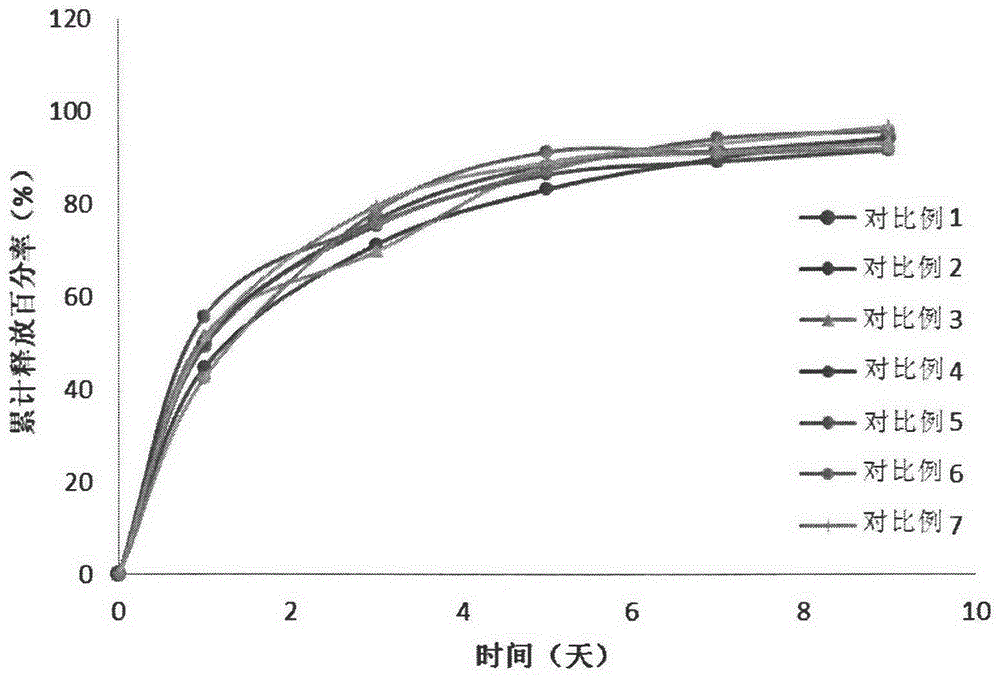
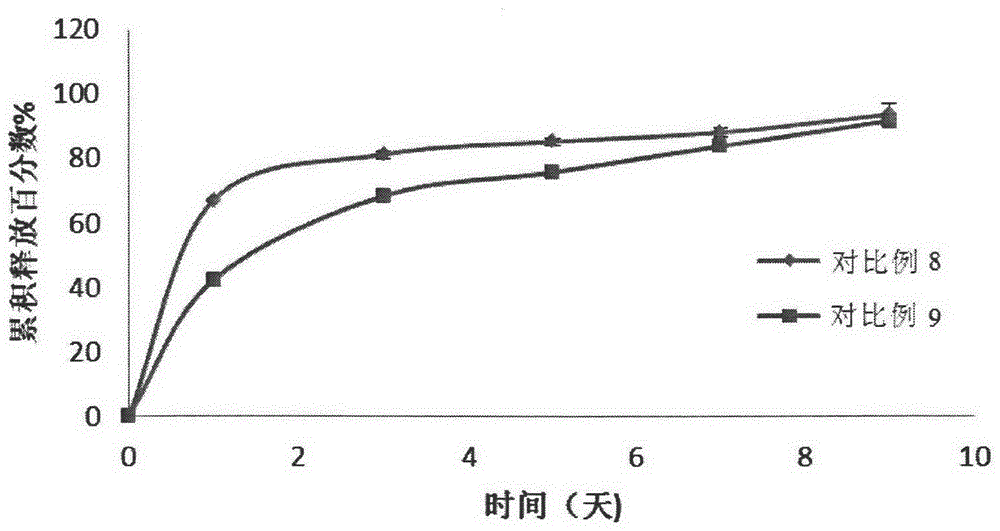
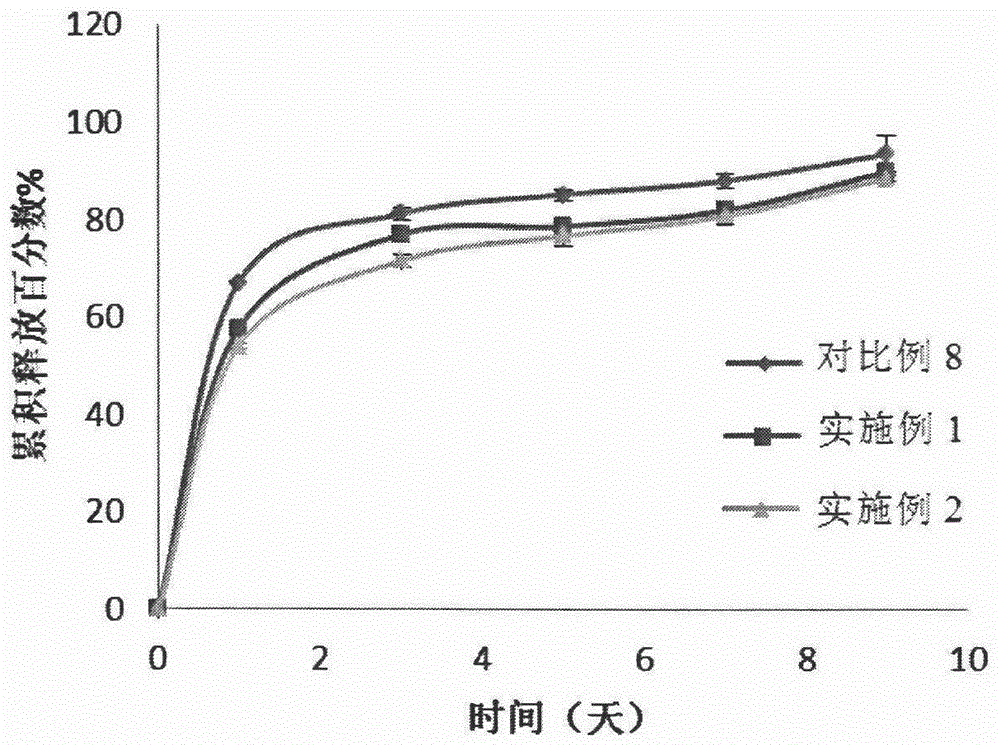
A thermosensitive gel pharmaceutical preparation and preparation method thereof
A temperature-sensitive gel and pharmaceutical preparation technology, which is applied in the directions of drug combination, pharmaceutical formulation, aerosol delivery, etc., can solve the problem of not achieving the sustained release effect of the preparation, achieve good reference value and application significance, and improve medication compliance. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-2
[0083] Polymer A: hydrophilic PLGA-PEG-PLGA copolymer (polymer A, 1571-1500-1571, the molecular weight of the block is the number average molecular weight, the block molar ratio LA / GA=4, the molecular weight distribution coefficient is 1.15) ; Polymer B: appropriate amount of hydrophobic PLGA-PEG-PLAG copolymer (polymer B, 1255-1500-1255, the molecular weight of the block is the number average molecular weight, the block molar ratio LA / GA=4, the molecular weight distribution coefficient is 1.23 ).
[0084] Wherein, the synthesis process of polymers A and B refers to the synthesis methods in Examples 1 and 3 of the Chinese patent application with application number 200910049664.6.
[0085] Get polymer A and polymer B, add deionized water, dissolve under magnetic stirring, configure polymer, the weight ratio of polymer A / polymer B is 1 / 1, and the total polymer concentration is 25% (w / The aqueous solution of w) was passed through a 0.22 μm filter membrane for later use, and was...
Embodiment 3-4
[0090] Polymer A: hydrophilic PLGA-PEG-PLGA copolymer (polymer A, 1571-1500-1571, the molecular weight of the block is the number average molecular weight, the block molar ratio LA / GA=4, the molecular weight distribution coefficient is 1.15) ; Polymer B: appropriate amount of hydrophobic PLGA-PEG-PLAG copolymer (polymer B, 1255-1500-1255, the molecular weight of the block is the number average molecular weight, the block molar ratio LA / GA=4, the molecular weight distribution coefficient is 1.23 ).
[0091] Wherein, the synthesis process of polymers A and B refers to the synthesis methods in Examples 1 and 3 of the Chinese patent application with application number 200910049664.6.
[0092] Get polymer A and polymer B, add deionized water, dissolve under magnetic stirring, configure polymer, the weight ratio of polymer A / polymer B is respectively 1 / 1, and total polymer concentration is 25% (w / The aqueous solution of w) was passed through a 0.22 μm filter membrane for later use...
Embodiment 5-6
[0097] Polymer A: hydrophilic PLGA-PEG-PLGA copolymer (polymer A, 1571-1500-1571, the molecular weight of the block is the number average molecular weight, the block molar ratio LA / GA=4, the molecular weight distribution coefficient is 1.15) ; Polymer B: appropriate amount of hydrophobic PLGA-PEG-PLAG copolymer (polymer B, 1255-1500-1255, the molecular weight of the block is the number average molecular weight, the block molar ratio LA / GA=4, the molecular weight distribution coefficient is 1.23 ).
[0098] Wherein, the synthesis process of polymers A and B refers to the synthesis methods in Examples 1 and 3 of the Chinese patent application with application number 200910049664.6.
[0099] Get polymer A and polymer B, add deionized water, dissolve under magnetic stirring, configure polymer, the weight ratio of polymer A / polymer B is respectively 1 / 1, and total polymer concentration is 25% (w / The aqueous solution of w) was passed through a 0.22 μm filter membrane for later use...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com