Preparation method of triptorelin acetate sustained-release microsphere
A technology of triptorelin acetate and sustained-release microspheres, which can be applied to medical preparations containing active ingredients, pharmaceutical formulas, endocrine system diseases, etc. Reduced diffusion, surface smoothing effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
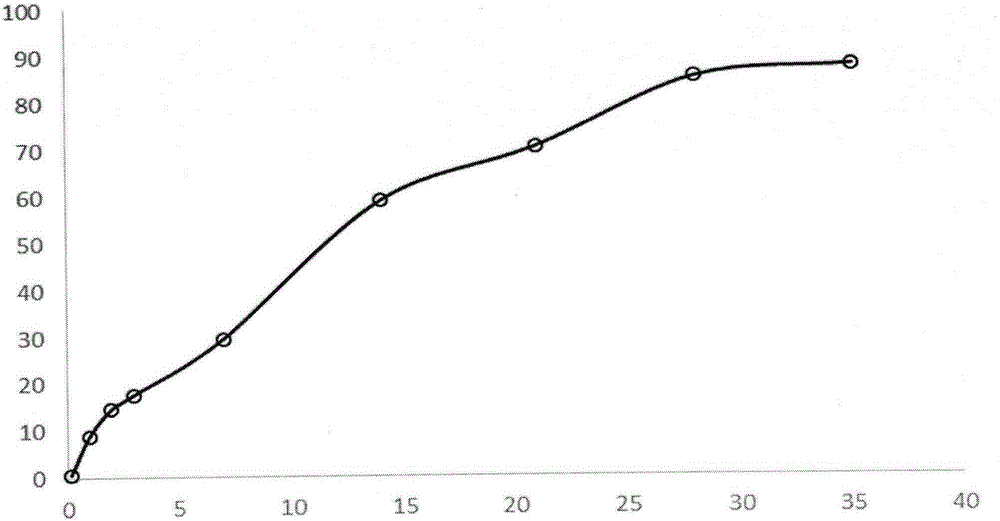
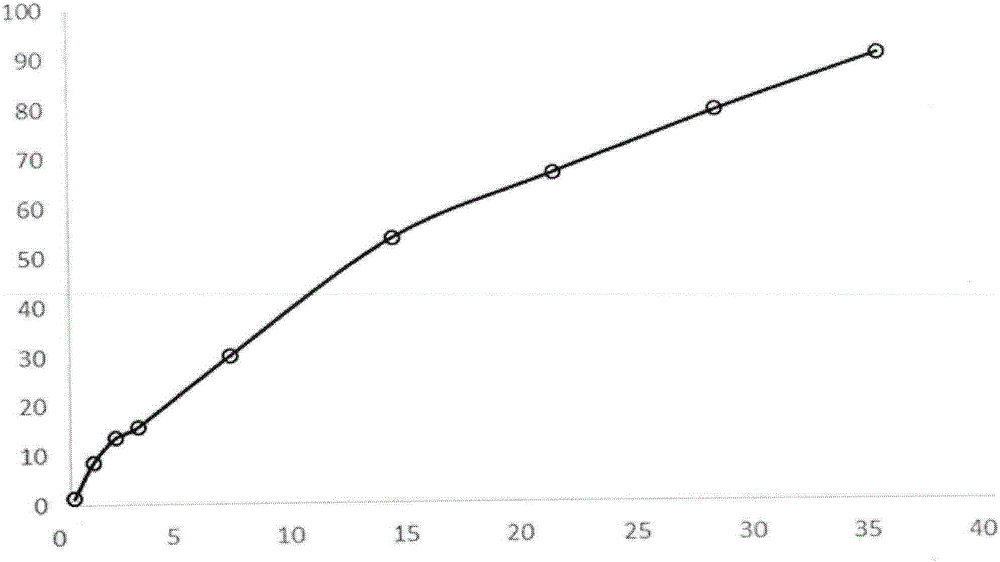
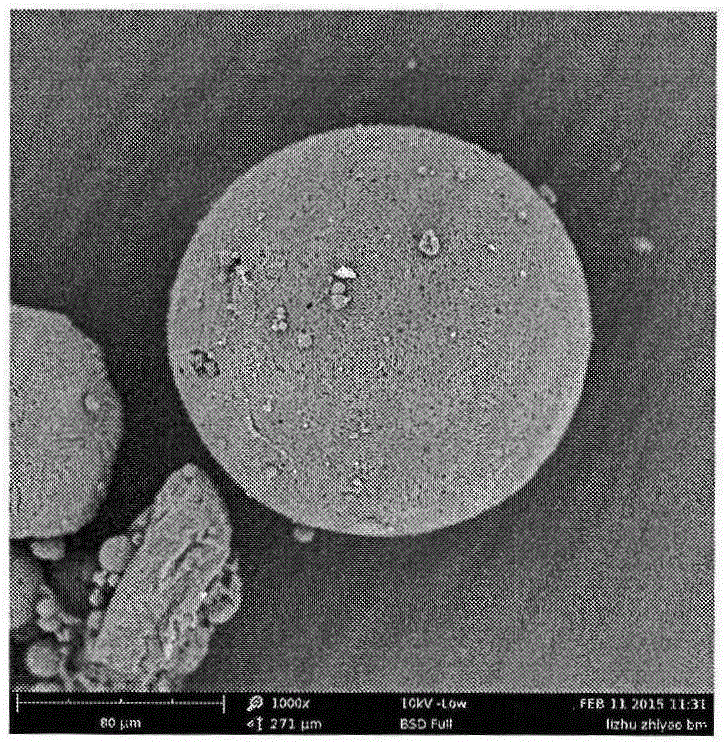
[0030] Weigh 1 g of triptorelin acetate (Ruilin Bachem company, batch number 1209001) and add water to make a drug solution with a concentration of 40%, dissolve 8 g of PLGA5050 with 25 ml of ethyl acetate and 10 ml of benzyl alcohol, and mix the two with ultrasound for 2 minutes to form a white Homogenized colostrum. The colostrum was added to 1000 ml of 0.5% PVA solution (containing 1% benzyl alcohol and 1% ethyl acetate) at 6°C through a syringe at 1500 rpm homogenization, and homogeneously emulsified for 2 min to obtain a double emulsion. The compound emulsion was moved to a cantilever mixer with a rotating speed of 600 rpm, stirred for 1 hour, then heated to 45° for 1 hour, then lowered to 10° for sieve filtration, and freeze-dried to obtain powdered microspheres. The drug loading of triptorelin acetate microspheres was 10.3%, the burst release was 9% in 1 day, and the pores of the microspheres were continuous and intact, and the release was stable for 28 days.
Embodiment 2
[0032] Weigh 1 g of triptorelin acetate (Ruilin Bachem company, batch number 1209001) and add water to make a drug solution with a concentration of 40%, dissolve 8 g of PLGA5050 with 23.5 ml of ethyl acetate and 3.3 ml of benzyl alcohol, and mix the two with ultrasonics for 2 min. A white homogeneous colostrum is formed. The colostrum was added to 1000 ml of 0.5% PVA solution (containing 1% benzyl alcohol and 1% ethyl acetate) at 6°C through a syringe at 1500 rpm homogenization, and homogeneously emulsified for 2 min to obtain a double emulsion. The compound emulsion was moved to a cantilever mixer with a rotating speed of 600 rpm, stirred for 1 hour, then heated to 45° for 1 hour, then lowered to 10° for sieve filtration, and freeze-dried to obtain powdered microspheres. The drug loading of triptorelin acetate microspheres was 10.8%, the burst release was 8% in 1 day, the pores of the microspheres were continuous and intact, and the release was stable for 28 days.
Embodiment 3
[0034] Weigh 1 g of triptorelin acetate (Ruilin Bachem company, batch number 1209001) and add water to make a drug solution with a concentration of 40%, dissolve 8 g of PLGA5050 with 19 ml of ethyl acetate and 7.6 ml of benzyl alcohol, and mix the two with ultrasound for 2 min to form The white homogeneous colostrum was added to 1000 ml of 0.5% PVA solution (containing 1% benzyl alcohol and 1% ethyl acetate) at 6°C through a syringe at 1500 rpm homogenization, and homogeneously emulsified for 2 minutes to obtain a double emulsion. The compound emulsion was moved to a cantilever mixer with a rotating speed of 600 rpm, stirred for 1 hour, then heated to 40° for 1 hour, then lowered to 10° for sieve filtration, and freeze-dried to obtain powdered microspheres. The drug loading of triptorelin acetate microspheres was 10.6%, the burst release in 1 day was 9%, the pores of the microspheres were continuous and intact, and the release was stable for 28 days.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com