Preparation method of goserelin acetate microspheres
A technology of goserelin acetate and microspheres, which can be applied to medical preparations containing active ingredients, microcapsules, nanocapsules, etc., can solve the problem of reducing the residual amount of organic solvents, reducing the residual amount of organic solvents, and the phenomenon of drug burst release and other problems, to achieve the effect of low solvent residue, long release cycle and smooth release curve
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
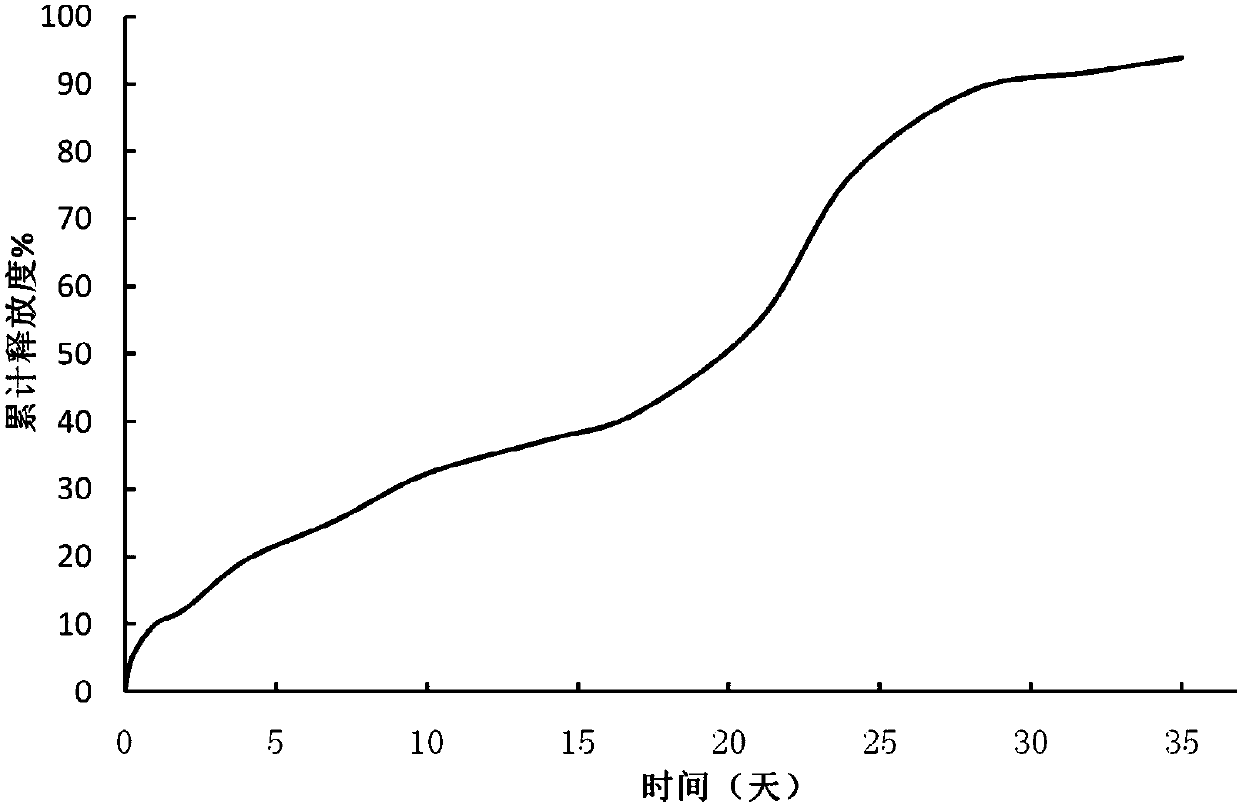
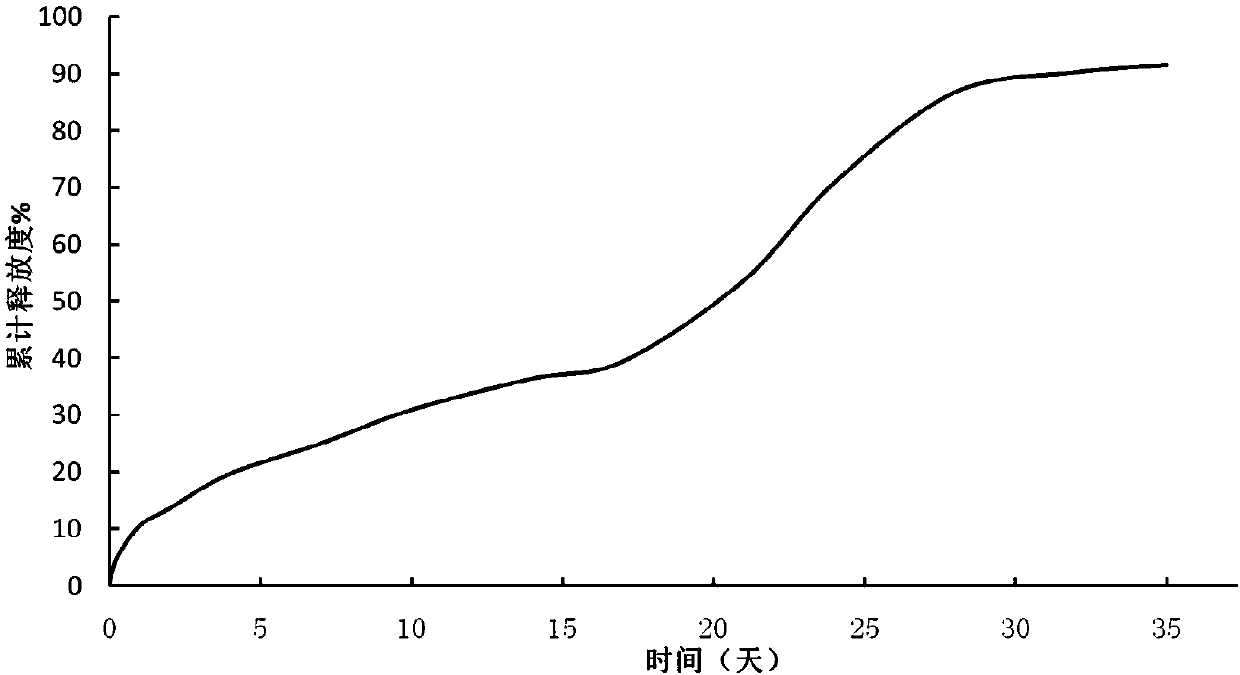
[0041] Weigh 0.2 g of goserelin acetate, dissolve 4 g of PLGA 7525 in 196 g of dichloromethane, add goserelin acetate into the PLGA in dichloromethane solution, and disperse homogeneously at 1000 rpm for 2 minutes to form a white suspension. The suspension was placed in an ice-water bath, under stirring at 500 rpm, simethicone at 5° C. was added through a syringe, and kept for 1 minute to form microsphere embryos. Transfer the mixture containing the microspheroids to n-heptane at 6 °C at 800 rpm, and stir for 60 minutes to complete the quench hardening. The hardened microspheres were collected by filtration, washed with an appropriate amount of methanol, vacuum-dried and lyophilized to obtain microsphere powder. Solvent residues: 0.12% dichloromethane, 0.75% n-heptane, 0.45% methanol.
Embodiment 2
[0043]Weigh 0.2g of goserelin acetate, dissolve 4g of PLGA 7525 in 133g of dichloromethane, add goserelin acetate into the solution of PLGA in dichloromethane, and disperse homogeneously at 1000rpm for 2 minutes to form a white suspension. The suspension was placed in an ice-water bath, under stirring at 500 rpm, simethicone at 7° C. was added through a syringe, and kept for 1 minute to form microsphere embryos. The mixture containing microspheroids was transferred to n-heptane at 5 °C at 800 rpm, and quenched and hardened after stirring for 60 minutes. The hardened microspheres were collected by filtration, washed with an appropriate amount of methanol, vacuum-dried and lyophilized to obtain microsphere powder. Solvent residues: 0.07% dichloromethane, 0.38% n-heptane, 0.61% methanol.
Embodiment 3
[0045] Weigh 0.2 g of goserelin acetate, dissolve 4 g of PLGA 7525 in 96 g of dichloromethane, add goserelin acetate into the PLGA in dichloromethane solution, and disperse homogeneously at 1000 rpm for 2 minutes to form a white suspension. The suspension was placed in an ice-water bath, under stirring at 500 rpm, simethicone at 6° C. was added through a syringe, and kept for 1 minute to form microsphere embryos. The mixture containing microspheroids was transferred to n-heptane at 8°C with a rotation speed of 800rpm, and quenched and hardened after stirring for 60 minutes. The hardened microspheres were collected by filtration, washed with an appropriate amount of methanol, vacuum-dried and lyophilized to obtain microsphere powder. Solvent residue: dichloromethane 0.05%, n-heptane 0.53%, methanol 0.34%
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com