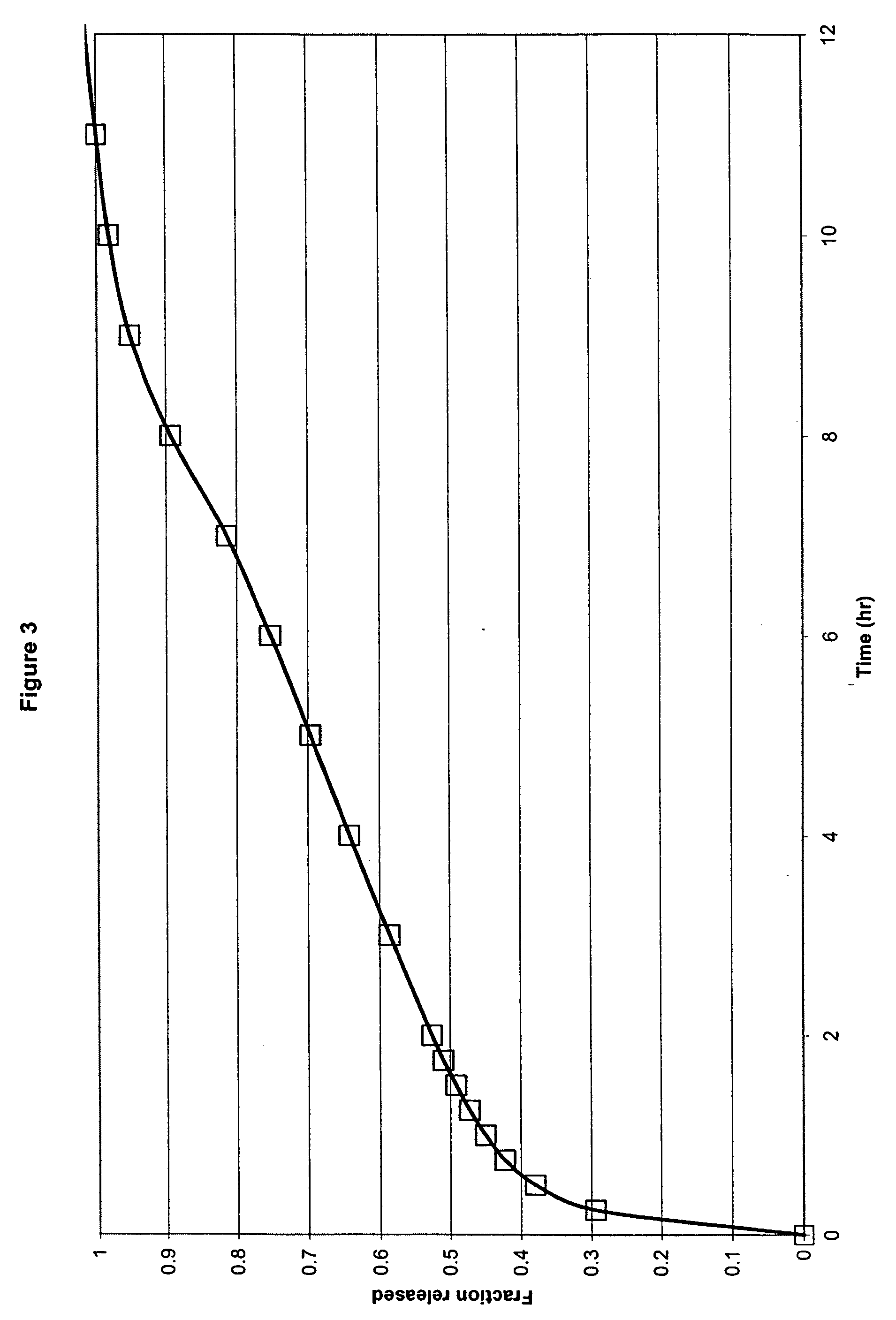
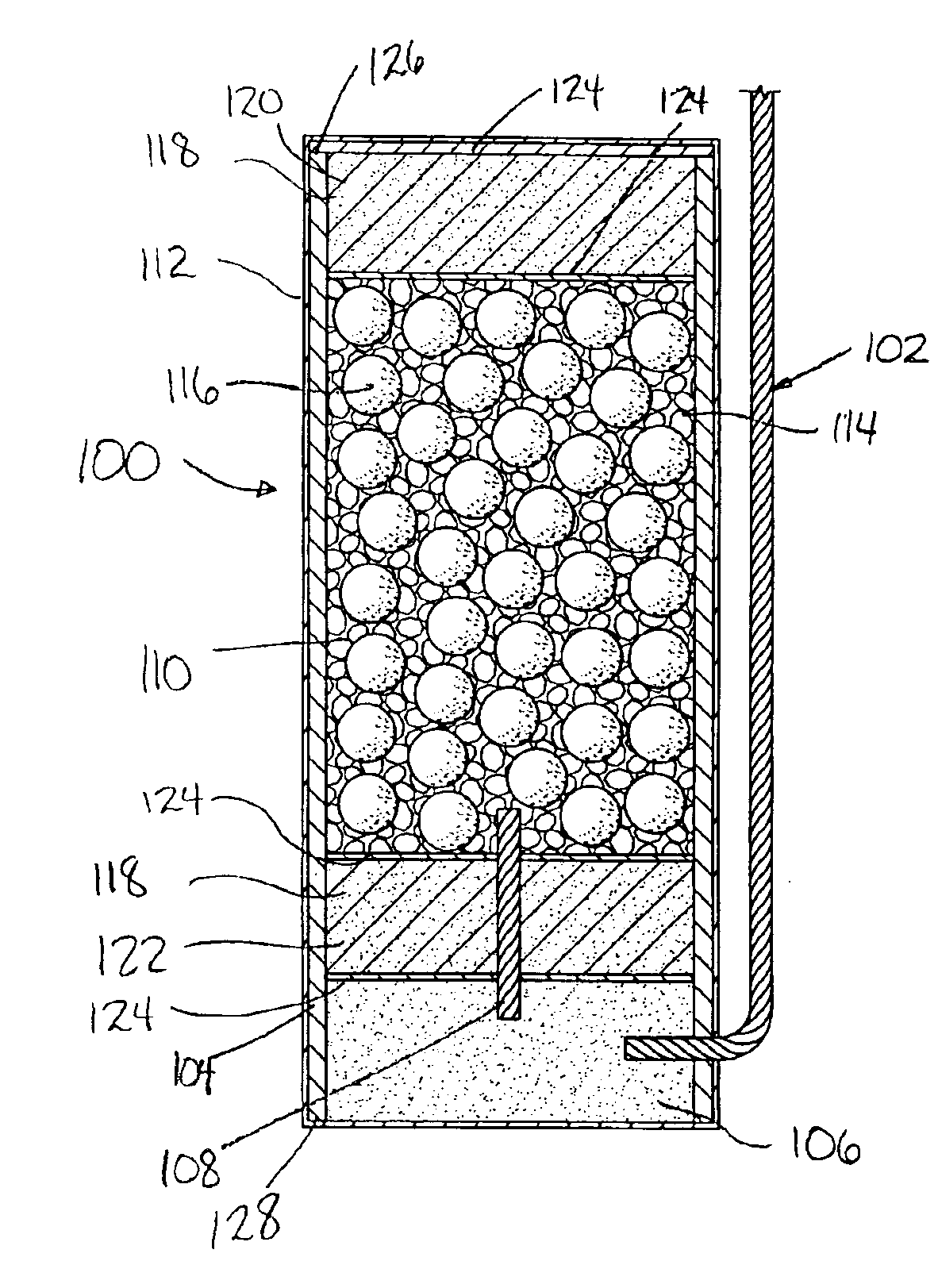
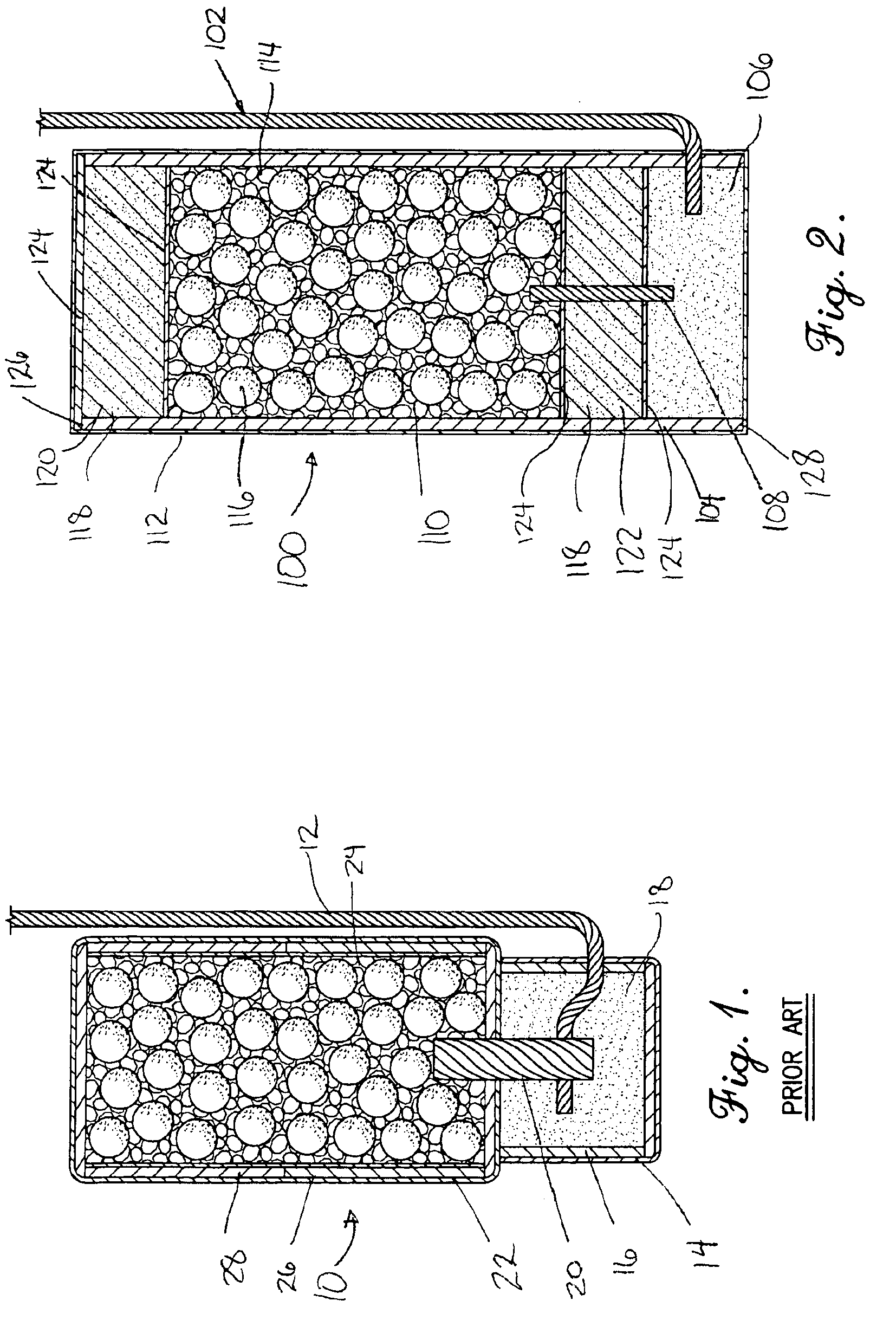
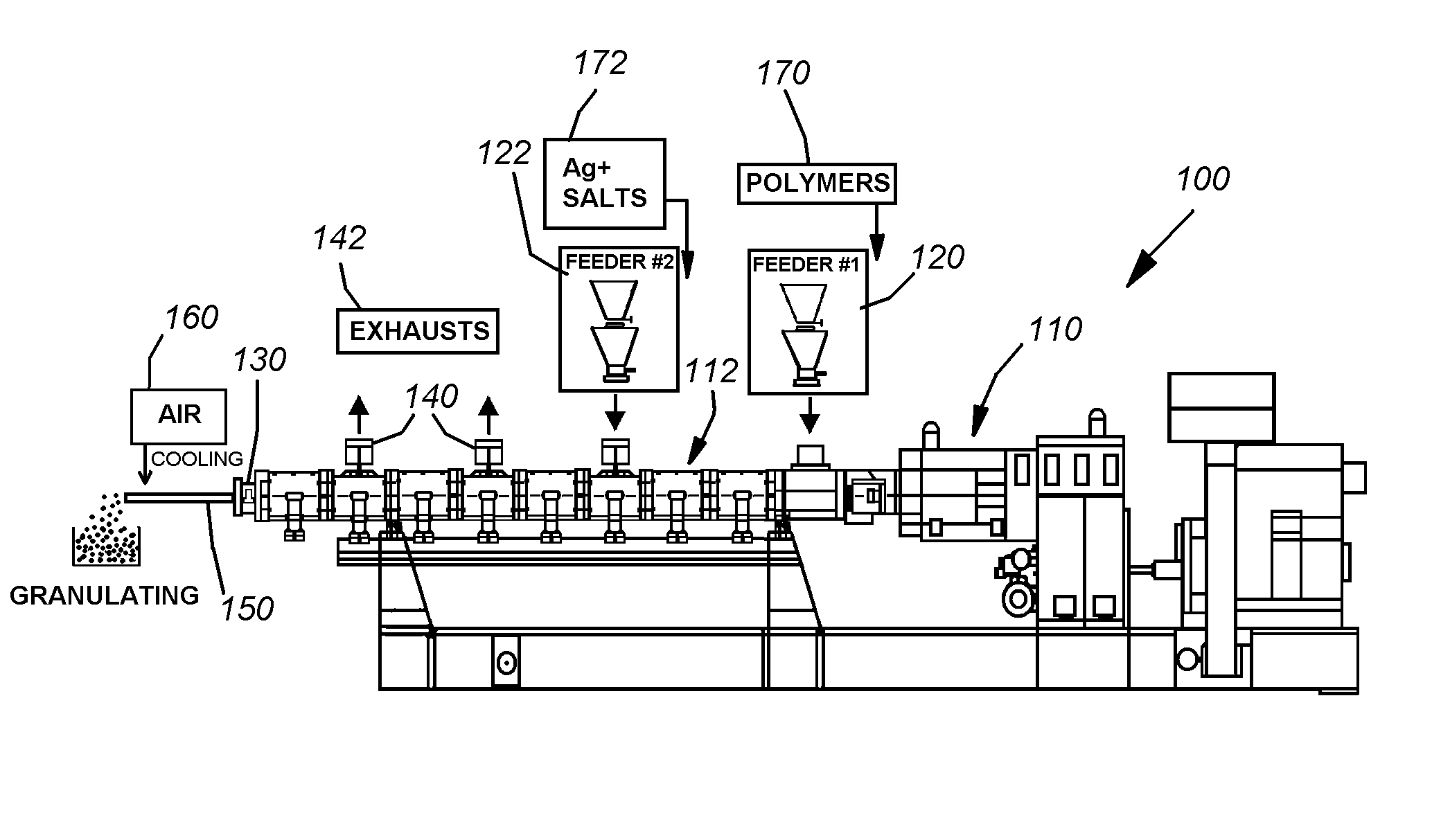
This invention provides a modified catheter biomaterial that provides both immediate, and long-term microbiocidal effects on otherwise antibiotic-resistant strains of microorganisms. The material, which exhibits good mechanical performance characteristics for medical devices, is composed of a hydrophobic polyurethane (PU), a hydrophilic polyethylene vinyl acetate (PEVA), a soluble silver salt and a sparsely-soluble silver salt. The hydrophobic polyurethane provides the good physical properties, the PEVA the hydrophilicity necessary to allow some water ingress into the catheter, the soluble silver salt for an immediate burst effect, and the sparsely-soluble silver salt for sustained-release over many months postimplantation.
Owner:SZYCHER MICHAEL
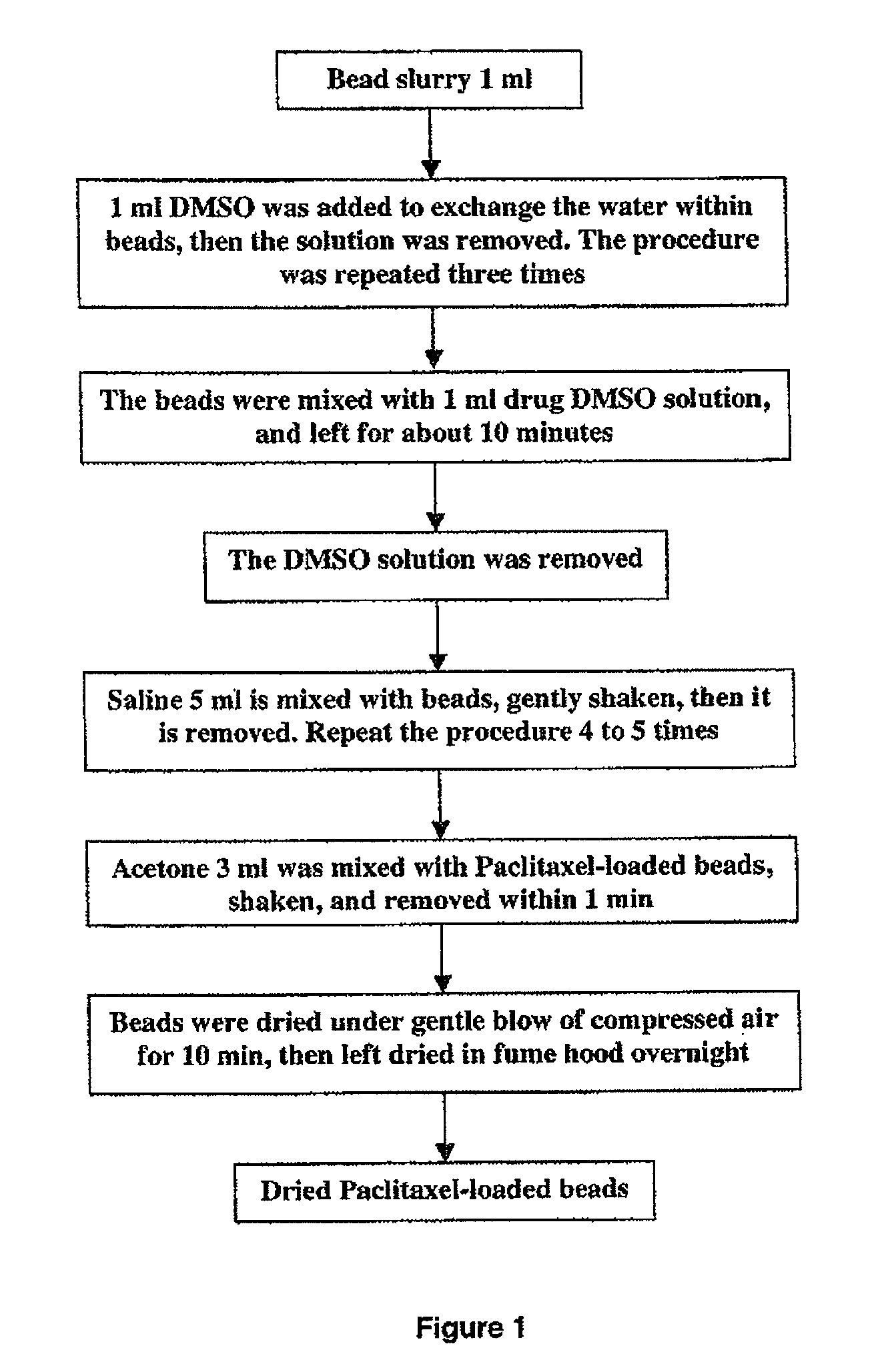
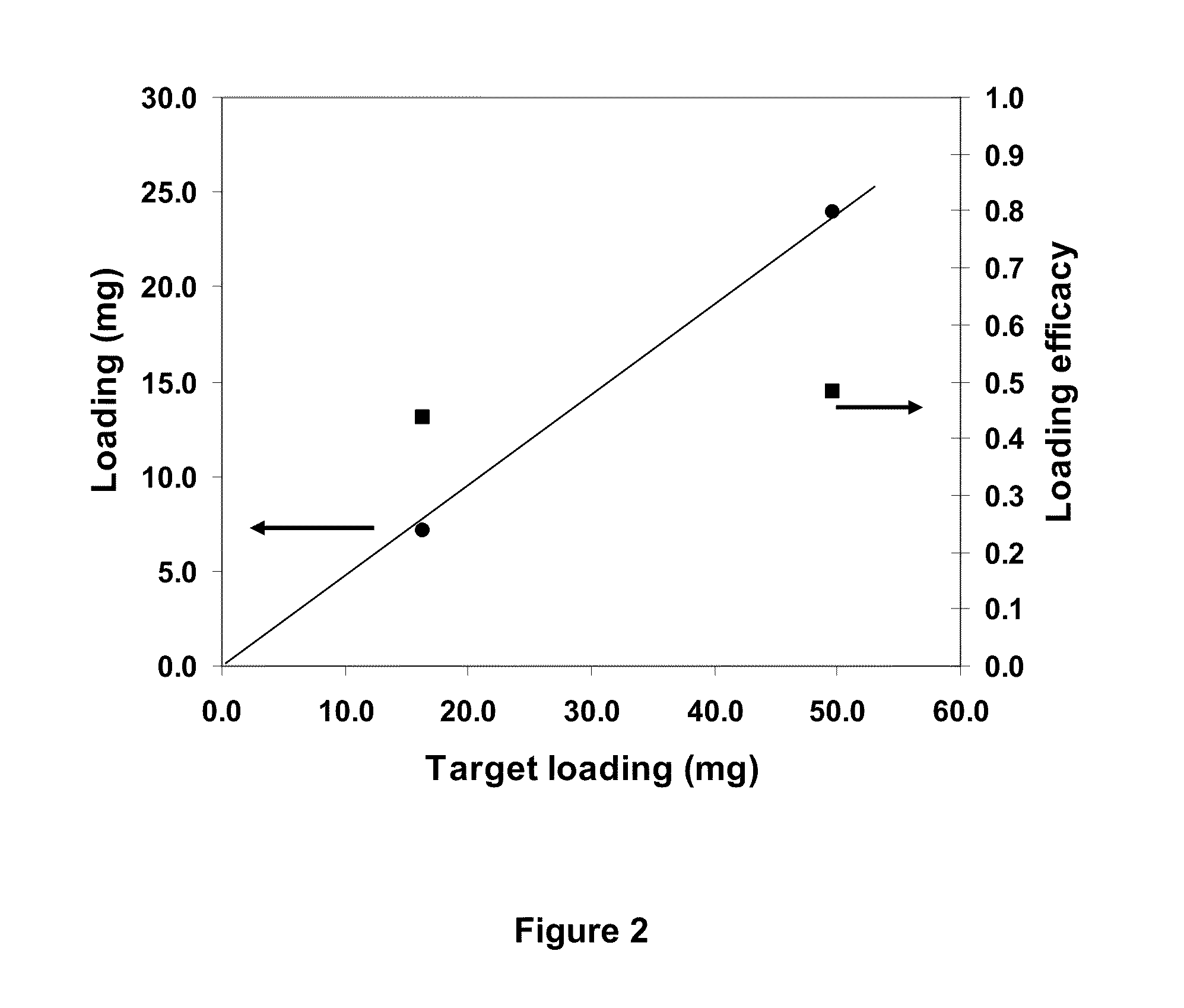
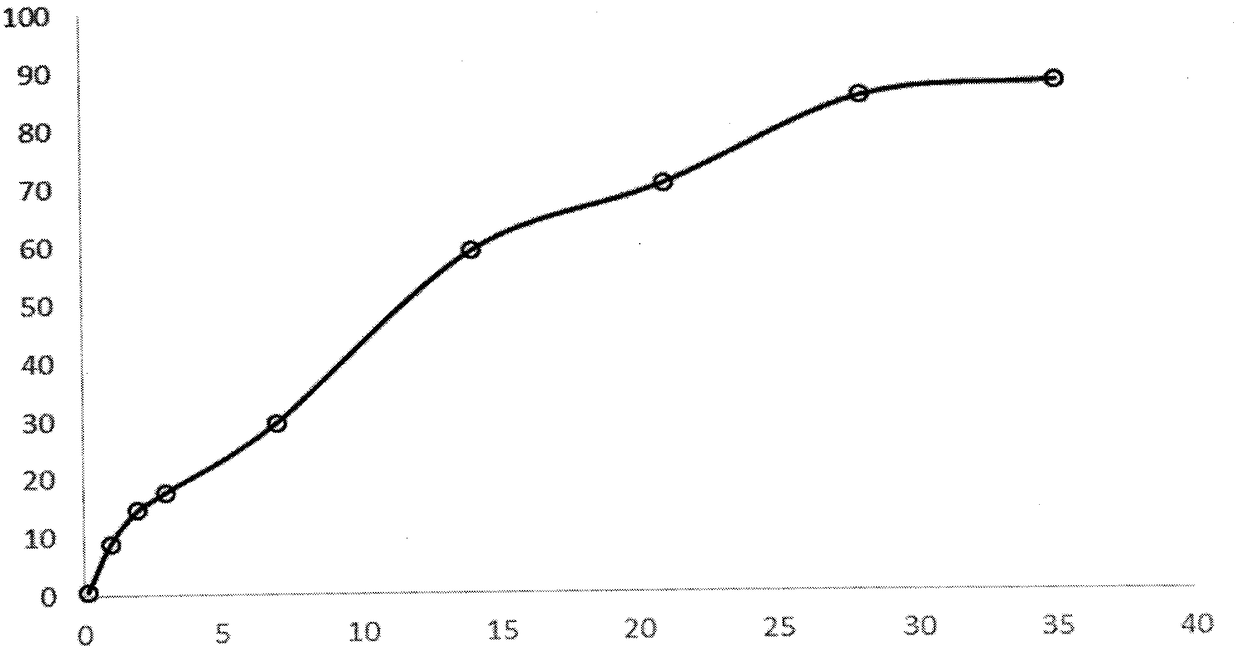
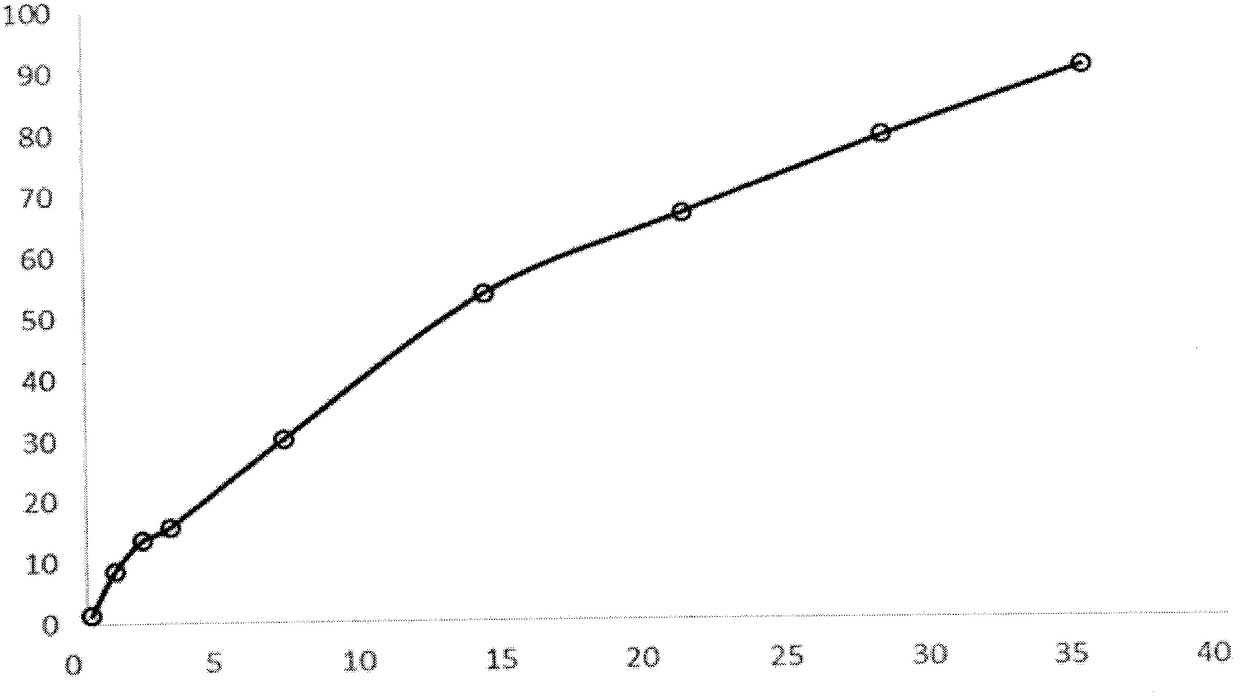
Loading of hydrophobic drugs into hydrophilic polymer delivery systems
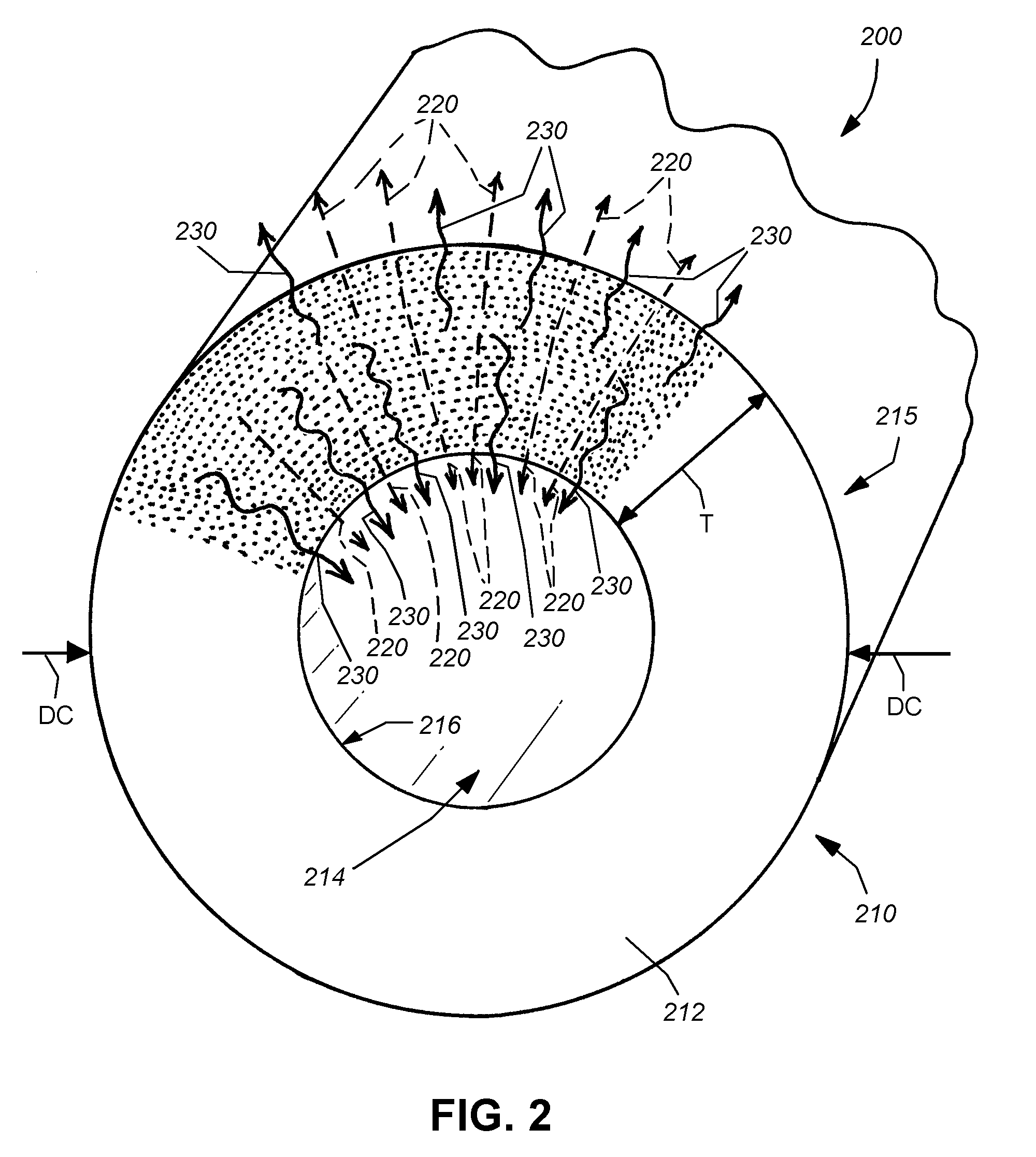
A process is described for loading hydrophilic polymer particles with a water-insoluble solvent-soluble drug. The particles are preferably embolic agents. The method provides particles having little or no drug at the surface and in a surface layer, whereby the burst effect is minimised. The drug is precipitated in the core of the particle, leading to extended release. The drug is, for instance, paclitaxel, rapamycin, dexamethasone or ibuprofen.
Owner:BIOCOMPATIBLES UK LTD
Method for increasing entrapment rate of polylactic acid microspheres to water soluble protein
InactiveCN101693111AReduce inactivationHigh encapsulation efficiencyPowder deliveryPeptide/protein ingredientsMicrosphereBurst effect
A method for increasing entrapment rate of polylactic acid microspheres to water soluble protein belongs to the field of medicinal preparation. On the basis of the existing multiple emulsion method (W / O / W), water soluble amphiphilic polymer carboxymethyl chitosan (O-CMC) of which the mass fraction ranges from 0.1% to 5% is added in internal water phase medicated solution used for forming multiple emulsion, wherein the molecular weight of the water soluble amphiphilic polymer carboxymethyl chitosan ranges from 15K to 30K, the degree of carboxymethylation ranges from 0.6 to 1, and the degree of deacetylation is 92%. Further, osmotically active substance NaCl or mannitol is added in external water phase solution used for forming multiple emulsions, wherein the mass fraction of NaCl ranges from 0.5% to 1.5%, and the mass fraction of mannitol ranges from 2% to 5%. The method reduces stability of protein during processes of preparation, storage and using of microspheres, and increases the entrapment rate of microspheres to protein and inhibits 'burst effect' of microspheres at initial stage of releasing.
Owner:TSINGHUA UNIV
Poorly soluble antineoplastic drug micelle preparation and preparation method thereof
InactiveCN104856974AGood water solubilitySmall toxicityOrganic active ingredientsHeavy metal active ingredientsSolubilityBurst effect
The invention provides a copolymer drug-loaded micelle preparation which comprises a poorly soluble antineoplastic drug and a copolymer carrier material. A poorly soluble antineoplastic drug micelle injection is prepared through using methoxy polyethylene glycol 2000-polylactide (53 / 47) diblock copolymer as a carrier material, embedding the poorly soluble antineoplastic drug in micelle, and performing spray drying or freeze drying. According to the invention, the poorly soluble antineoplastic drug micelle preparation not only greatly increases the solubility of poorly soluble antineoplastic drug and improves efficacy, but also prolongs the circulation time of drug in the body, improves the drug activity, reduces the burst effect and increases the bioavailability.
Owner:海南路易丹尼生物科技有限公司
Preparation method of vesicle-loaded multivescular liposome
ActiveCN106924185AAddress common cases of sudden releaseMaintain biological activityOrganic active ingredientsSenses disorderLipid formationExocytic vesicle
The invention relates to a preparation method of a vesicle-loaded multivescular liposome. The preparation method comprises the steps of adopting nanoscale vesica and an osmotic pressure regulator as an inner water phase and an organic solvent of a lipid with good biocompatibility as an oil phase, dispersing the inner water phase into the oil phase to form a W / O primary emulsion; dispersing the primary emulsion into an outer water phase containing the osmotic pressure regulator and an auxiliary emulsifier to form a W / O / W compound emulsion; and transferring the compound emulsion to the same outer water phase and removing an organic solvent through rotary evaporation or nitrogen introduction to obtain a multivescular liposome suspension. According to a multivescular liposome preparation, the encapsulation efficiency of a loaded drug can be ensured, the biological activity of biomacromolecules can be improved, the immunogenicity is reduced, the burst effect is reduced and sustained slow release of the drug is ensured.
Owner:YANTAI UNIV
Modified release ibuprofen dosage form
The present invention is a solid dosage form for oral administration of ibuprofen comprising a modified release formulation of ibuprofen which provides an immediate burst effect and thereafter a sustained release of sufficient ibuprofen to maintain blood levels at least 6.4 g / ml over an extended period of at least 8 hours following administration of a single dose. The dosage form releases ibuprofen at a rate sufficient to initially deliver a effective amount of ibuprofen within about 2.0 hours following administration. The dosage form then subsequently delivers the remaining amount of ibuprofen at a relatively constant rate sufficient to maintain a level of ibuprofen over a predetermined delivery period of for at least 8 hours.
Owner:骏达医药科技有限公司
Fireworks artillery shell
InactiveUS20050066837A1Increases burst presentationGreat audible reportFirework flares/torchesAerial display rocketsBurst effectFireworks
A fireworks artillery shell for use as a consumer firework which may be propelled by the use of a mortar is provided which includes a casing, a lift charge, an effects charge, a timing fuse and an ignition fuse, and seals. The seals are provided within the casing above and below the effects charge to increase the burst effect of the effects charge. The lift charge is positioned within the casing and below the lower seal, and upon ignition, lifts the fireworks artillery shell into the air. The seals promote a harder break and more explosive effect from the effects charge without interfering with the lifting charge.
Owner:JAKES FIREWORKS
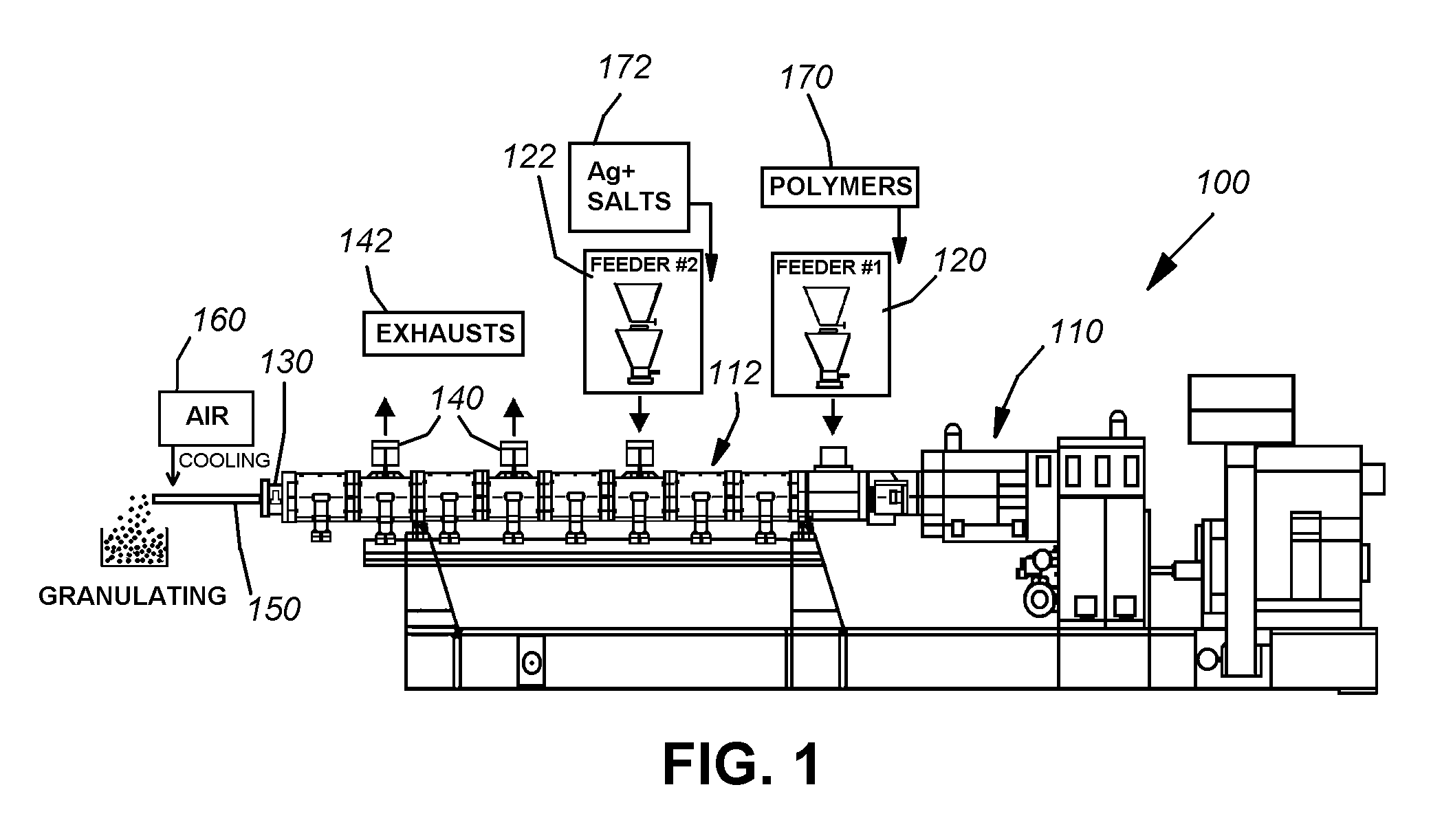
Loading of hydrophobic drugs into hydrophilic polymer delivery systems
A process is described for loading hydrophilic polymer particles with a water-insoluble solvent-soluble drug. The particles are preferably embolic agents. The method provides particles having little or no drug at the surface and in a surface layer, whereby the burst effect is minimised. The drug is precipitated in the core of the particle, leading to extended release. The drug is, for instance, paclitaxel, rapamycin, dexamethasone or ibuprofen.
Owner:BOSTON SCI MEDICAL DEVICE LTD
A kind of preparation method of triptorelin acetate sustained-release microspheres
ActiveCN105169366BSmall burst effectSlow release curvePowder deliveryPeptide/protein ingredientsDrugs solutionMicrosphere
The invention relates to a preparation method of triptorelin acetate sustained-release microspheres, comprising the following steps: step 1) adding water to triptorelin acetate to form a drug solution A; adding PLGA to an organic solvent to form a solution B; step 2) mixing Solution A and solution B are mixed and ultrasonicated to form colostrum, and the colostrum is added to a PVA aqueous solution saturated with an organic mixed solvent, and homogeneously emulsified to obtain double emulsion; Step 3) Stir the double emulsion at room temperature for 1 hour and then heat up to 40°C-45°C The temperature was kept at ℃ for 1 hour, and then the temperature was lowered to 10 ℃, and the particles were collected by sieving and freeze-dried. On the one hand, the technology involved in the present invention can overcome the problem of increased adverse drug reactions caused by the sudden release of triptorelin acetate microspheres; on the other hand, the blood drug concentration of the prepared microspheres is very smooth and stable, and is suitable for long-term administration treat.
Owner:LIVZON PHARM GRP INC
Preparation method of oral slow/controlled-release preparation containing febuxostat
ActiveCN101773498BImprove securityEffective plasma concentrationOrganic active ingredientsSkeletal disorderAdhesiveImmediate release
Owner:QINGDAO HUANGHAI PHARM CO LTD
Method for dyeing interface of bursting body
The invention relates to a method for dyeing an interface of a bursting body. In the method, a container filled with dye of a certain color is placed on the bottom of a blast hole to be burst; when the blast hole of a dye bag is burst, the container can be cracked under the action of the burst pressure of an explosive and release the dye, the dye can dye ores or rocks near the bottom of the blasthole, and then the dyed ores or rocks become dyeing interfaces of bursting ore loosing bodies. By using the method, a specific interface mark of a burst body can be formed, which can provide the change information of the interfaces of the burst ores and rocks of an underground mine in a closed space, can provide a new means for ore drawing control management and research in a stope caving method of the underground mine, and also provide a new evidence for bursting effect analysis.
Owner:KUNMING UNIV OF SCI & TECH
Emulsion explosive and preparation method thereof
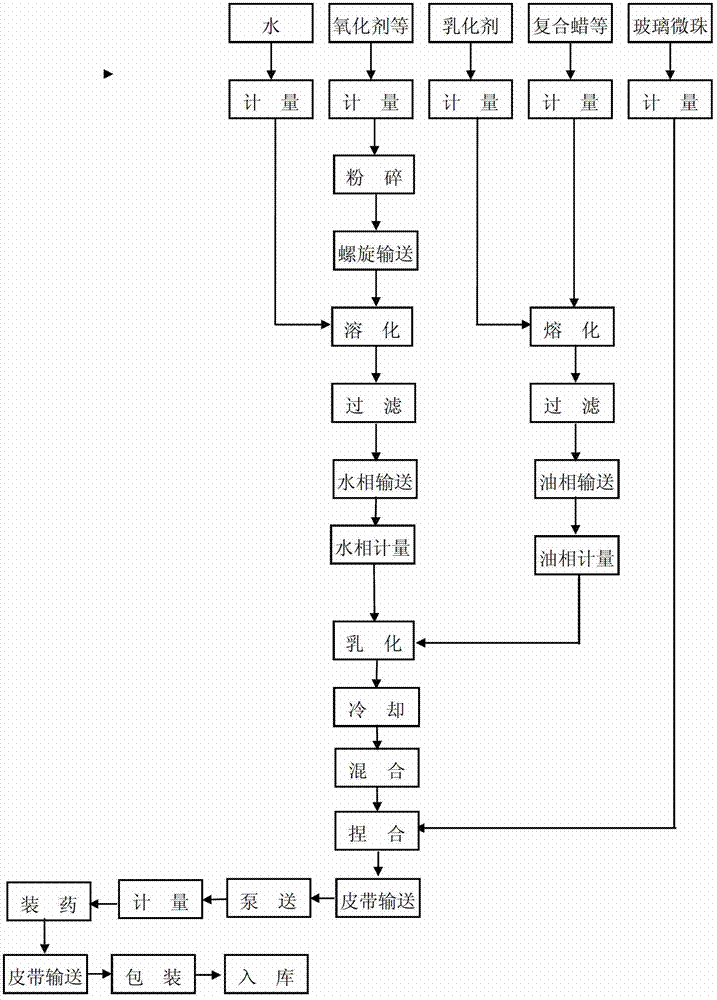
ActiveCN103172477ALong storage periodReduce storage costsExplosive working-up apparatusNon-explosive/non-thermic compositionsWaxBurst effect
The invention relates to an emulsion explosive and a preparation method thereof. The emulsion explosive comprises the following components in parts by weight: 70-80 parts of ammonium nitrate, 5-6 parts of sodium nitrate, 10-15 parts of water, 3-4 parts of composite wax, 0.3-0.8 part of engine oil, 1-1.7 parts of composite emulsifying agent, 0.5-1 part of polymer emulsifying agent and 1.2%-1.7% (based on total weight of the components) of glass beads, wherein the composite emulsifying agent is a mixture of Span 80 and T152 emulsifying agent in a weight ratio of 6 to 4. According to the emulsion explosive disclosed by the invention, various indexes satisfy the relevant national regulations, and the performances reach three international standards, the gap distance for sympathetic detonation reaches 5, the performance quality of the explosive is improved and the burst effect is ideal. Moreover, the high polymer emulsifying agent is added to the explosive formula, so that the storage period of the emulsifying explosive is prolonged to one year from half a year, and therefore, the storage cost of the explosive is reduced. Meanwhile, the composite wax and the engine oil are used in the formula, so that the price is low, the resources are extensive and the production cost is reduced.
Owner:TIANJIN HONGTAI CHEM
Persimmon leaf general flavone sustained-release micropills and preparation method thereof
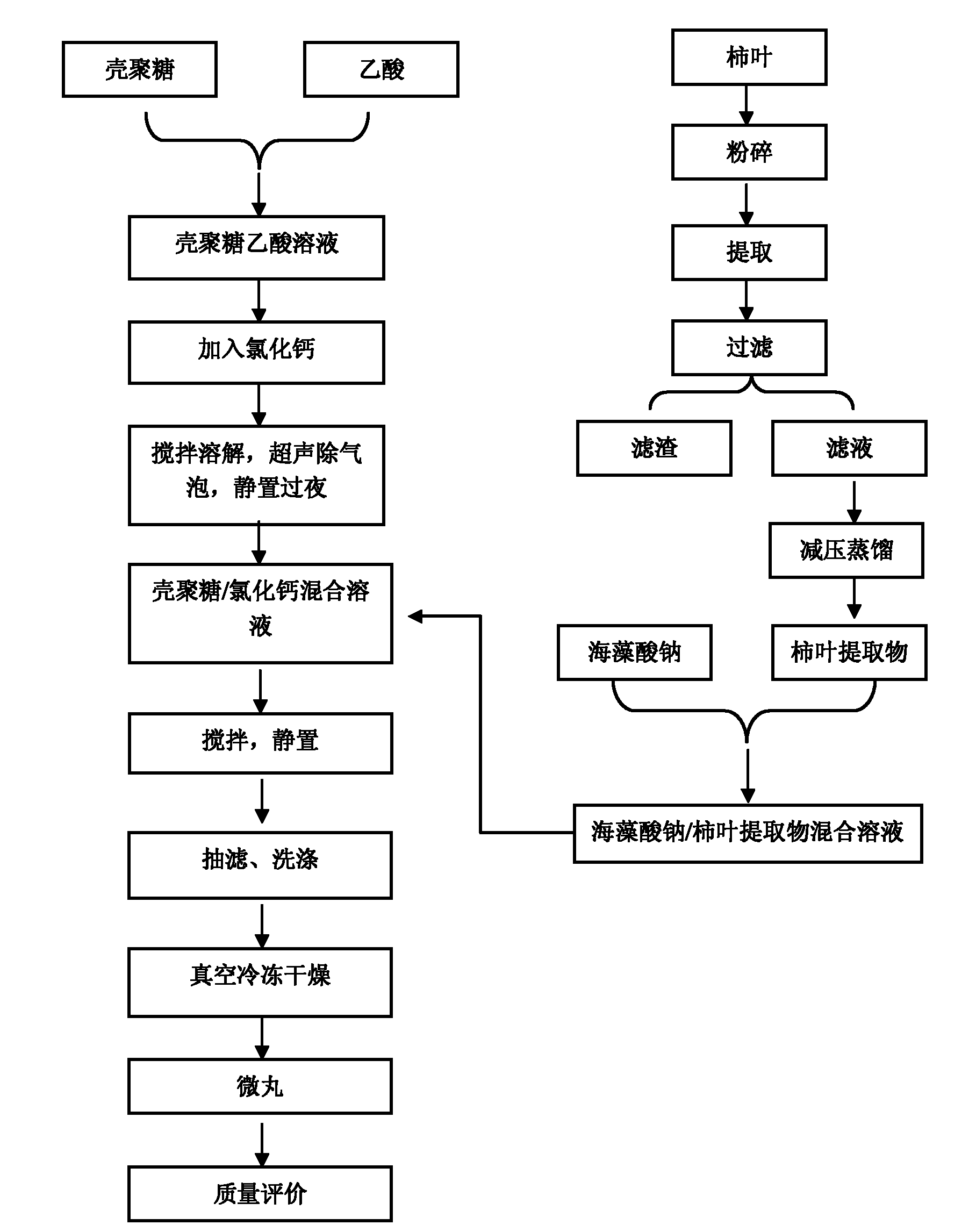
InactiveCN102178722AMeet the requirementsNo burst effectAntibacterial agentsMetabolism disorderBurst effectBULK ACTIVE INGREDIENT
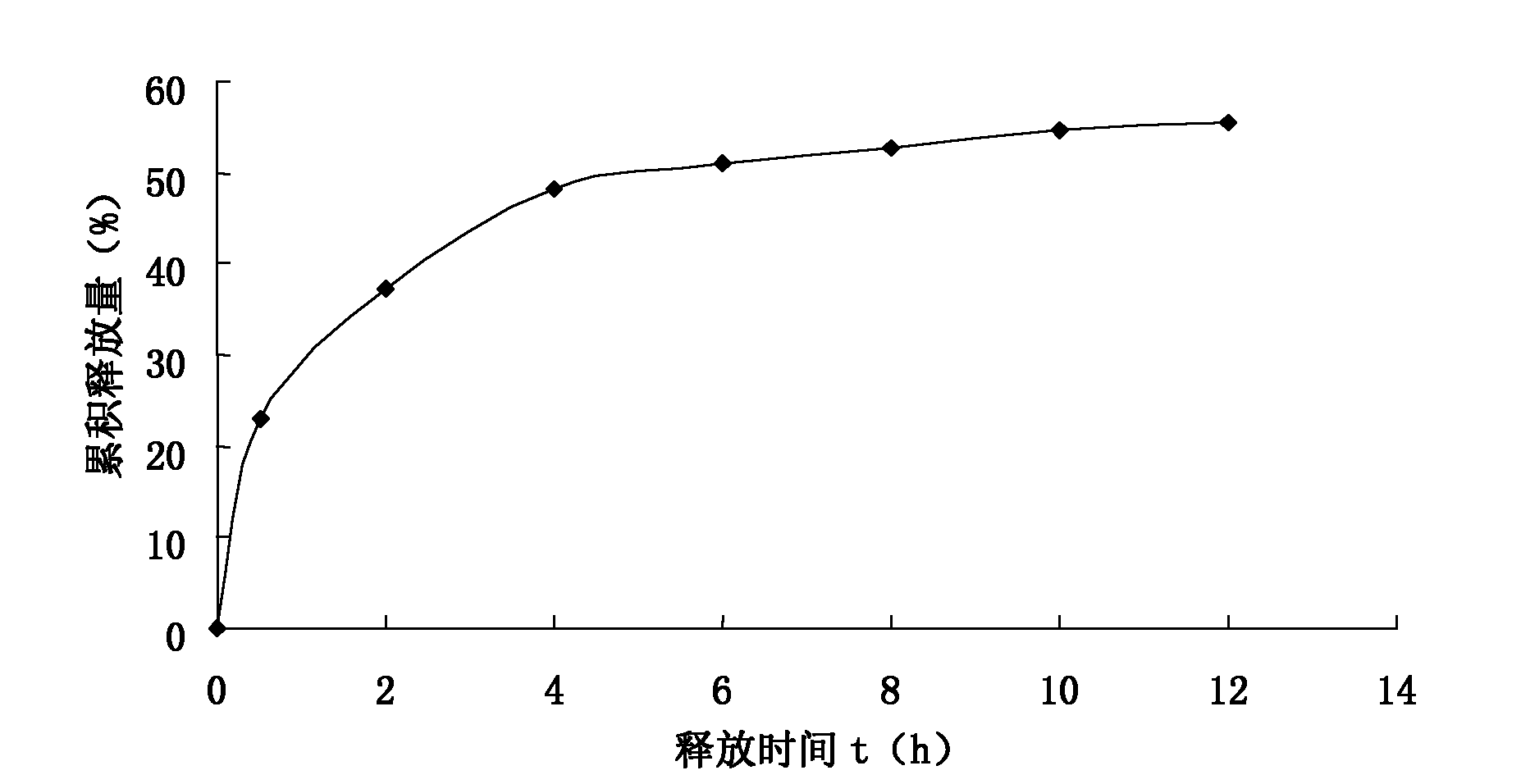
The invention discloses persimmon leaf general flavone sustained-release micropills and a preparation method thereof. The persimmon leaf general flavone sustained-release micropills comprise the following components in percentage by mass: 1 to 25 percent of persimmon leaf general flavone, 10 to 50 percent of sodium alginate, 10 to 40 percent of chitosan, 10 to 50 percent of calcium chloride, 0.5 to 5 percent of acetic acid and the balance of water. The persimmon leaf general flavone sustained-release micropills take the persimmon leaf general flavone as active ingredients and are a novel Chinese medicinal sustained-release preparation. General flavone is used as a release amount index, the accumulative release amount of the general flavone of the persimmon leaf general flavone sustained-release micropills in artificial gastric juice and phosphate buffer saline (PBS) buffer solution is 22.97 percent and 34.93 percent respectively within 0.5 hour, does not exceed 45 percent and does not have a burst effect, and results meet the requirement on sustained-release preparations in Chinese pharmacopoeia (the guiding principle of sustained release, controlled release and delayed release in XIXD of 2010 version).
Owner:SHAANXI UNIV OF SCI & TECH
Method for preparing oxaliplatin sustained release suppository
InactiveCN101543472ALow toxicityEliminate side effectsSuppositories deliveryOrganic non-active ingredientsWater bathsSide effect
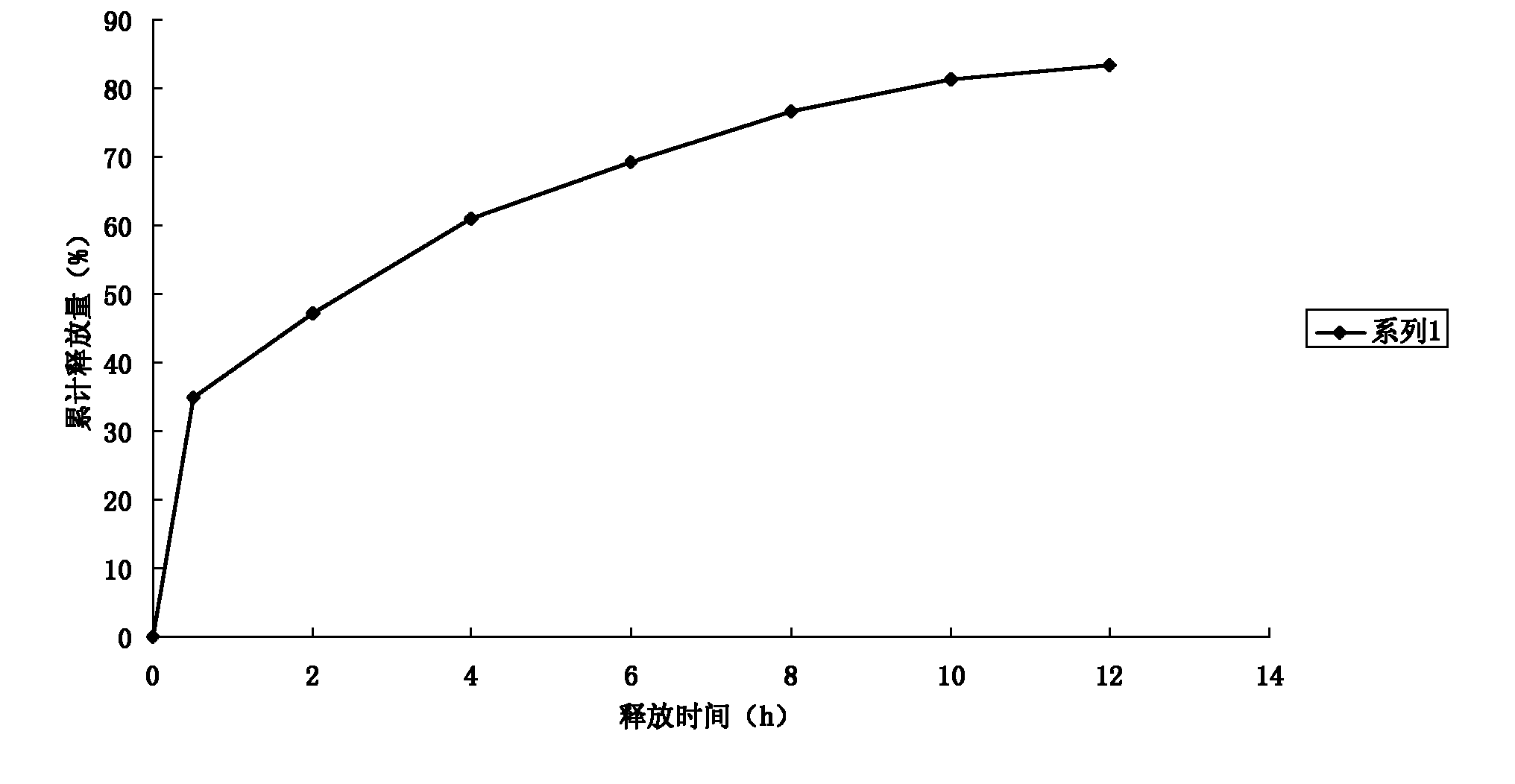
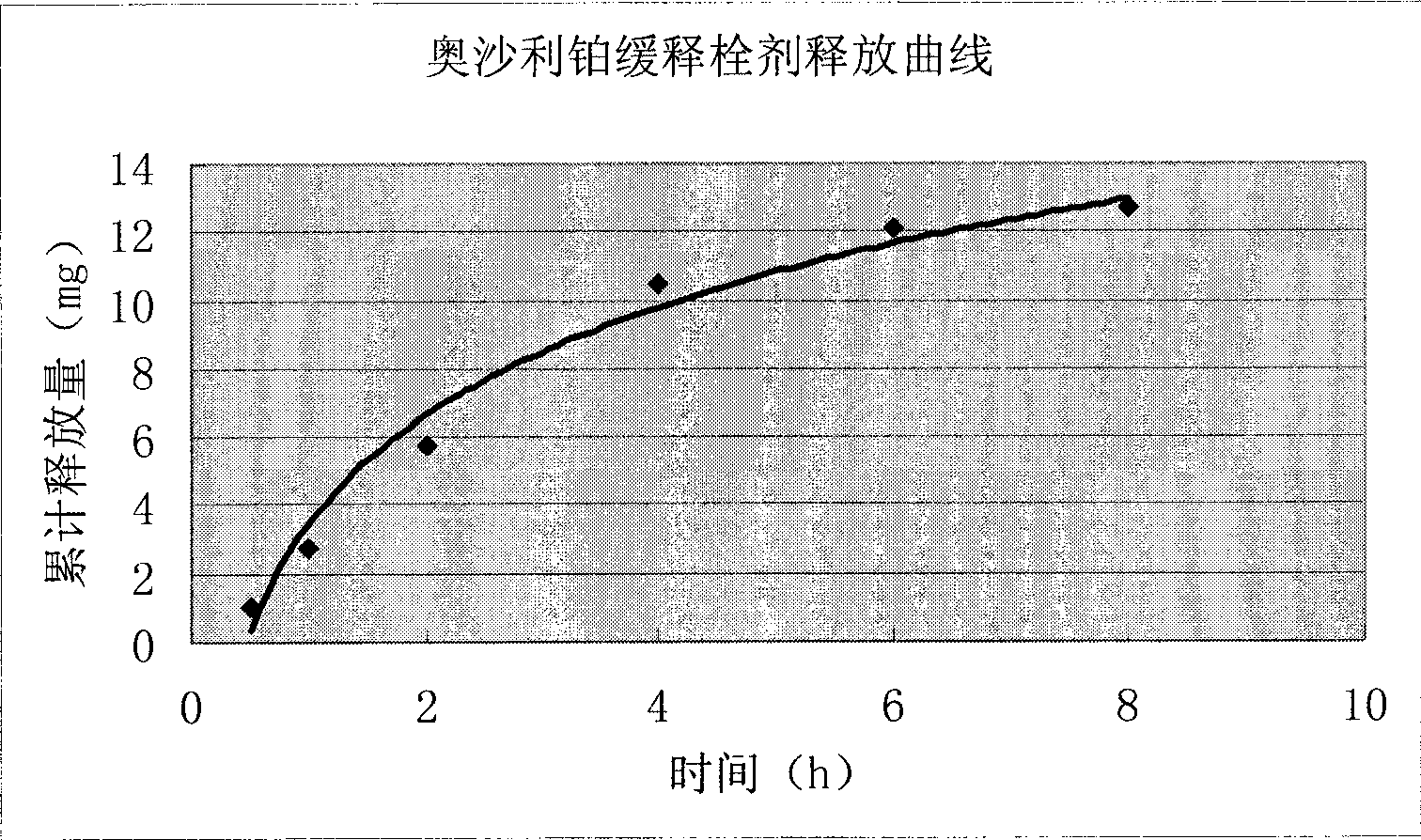
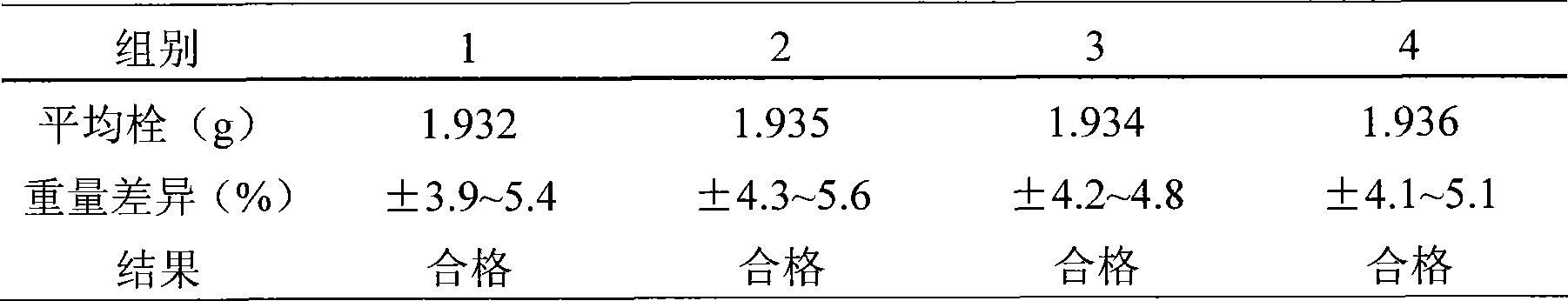
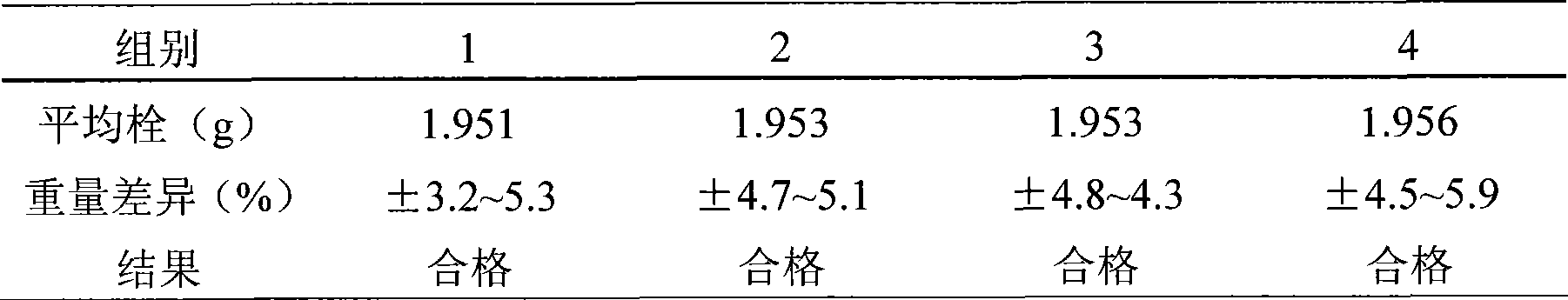
The invention discloses a method for preparing an oxaliplatin sustained release suppository, which comprises the following steps of: using an acetic acid solution with a mass concentration of between 0.5 and 5 percent to prepare an oxaliplatin solution with a concentration of between 0.5 and 5 mg / mL, taking chitosan, using an oxaliplatin acetic acid solution to dissolve the chitosan, adding span-80 or tween-80 and liquid paraffin, stirring and emulsifying the mixture, adding glutaric dialdehyde into the emulsified mixture, adding NaOH or ammonia water to adjust to ensure that the pH value is more than or equal to 9, stirring the mixture, crosslinking the mixture, centrifugally separating microspheres, and drying to obtain oxaliplatin microspheres; mixing polyethyleneglycol 4000 and polyethyleneglycol 6000 according to the mass ratio of 1-7: 1, and carrying out hot melting on the mixture in water bath with constant temperature; and adding the oxaliplatin microspheres which account for 0.01 to 0.5 times of the total mass of the polyethyleneglycol 4000 and the polyethyleneglycol 6000 into the mixture after the hot melting is finished, and obtaining the oxaliplatin sustained release suppository after the model filling and cooling. The preparation method is simple, and the prepared oxaliplatin sustained release suppository has no burst effect; and the medicament has sustained release characteristics, is slowly released and has sustained release effect. The oxaliplatin sustained release suppository can well play the antitumor effect of oxaliplatin, improve the antitumor activity of the oxaliplatin, and furthest reduce the toxic side effect of the oxaliplatin at the same time.
Owner:WUHAN UNIV OF TECH
Preparation method of low-agglomeration and anti-burst fibers for unshaped refractory materials
InactiveCN102924098AAccelerated exclusionShorten drying timeSolid waste managementFiberScreening techniques
The invention discloses a preparation method of low-agglomeration and anti-burst fibers for unshaped refractory materials, which belongs to the field of the refractory materials. The preparation method comprises the following steps: using waste paper (books, newspaper and paperboards and the like), waste hemp articles (hemp bags and hemp ropes and the like) as raw materials; adding a proper amount of an anti-agglomeration additive, placing into an efficient crusher to crush; and then adopting a screening technique to prepare the anti-burst fibers with different length-diameter ratios. The preparation method is simple process and convenient in operation; the obtained product is green and environment-friendly to the environment, the resource is recycled, and the cost performance is high; the internal vapor pressure inside the unshaped refractory materials can be effectively reduced so as to avoid burst; especially in quick-drying unshaped refractory materials with high strength, the anti-burst effect is more obvious.
Owner:TIANJIN POLYTECHNIC UNIV
A thermosensitive gel pharmaceutical preparation and preparation method thereof
ActiveCN103622902BImprove burst issuesImprove medication compliancePeptide/protein ingredientsMetabolism disorderAdjuvantBurst effect
The invention discloses a temperature-sensitive gel pharmaceutical preparation and a preparation method thereof. The raw material formula of the temperature-sensitive gel pharmaceutical preparation contains a drug, a temperature-sensitive gel composition, and an excipient C; the excipient C is one or more of metal salts, carbohydrates, and hydrophilic polymers for injection; The metal salt is a pharmaceutically acceptable metal salt that dissociates divalent metal cations in an aqueous solution; the drug is a substance A that exists in an anion form in an aqueous solution, and the substance A is a protein drug, a polypeptide One or more of class drugs and glycopeptide antibiotics. The preparation method comprises the following steps: according to the formula of raw materials, the drug is dissolved in the temperature-sensitive gel composition in the form of solution. The temperature-sensitive gel pharmaceutical preparation is especially suitable for water-soluble drugs, reduces the burst release effect, releases the drugs slowly, and achieves the purpose of sustained release.
Owner:SHANGHAI MODERN PHARMA ENG INVESTIGATION CENT
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com