Oral slow/controlled-release preparation containing febuxostat and preparation method thereof
A technology for controlled release preparations and febuxostat, which is applied in the directions of medical preparations containing active ingredients, pill delivery, pharmaceutical formulations, etc. Improve the efficacy, reduce the incidence of adverse reactions, and avoid the effect of sudden release effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
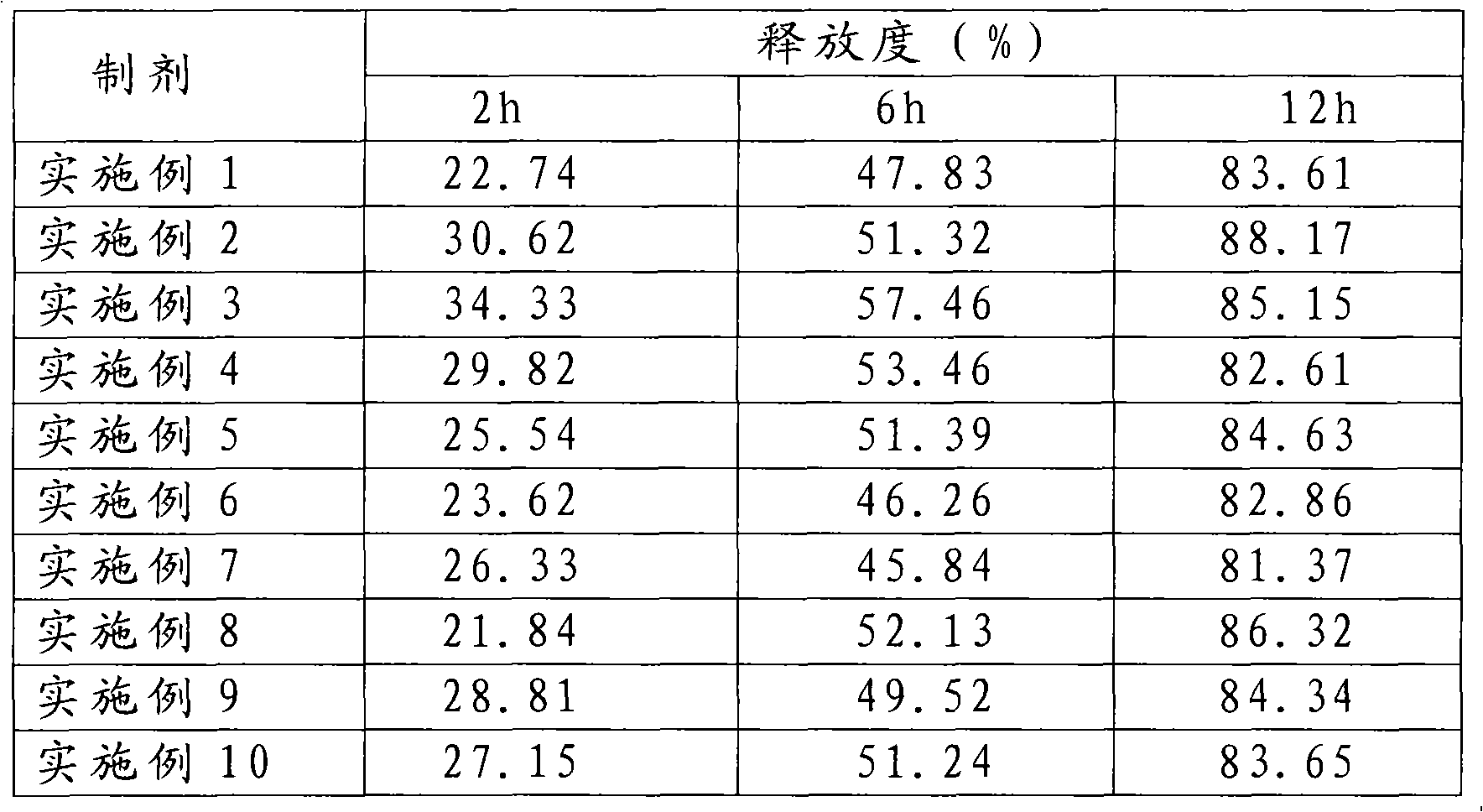
Examples
Embodiment 1
[0036] Example 1 Matrix-type sustained-release tablet
[0037] Febuxostat 80mg
[0038] Hypromellose (K15M) 15mg
[0039] Hypromellose (K4M) 15mg
[0040] Lactose 85mg
[0041] 5% Povidone K30 in 75% ethanol 60mg
[0043] (The 75% ethanol solution of 5% povidone K30 refers to the 75% ethanol solution containing 5% povidone K30 by mass);
[0044] Preparation
[0045] After crushing febuxostat, pass it through a 100-mesh sieve, and then mix it with hypromellose (K15M), hypromellose (K4M) and lactose, then add 5% povidone K30 in 75% ethanol solution , use HLSH2-6 wet mixing granulator to prepare soft materials, sieve wet granules with 24 mesh, dry the wet granules by blasting at 50 ℃ ~ 60 ℃, dry granules with a 22 mesh sieve, add magnesium stearate and mix After uniformity, use ZP10A tablet press for tableting.
Embodiment 2
[0046] Example 2 Coated sustained and controlled release tablet
[0047] Chip formula
[0048] Febuxostat 80mg
[0049] Calcium hydrogen phosphate 25mg
[0050] Microcrystalline Cellulose 110mg
[0051] 2% Hypromellose K15M in water 40mg
[0053] coating material
[0054] 15% ethyl cellulose dispersion in water 116mg
[0055] Preparation Process
[0056] After crushing febuxostat, pass it through a 100-mesh sieve, and then mix it with calcium hydrogen phosphate and microcrystalline cellulose evenly, add 2% hypromellose K15M aqueous solution, and use HLSH2-6 type wet mixing granulator to prepare Soft material, 24 mesh sieve to make wet granules, the wet granules are air-dried at 50 ℃ ~ 60 ℃, the dry granules are granulated with a 22 mesh sieve, after adding magnesium stearate and mixing evenly, use a ZP10A tablet machine for tableting, hardness Range 35-85N, and then coat with 15% aqueous ethyl cellulose dispersion.
Embodiment 3
[0057] Embodiment 3 skeleton type slow-release capsule
[0058] Febuxostat 80mg
[0059] Hypromellose (K15M) 15mg
[0060] Hypromellose (K4M) 15mg
[0061] Microcrystalline Cellulose 85mg
[0062] 5% Povidone K30 in 75% ethanol 60mg
[0063] Micronized silica gel 2mg
[0064] Preparation
[0065] After crushing febuxostat, pass it through a 100-mesh sieve, and then mix it with hypromellose (K15M), hypromellose (K4M), and microcrystalline cellulose, and then add 75% of 5% povidone K30. % ethanol solution, use HLSH2-6 wet mixing granulator to prepare soft material, and then add JBZ-300 multifunctional granulation coating machine to extrude and spheronize to prepare pellets. The pellets are prepared at 50℃~60℃ Blast dry, add micropowder silica gel and mix evenly, then fill into No. 0 capsule shell.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com