A kind of preparation method of triptorelin acetate sustained-release microspheres
A technology of triptorelin acetate and sustained-release microspheres, applied in endocrine system diseases, sexual diseases, powder delivery and other directions, can solve problems such as increase in adverse drug reactions, and achieve long-term drug use, reduce sudden release effects, The effect of slow release curve flattening
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
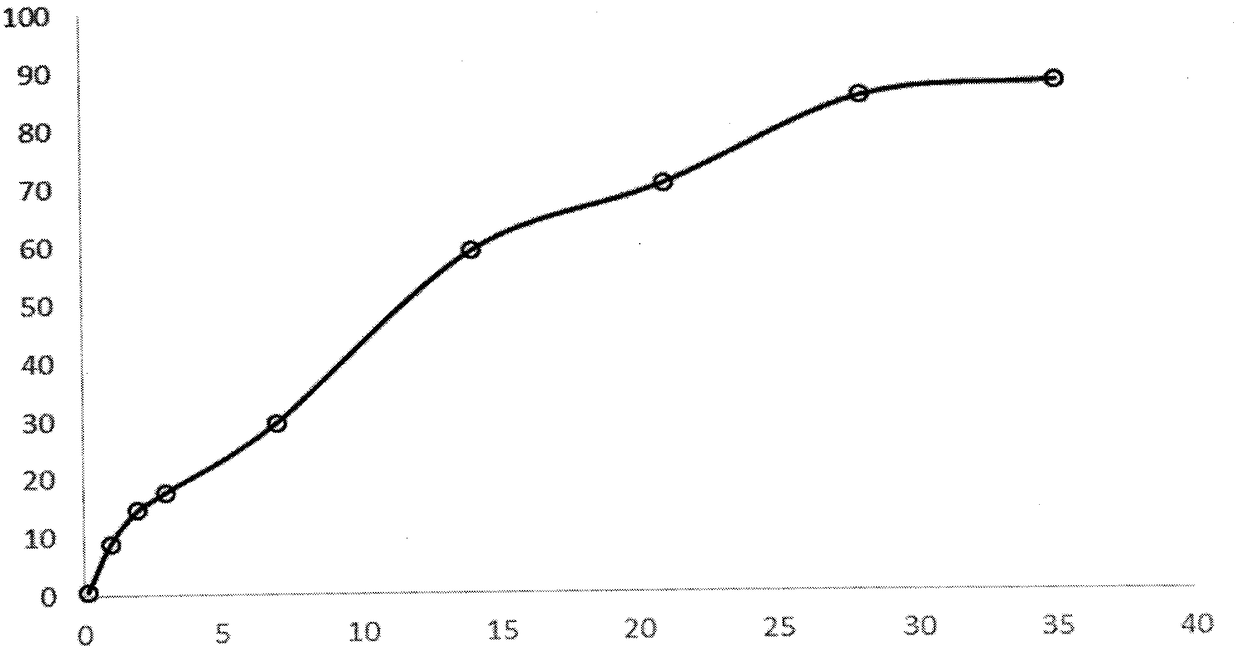
[0030] Take by weighing 1g of triptorelin acetate (Railin Bachem Company, lot number 1209001) and add water to make a drug solution with a concentration of 40%, dissolve 8g of PLGA5050 with 25ml of ethyl acetate and 10ml of benzyl alcohol, mix the two with ultrasound for 2min, and form White homogeneous colostrum. The colostrum was added into 1000 ml of 0.5% PVA solution (containing 1% benzyl alcohol and 1% ethyl acetate) at 6° C. through a syringe through a syringe at 1500 rpm, and homogeneously emulsified for 2 minutes to obtain double emulsion. Move the double emulsion to a cantilever mixer with a rotation speed of 600 rpm, stir for 1 hour, then raise the temperature to 45° and keep it for 1 hour, then lower the temperature to 10° to filter through a sieve, and freeze-dry to obtain powdered microspheres. The drug loading capacity of the triptorelin acetate microspheres was 10.3%, and the burst release rate was 9% in 1 day. The pores of the microspheres were continuous and c...
Embodiment 2
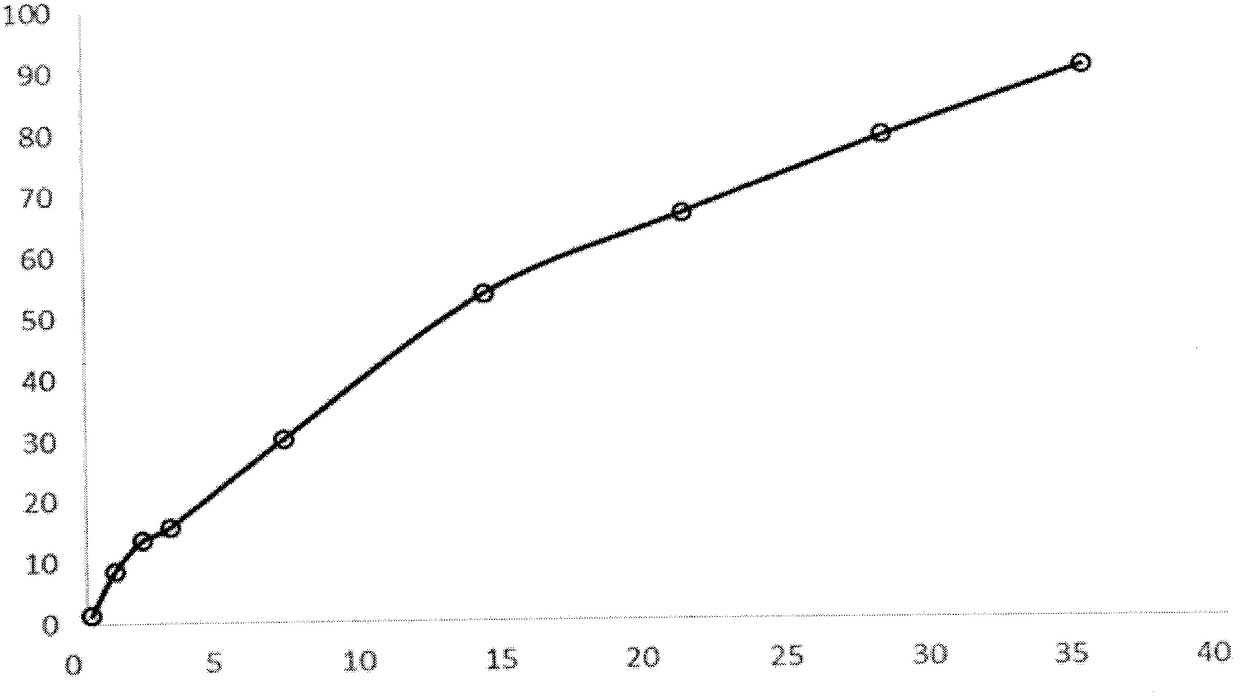
[0032] Weigh 1g of triptorelin acetate (Railin Bachem Co., lot number 1209001) and add water to make a drug solution with a concentration of 40%. Dissolve 8g of PLGA5050 with 23.5ml of ethyl acetate and 3.3ml of benzyl alcohol, mix the two and sonicate for 2min , forming white homogeneous colostrum. The colostrum was added into 1000 ml of 0.5% PVA solution (containing 1% benzyl alcohol and 1% ethyl acetate) at 6° C. through a syringe through a syringe at 1500 rpm, and homogeneously emulsified for 2 minutes to obtain double emulsion. Move the double emulsion to a cantilever mixer with a rotation speed of 600 rpm, stir for 1 hour, then raise the temperature to 45° and keep it for 1 hour, then lower the temperature to 10° to filter through a sieve, and freeze-dry to obtain powdered microspheres. The drug-loading capacity of the triptorelin acetate microspheres was 10.8%, and the burst release rate was 8% in 1 day. The pores of the microspheres were continuous and complete, and th...
Embodiment 3
[0034] Take by weighing 1g of triptorelin acetate (Railin Bachem Company, batch number 1209001) and add water to make a drug solution with a concentration of 40%, dissolve 8g of PLGA5050 with 19ml of ethyl acetate and 7.6ml of benzyl alcohol, and mix the two with ultrasound for 2min. Form white homogeneous colostrum. Colostrum is added into 1000ml of 0.5% PVA solution (containing 1% benzyl alcohol and 1% ethyl acetate) at 6° C. through a syringe under homogenization at 1500 rpm, and homogeneously emulsified for 2 minutes to obtain double emulsion. Move the double emulsion to a cantilever mixer with a rotation speed of 600 rpm, stir for 1 hour, then raise the temperature to 40° and keep it for 1 hour, then lower the temperature to 10° to filter through a sieve, and freeze-dry to obtain powdery microspheres. The drug loading capacity of the triptorelin acetate microspheres was 10.6%, and the burst release rate was 9% in 1 day. The pores of the microspheres were continuous and com...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com