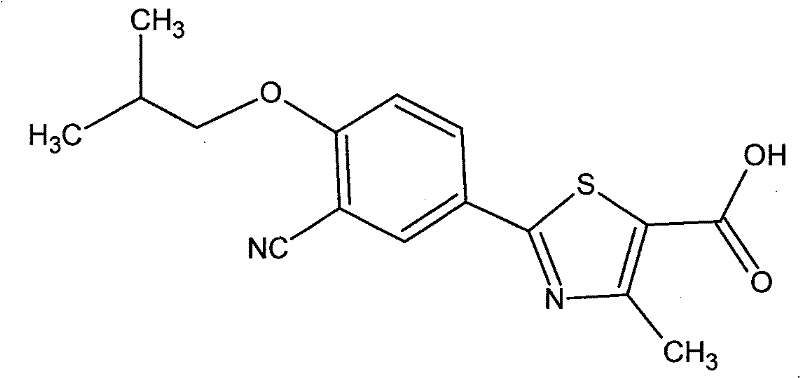
Preparation method of oral slow/controlled-release preparation containing febuxostat
A technology for controlled release preparations and febuxostat, which is applied in the directions of medical preparations containing active ingredients, pill delivery, pharmaceutical formulations, etc., to avoid burst release effects, improve safety, and reduce the incidence of adverse reactions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
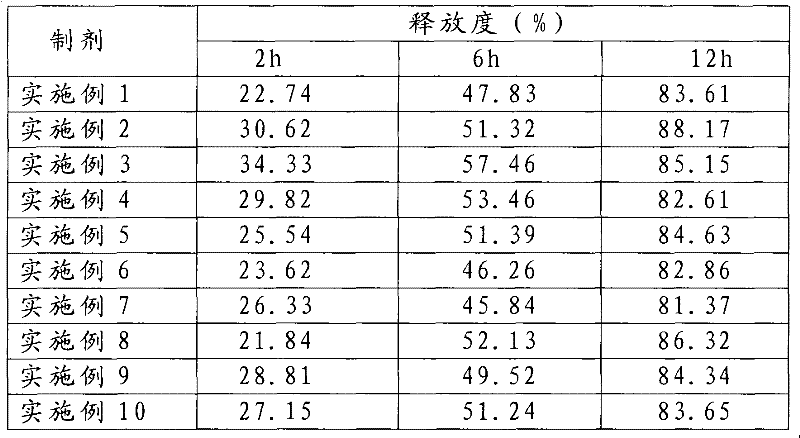
Examples
Embodiment 1
[0037] Embodiment 1 skeleton type sustained and controlled release tablet
[0038] Febuxostat 80mg
[0039] Hypromellose (K15M) 15mg
[0040] Hypromellose (K4M) 15mg
[0041] Lactose 85mg
[0042] 5% povidone K30 in 75% ethanol solution 60mg
[0043] Magnesium Stearate 2mg
[0044] (The 75% ethanol solution of 5% povidone K30 refers to that in the ethanol solution that the mass percentage concentration is 75%, containing the povidone K30 that the mass percentage is 5%);
[0045] Preparation
[0046]After crushing febuxostat, pass through a 100-mesh sieve, mix well with hypromellose (K15M), hypromellose (K4M) and lactose, add 5% povidone K30 in 75% ethanol solution , using HLSH2-6 wet mixing granulator to prepare soft materials, 24 mesh sieve to make wet granules, wet granules to be air-dried at 50℃~60℃, dry granules to granulate with 22 mesh sieves, add magnesium stearate and mix After homogeneity, use ZP10A tablet press for tablet compression.
Embodiment 2
[0047] Embodiment 2 Coating type sustained and controlled release tablet
[0048] Tablet formulation
[0049] Febuxostat 80mg
[0050] Calcium hydrogen phosphate 25mg
[0051] Microcrystalline Cellulose 110mg
[0052] 2% hypromellose K15M aqueous solution 40mg
[0053] Magnesium Stearate 2mg
[0054] Coating material
[0055] 15% aqueous dispersion of ethylcellulose 116mg
[0056] Preparation Process
[0057] After pulverizing febuxostat, passing through a 100-mesh sieve, mixing evenly with calcium hydrogen phosphate and microcrystalline cellulose, adding 2% hypromellose K15M aqueous solution, and preparing it with a HLSH2-6 wet mixing granulator Soft material, 24 mesh sieve to make wet granules, wet granules are air-dried at 50℃~60℃, dry granules are granulated with 22 mesh sieves, after adding magnesium stearate and mixing evenly, use ZP10A tablet machine for tableting, hardness The range is 35-85N, and then coated with 15% ethylcellulose aqueous dispersion.
Embodiment 3
[0058] Embodiment 3 skeleton type sustained and controlled release capsules
[0059] Febuxostat 80mg
[0060] Hypromellose (K15M) 15mg
[0061] Hypromellose (K4M) 15mg
[0062] Microcrystalline Cellulose 85mg
[0063] 5% povidone K30 in 75% ethanol solution 60mg
[0064] Micronized silica gel 2mg
[0065] Preparation
[0066] After pulverizing febuxostat, pass it through a 100-mesh sieve, mix it with hypromellose (K15M), hypromellose (K4M) and microcrystalline cellulose evenly, add 75% of 5% povidone K30 % ethanol solution, use HLSH2-6 wet mixing granulator to prepare soft materials, then add JBZ-300 multifunctional granulation and coating machine to extrude and spheronize to prepare pellets. Blow dry, add micropowder silica gel and mix evenly, then fill into No. 0 capsule shell.
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com