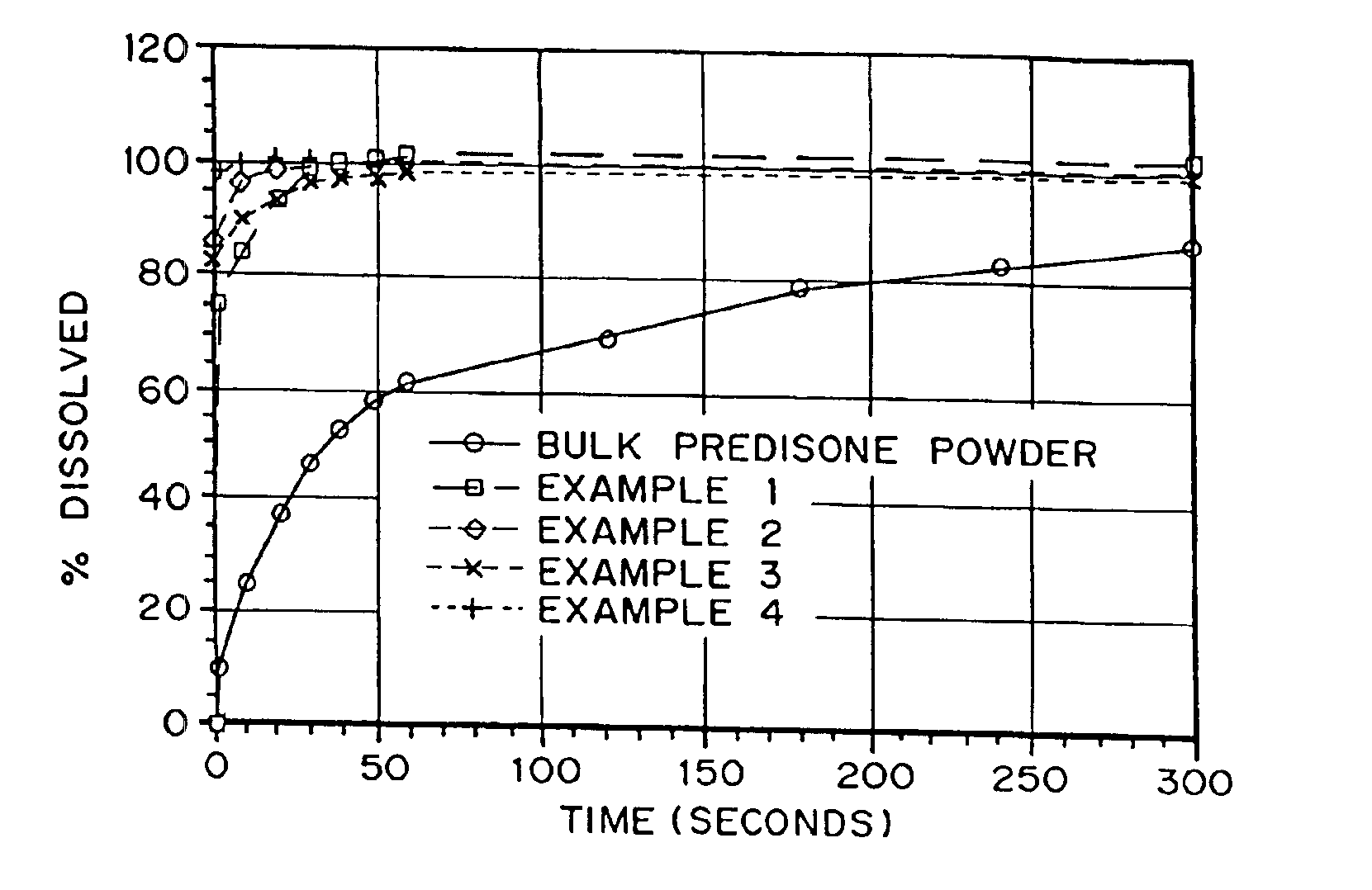
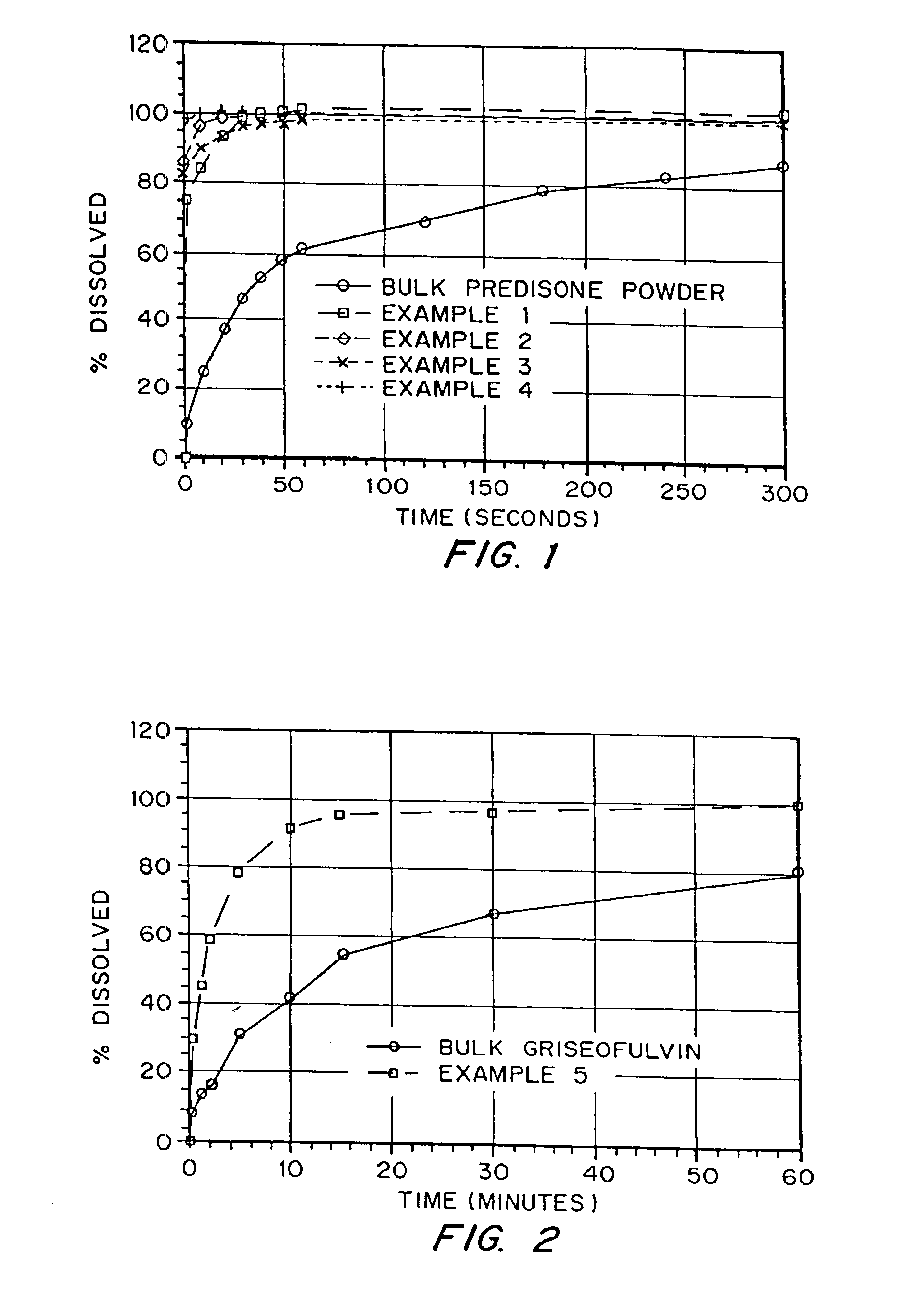
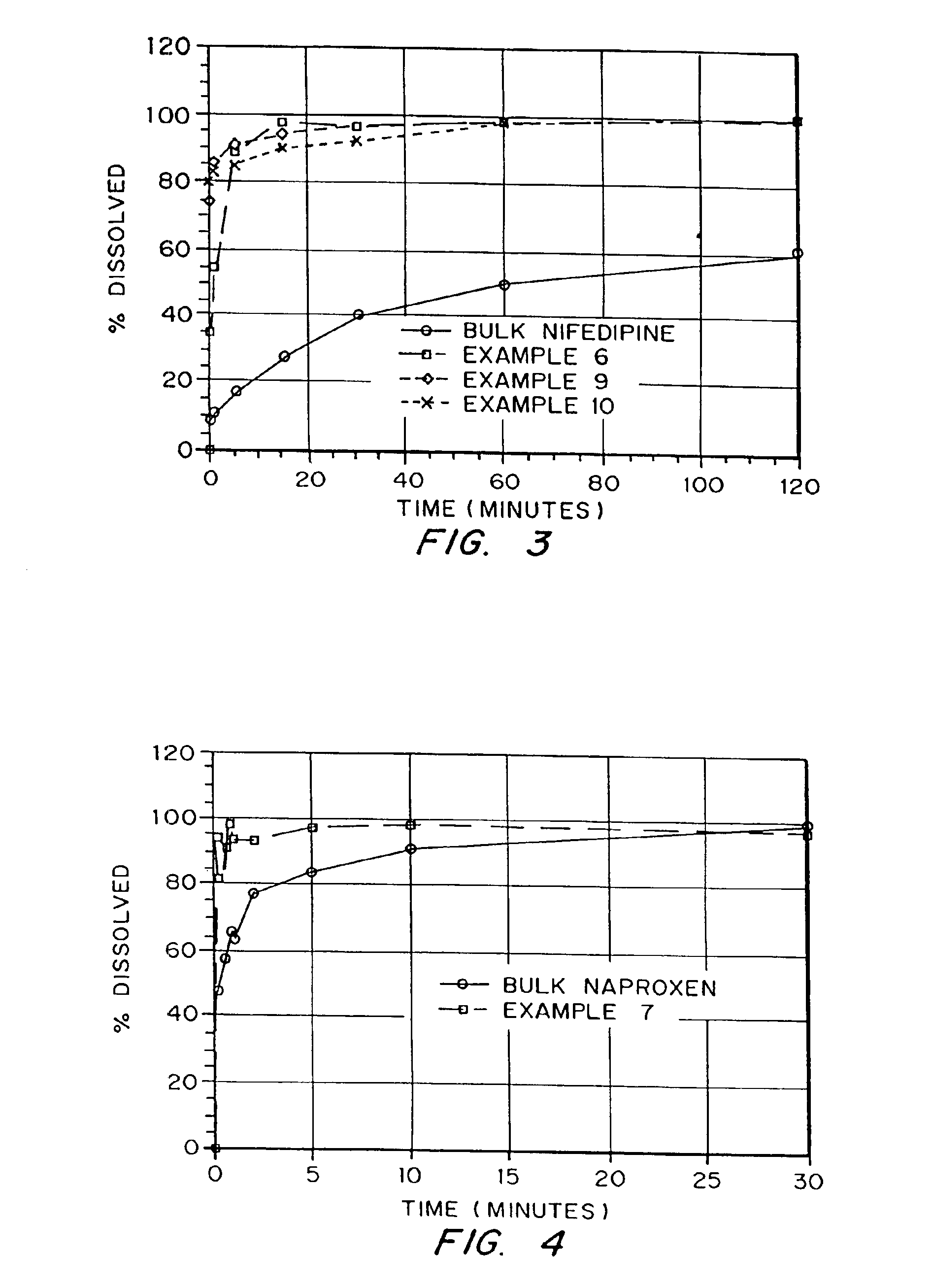
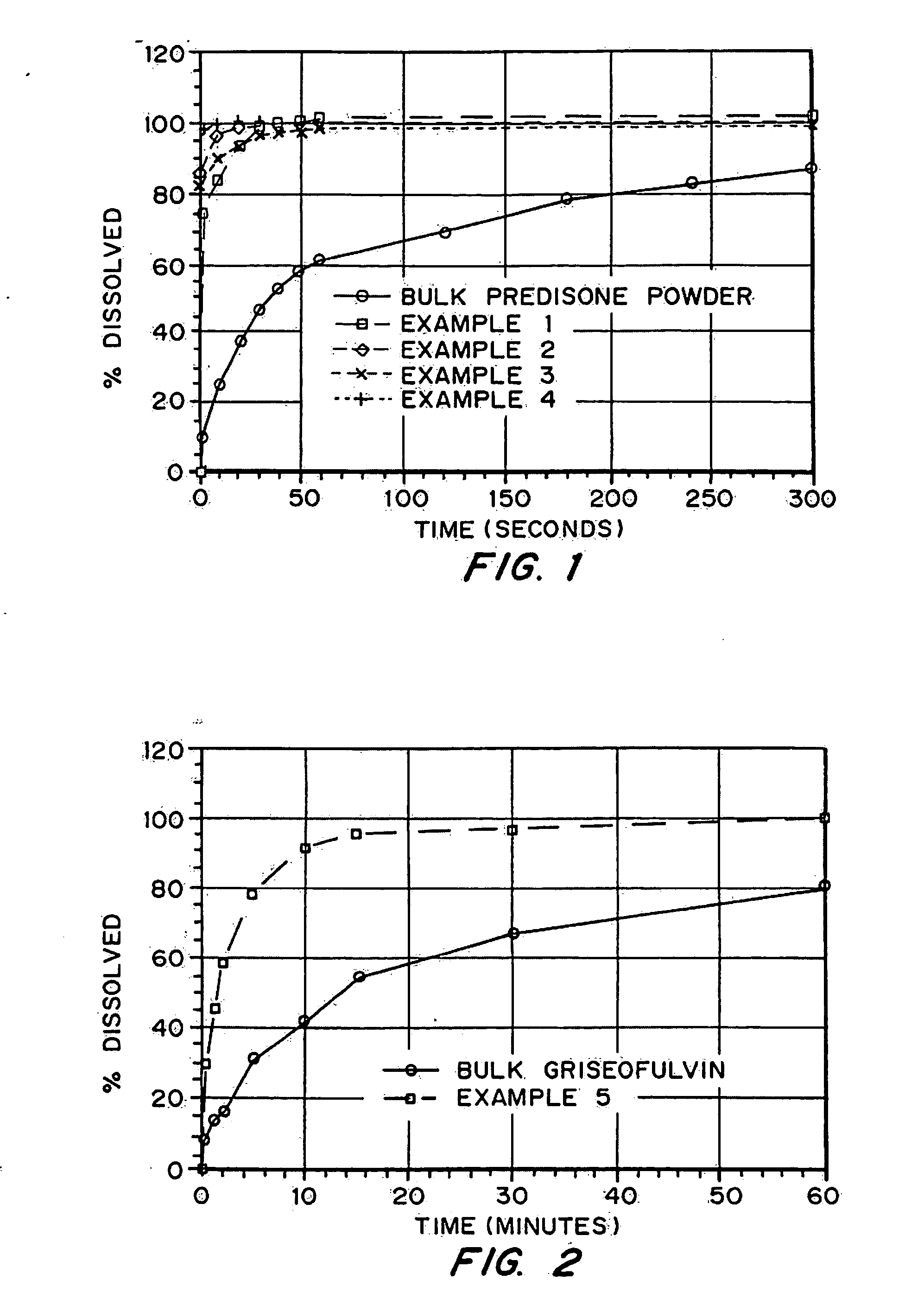
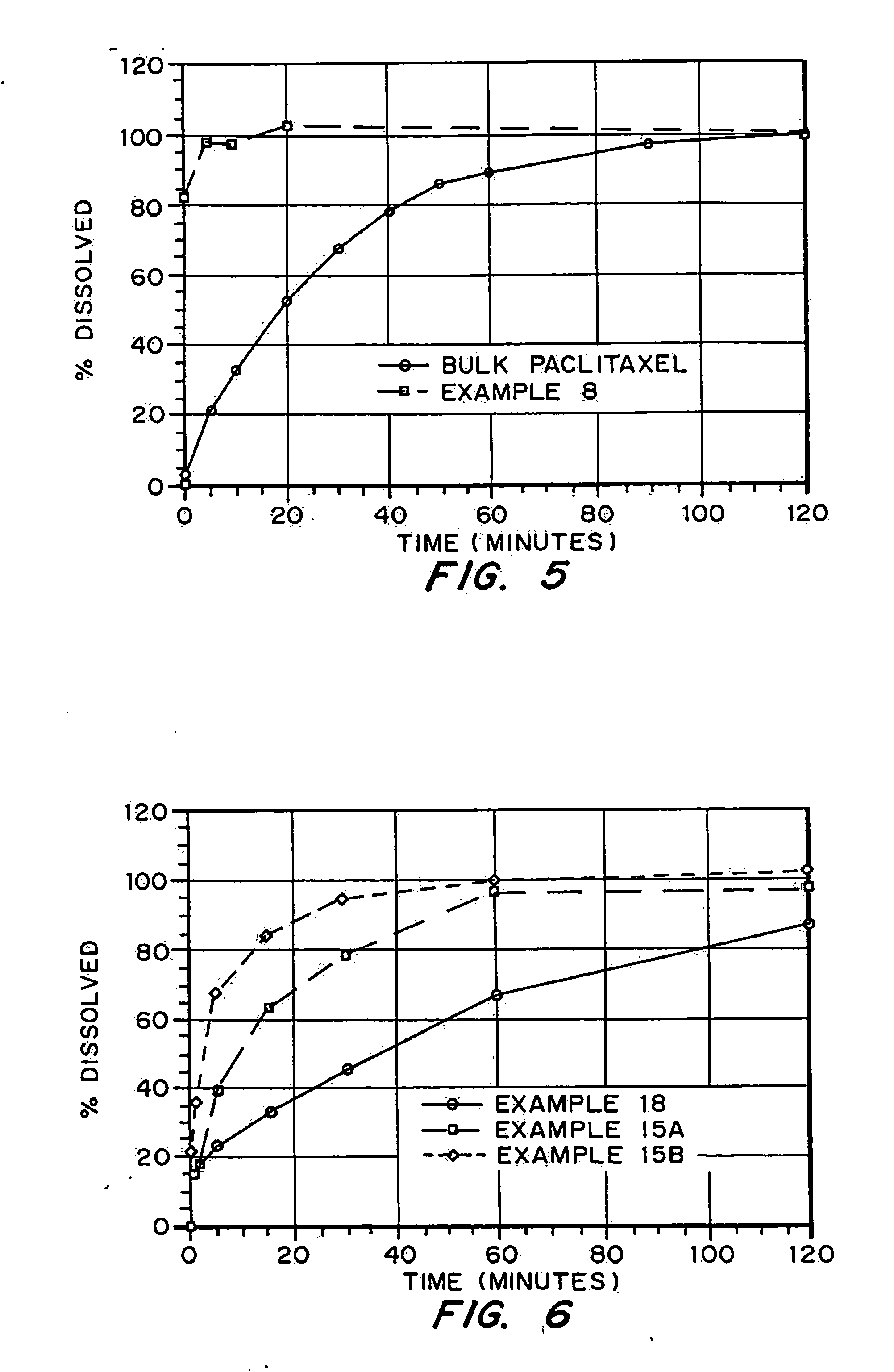
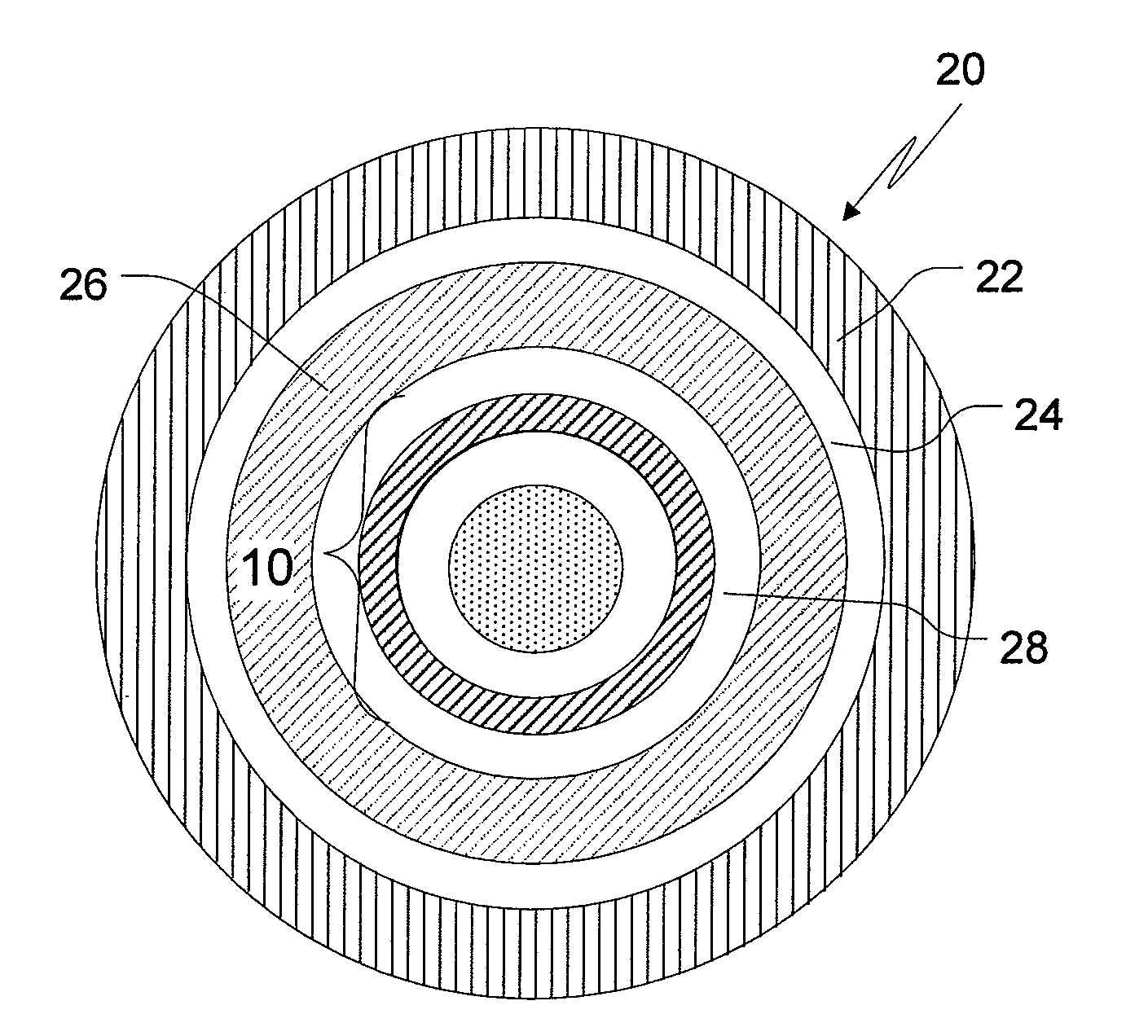
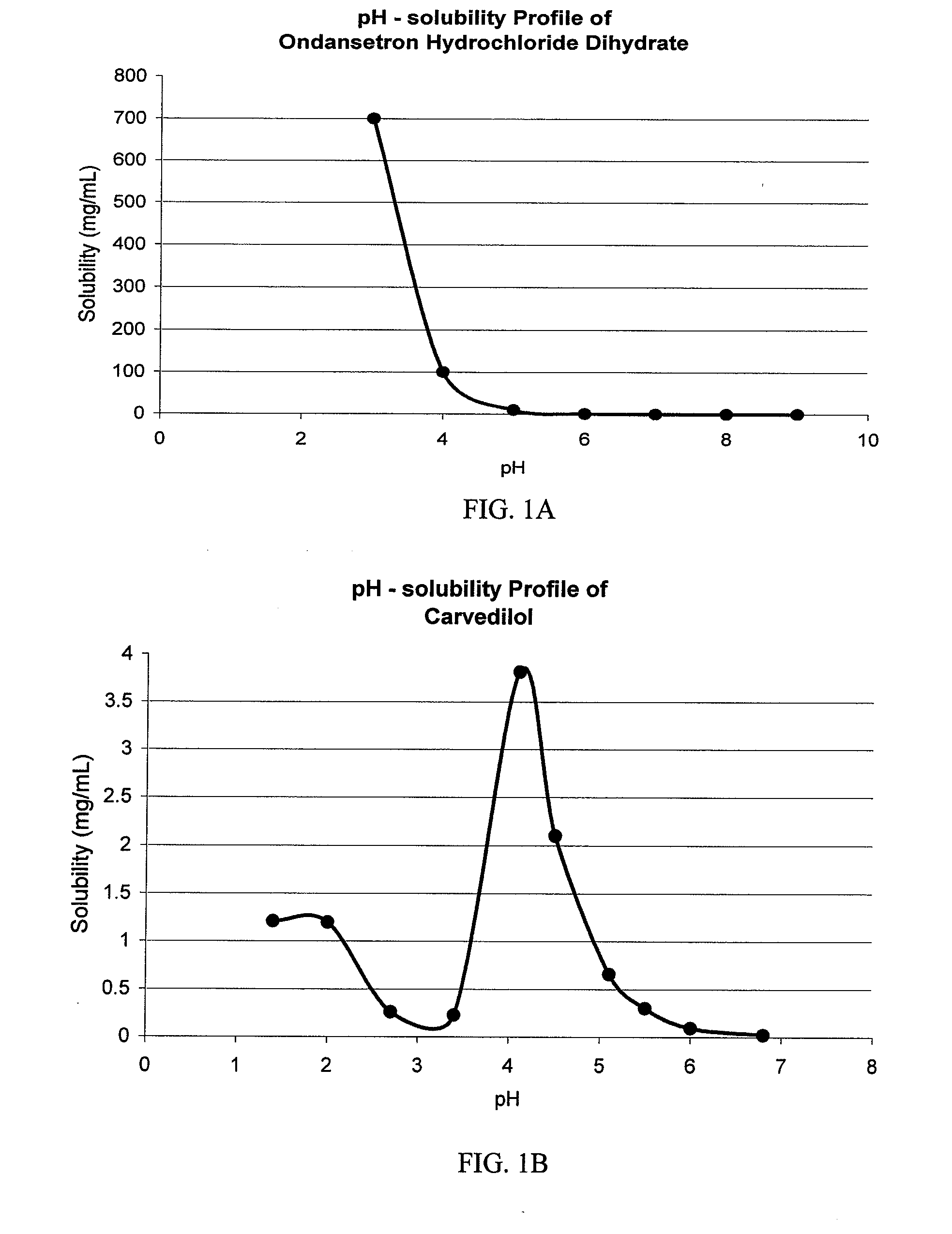
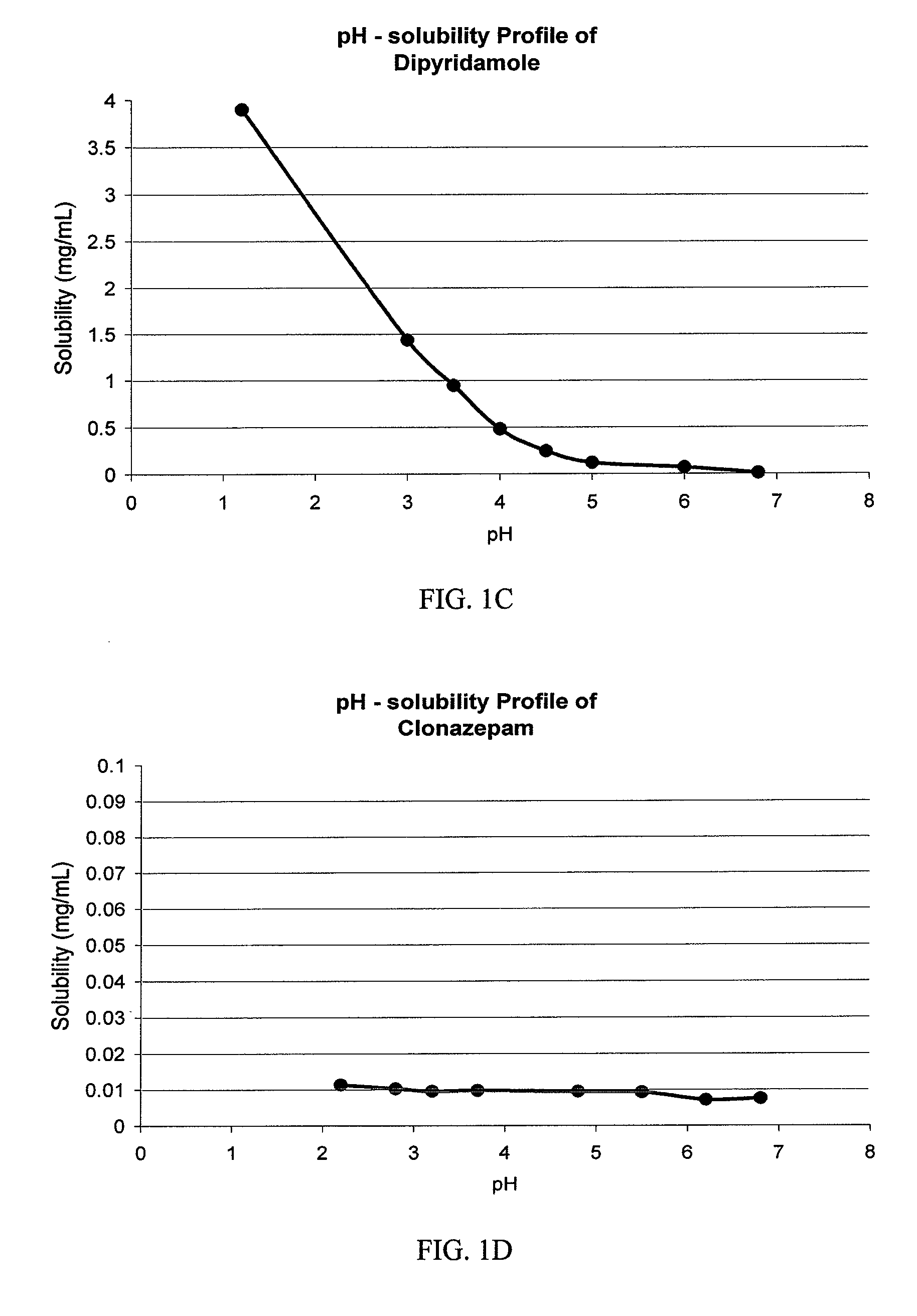
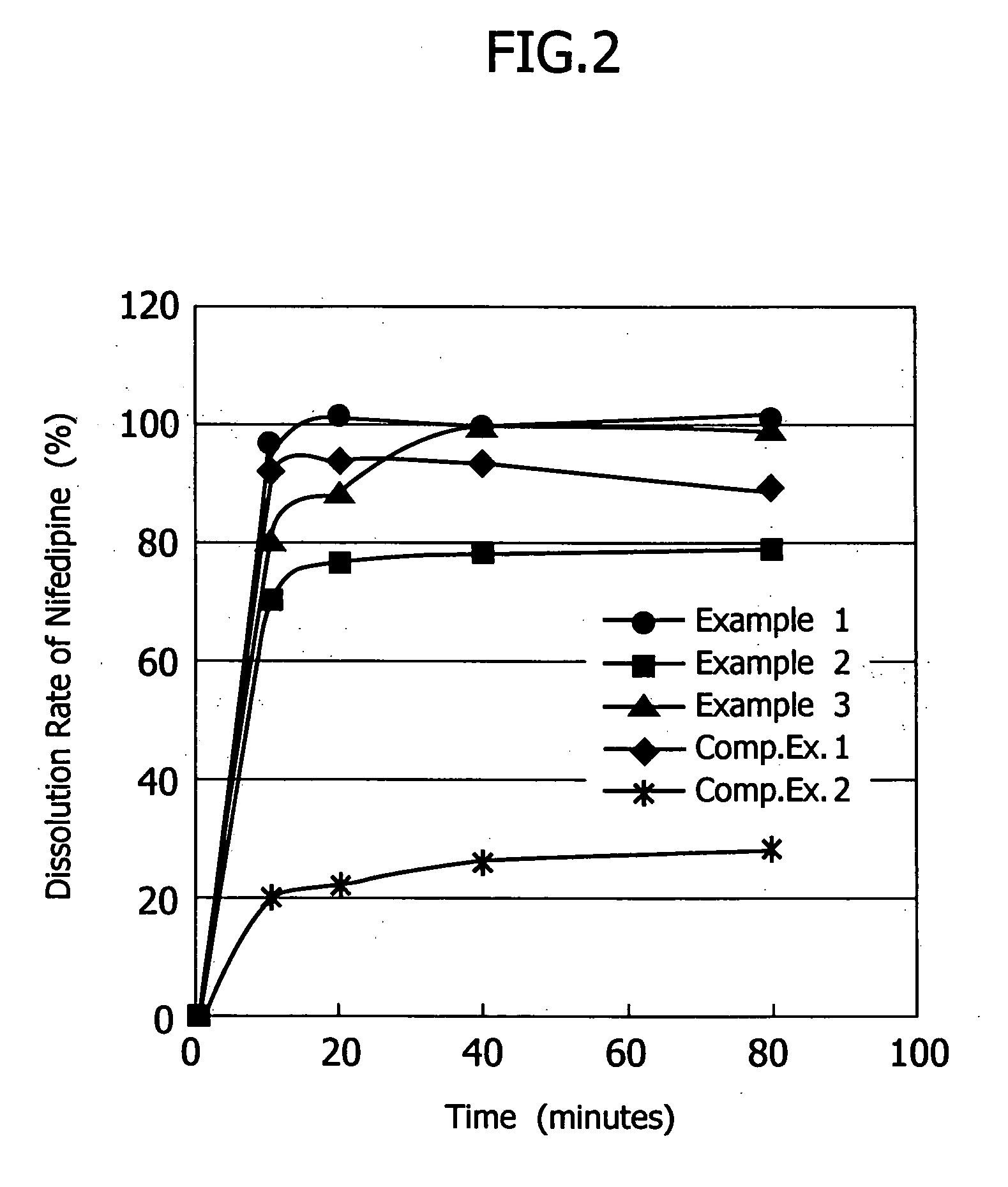
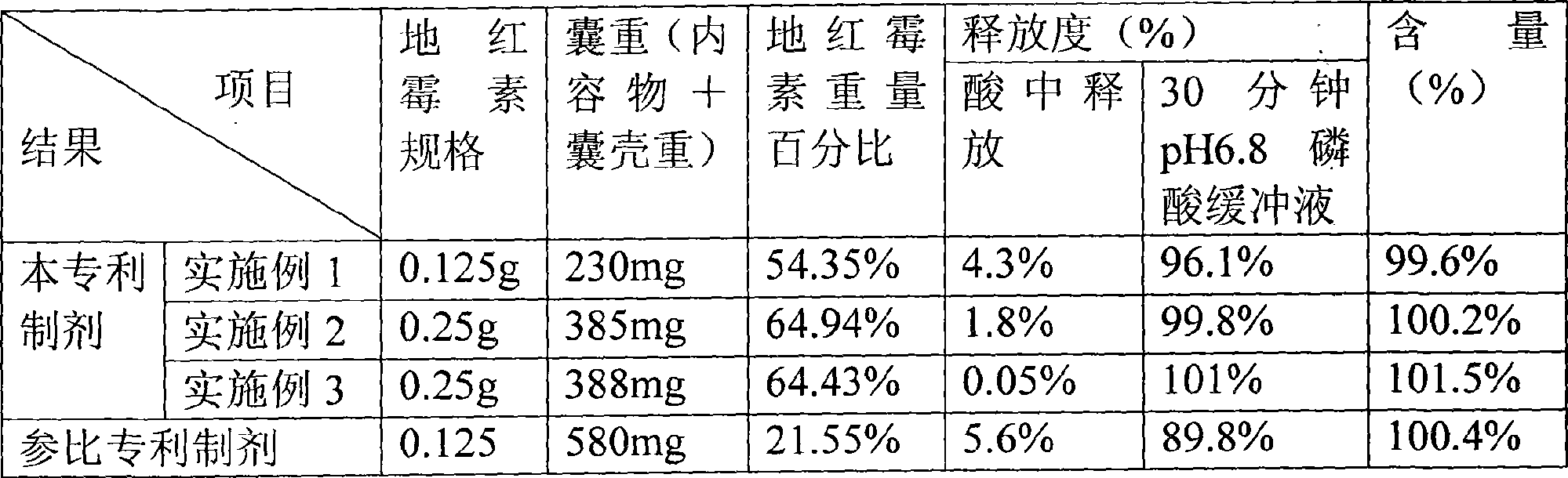
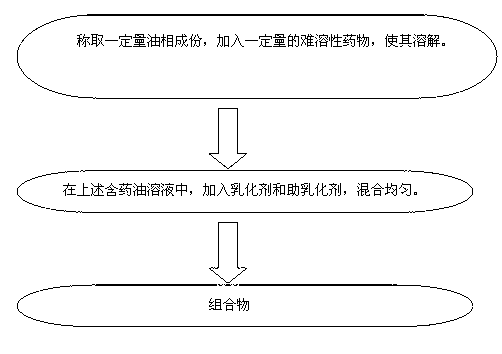
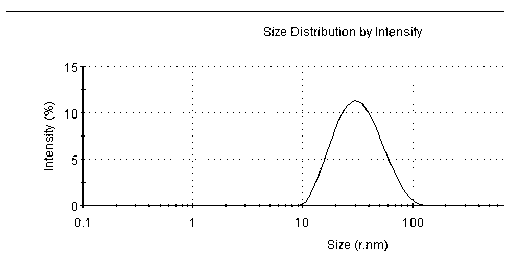
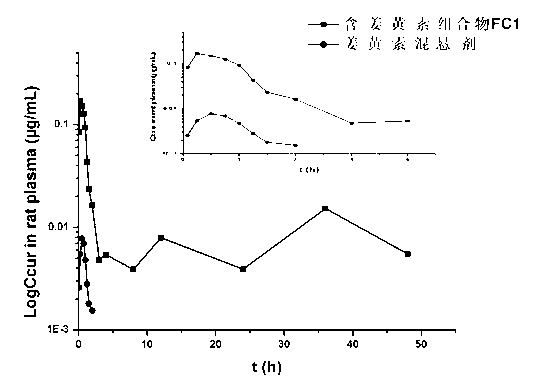
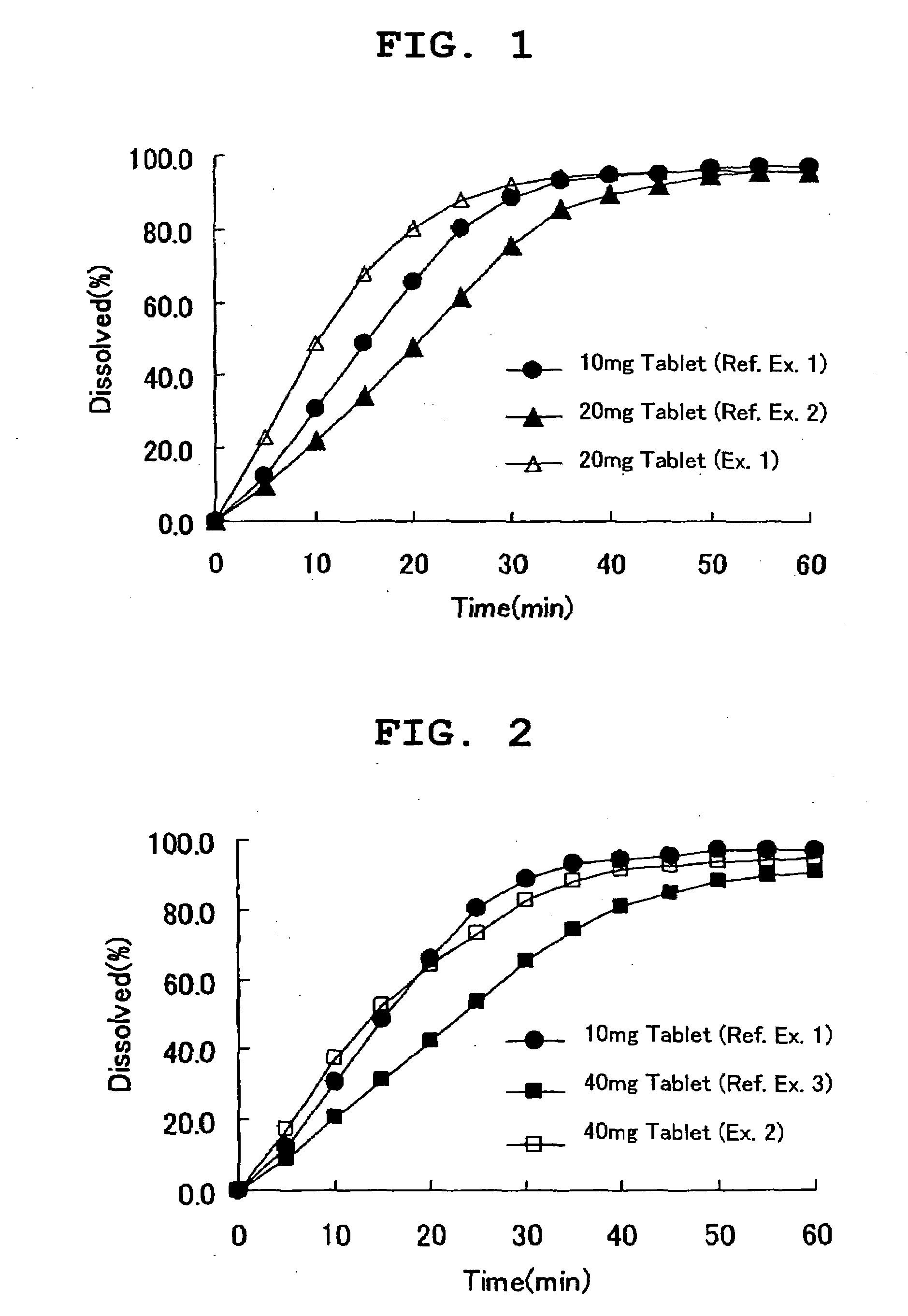
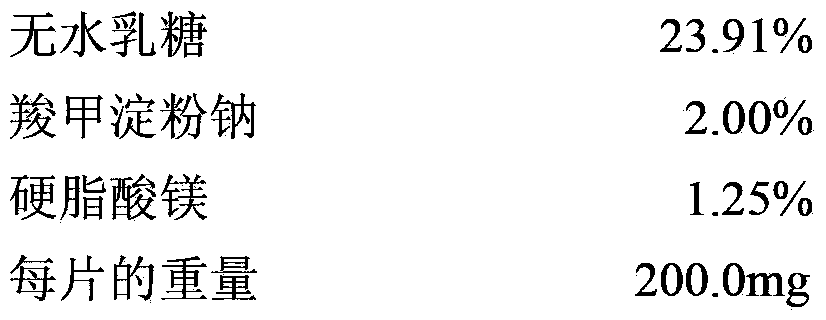Patents
Literature
488 results about "Drug Dissolution" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
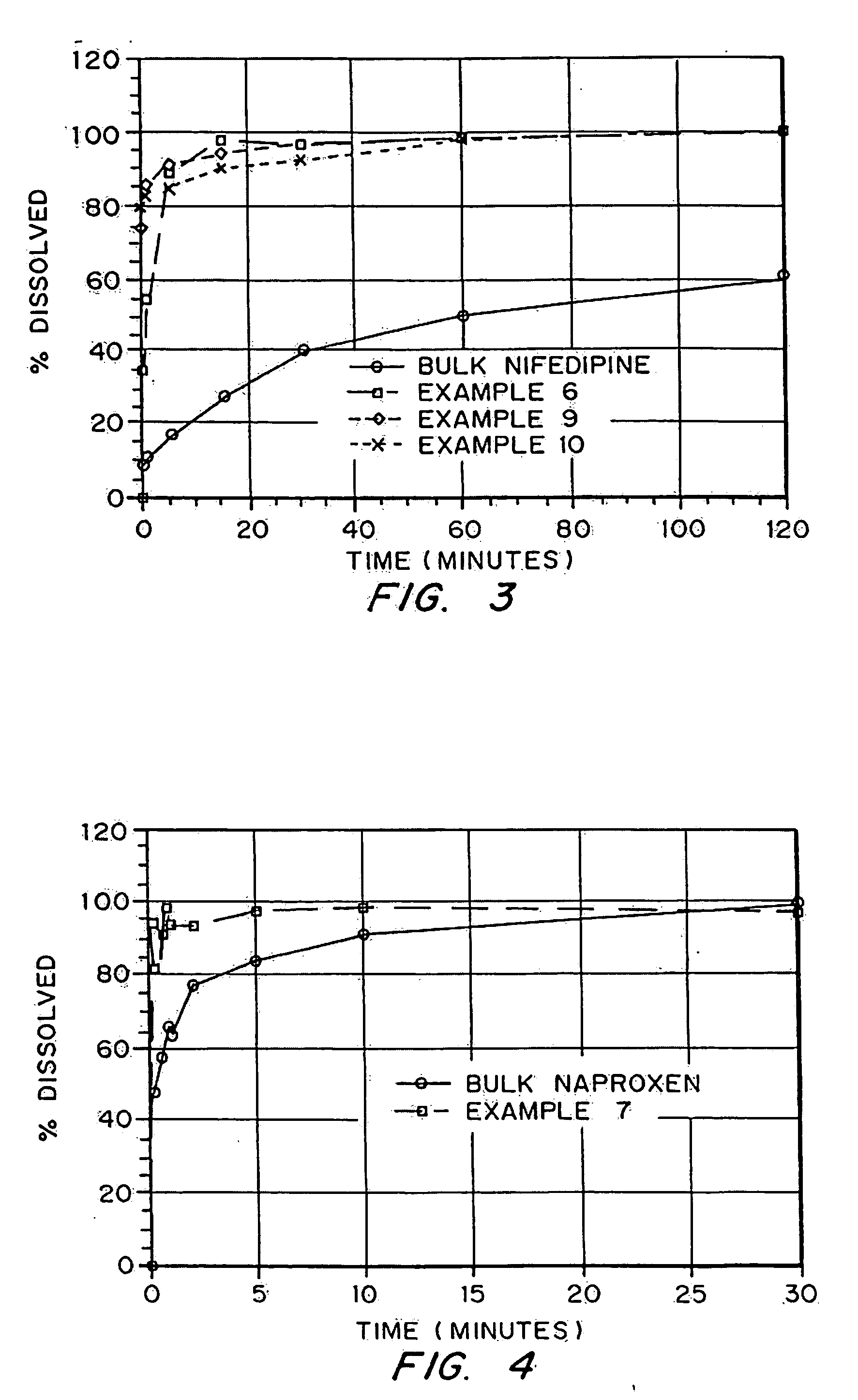
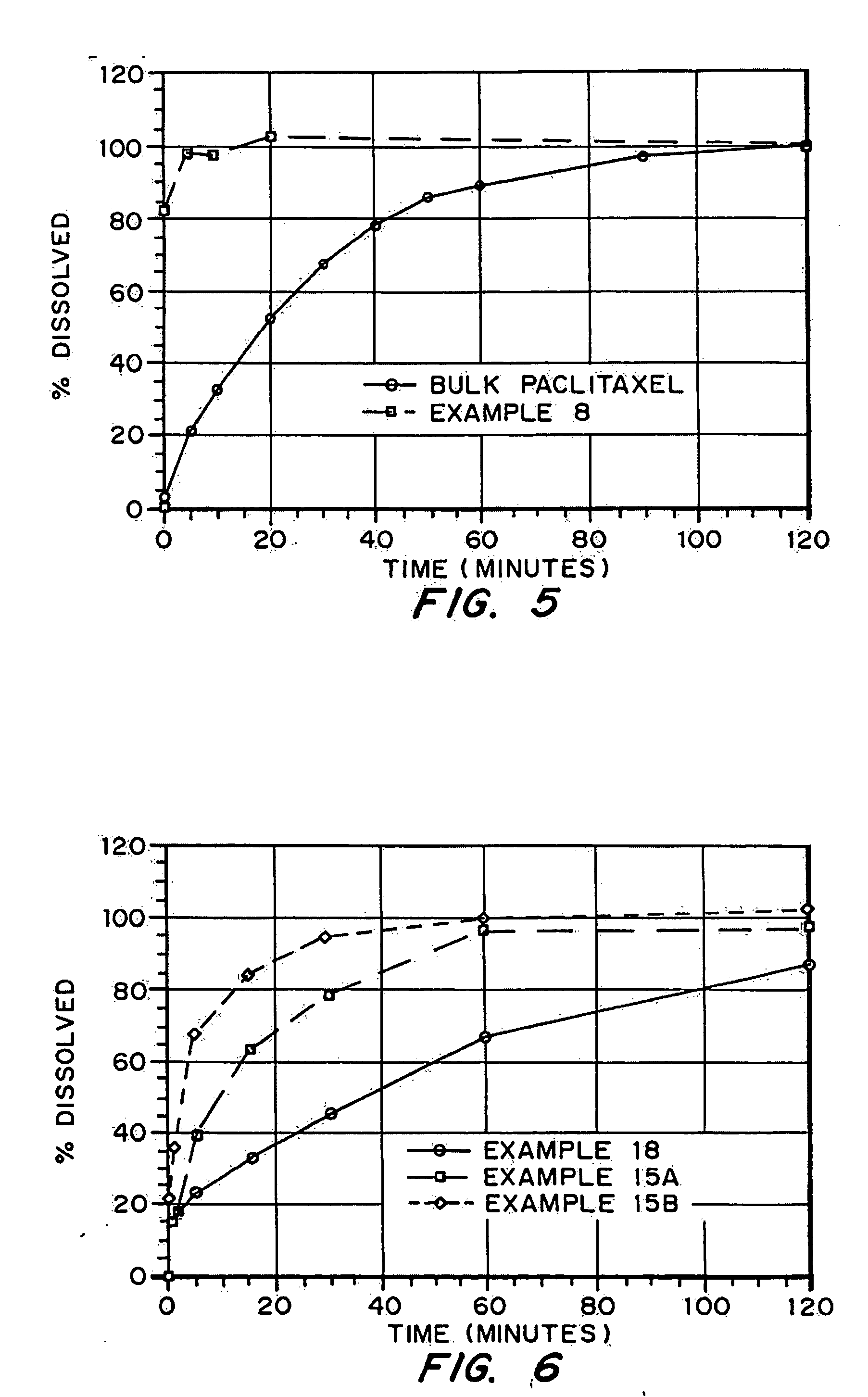
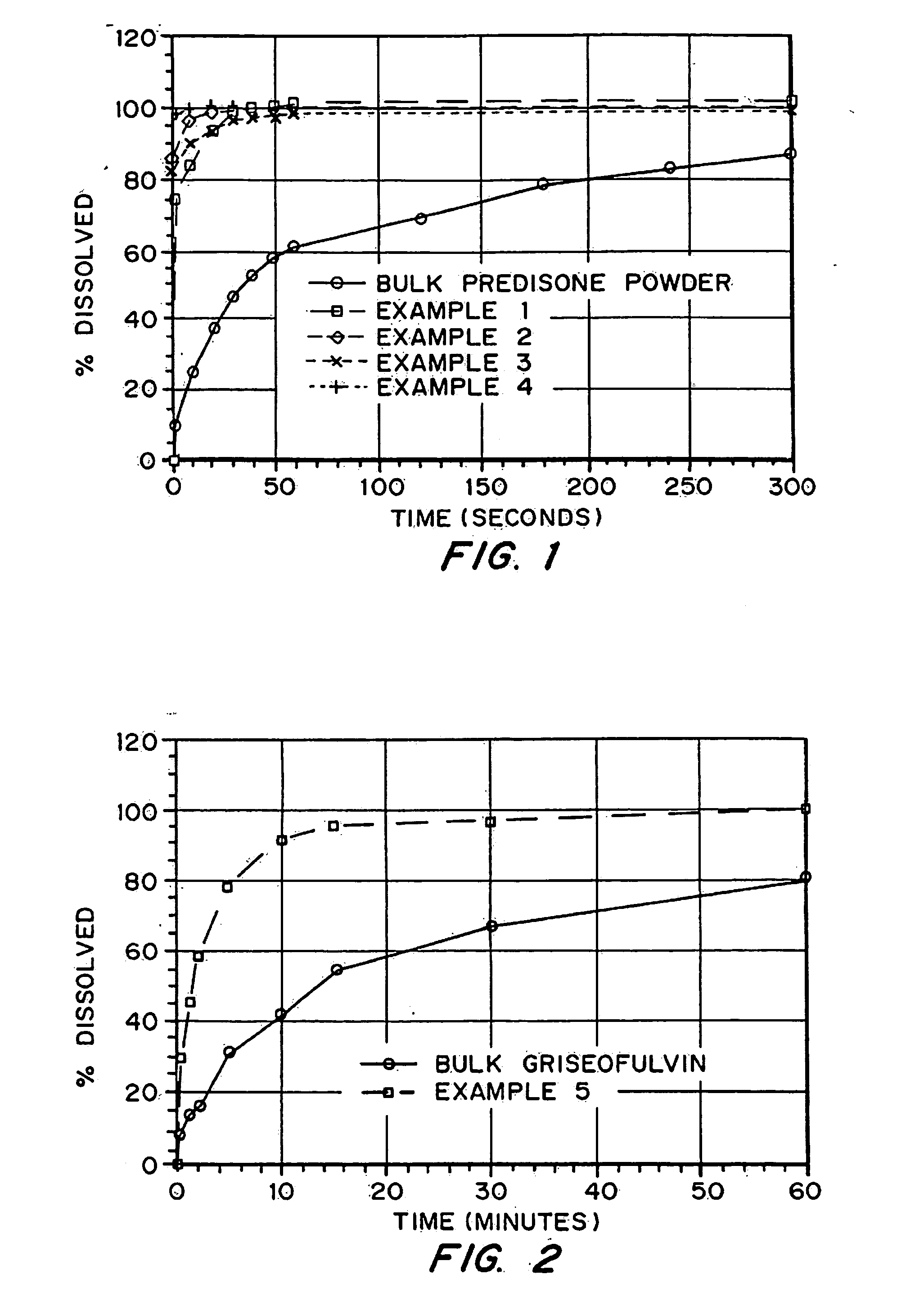
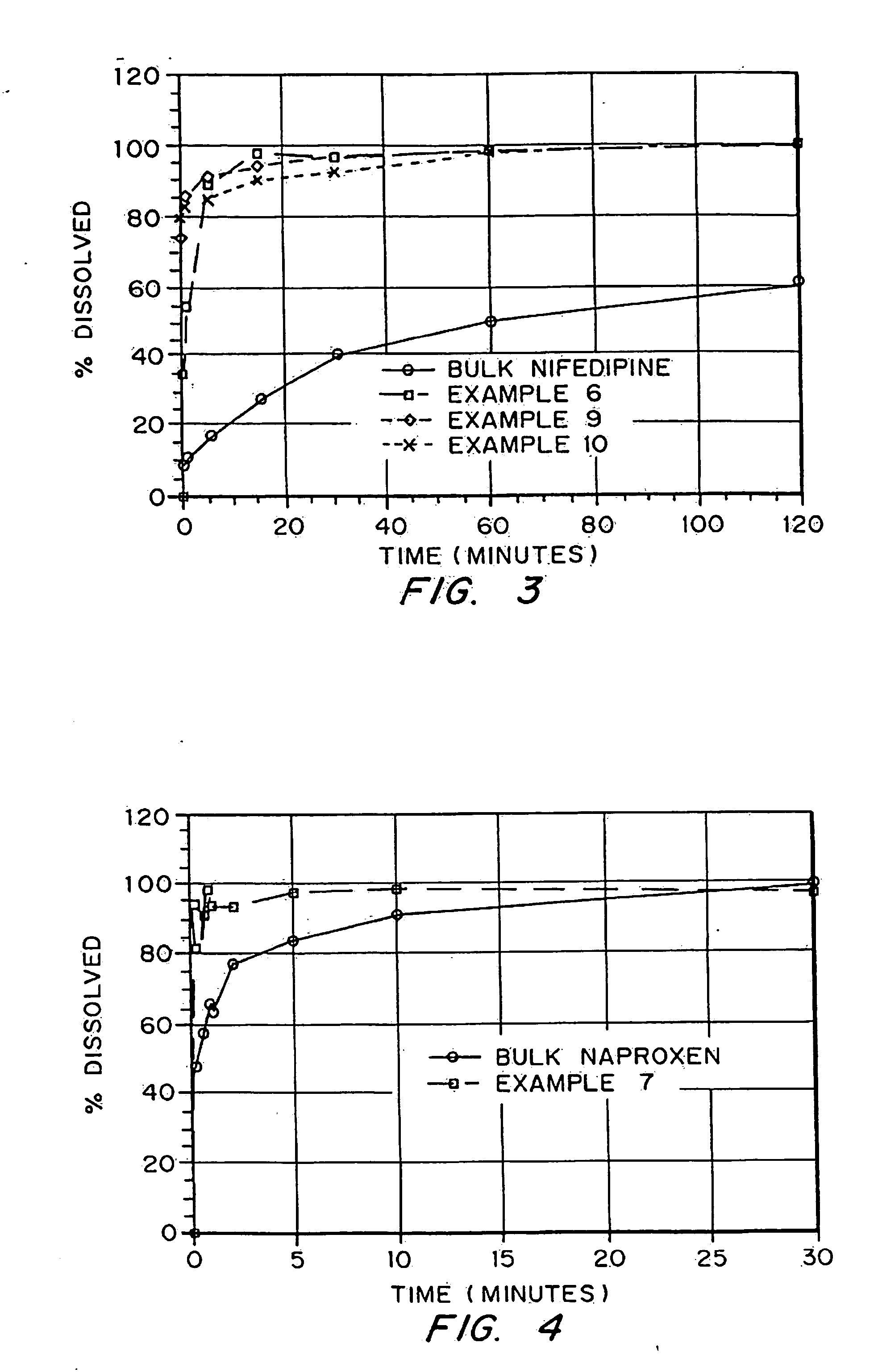
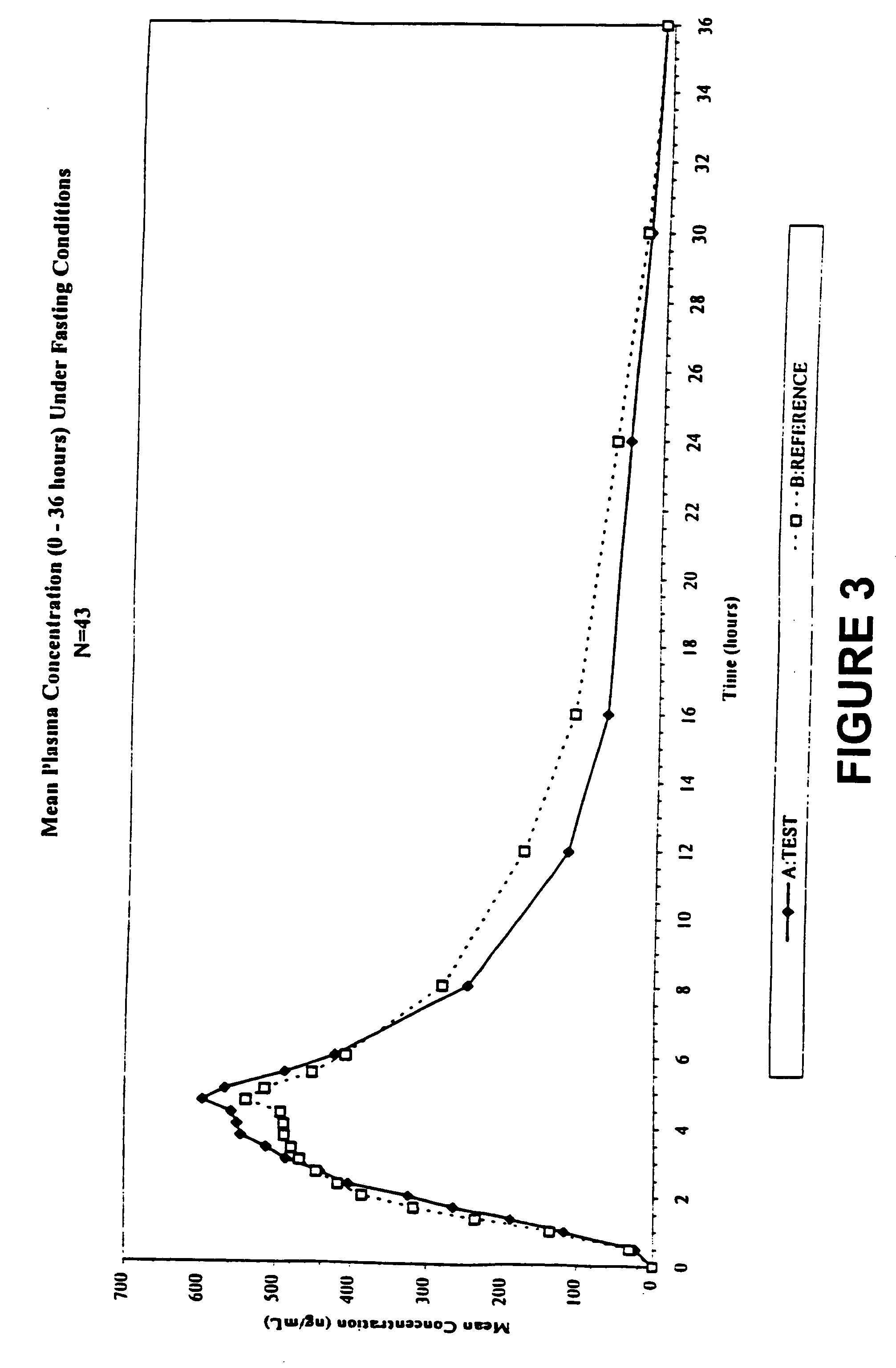
In the pharmaceutical industry, drug dissolution testing is routinely used to provide critical in vitro drug release information for both quality control purposes, i.e., to assess batch-to-batch consistency of solid oral dosage forms such as tablets, and drug development, i.e., to predict in vivo drug release profiles.
Porous drug matrices and methods of manufacture thereof
InactiveUS6932983B1Lower the volumePrevent precipitationPowder deliveryNanotechDrugs solutionWater soluble drug
Drugs, especially low aqueous solubility drugs, are provided in a porous matrix form, preferably microparticles, which enhances dissolution of the drug in aqueous media. The drug matrices preferably are made using a process that includes (i) dissolving a drug, preferably a drug having low aqueous solubility, in a volatile solvent to form a drug solution, (ii) combining at least one pore forming agent with the drug solution to form an emulsion, suspension, or second solution, and (iii) removing the volatile solvent and pore forming agent from the emulsion, suspension, or second solution to yield the porous matrix of drug. The pore forming agent can be either a volatile liquid that is immiscible with the drug solvent or a volatile solid compound, preferably a volatile salt. In a preferred embodiment, spray drying is used to remove the solvents and the pore forming agent. The resulting porous matrix has a faster rate of dissolution following administration to a patient, as compared to non-porous matrix forms of the drug. In a preferred embodiment, microparticles of the porous drug matrix are reconstituted with an aqueous medium and administered parenterally, or processed using standard techniques into tablets or capsules for oral administration.
Owner:ACUSPHERE INC
Porous drug matrices and methods of manufacture thereof
InactiveUS20050048116A1Fast dissolutionHigh dissolution ratePowder deliveryGranular deliveryDrugs solutionMicroparticle
Drugs, especially low aqueous solubility drugs, are provided in a porous matrix form, preferably microparticles, which enhances dissolution of the drug in aqueous media. The drug matrices preferably are made using a process that includes (i) dissolving a drug, preferably a drug having low aqueous solubility, in a volatile solvent to form a drug solution, (ii) combining at least one pore forming agent with the drug solution to form an emulsion, suspension, or second solution and hydrophilic or hydrophobic excipients that stabilize the drug and inhibit crystallization, and (iii) removing the volatile solvent and pore forming agent from the emulsion, suspension, or second solution to yield the porous matrix of drug. Hydrophobic or hydrophilic excipients may be selected to stabilize the drug in crystalline form by inhibiting crystal growth or to stabilize the drug in amorphous form by preventing crystallization. The pore forming agent can be either a volatile liquid that is immiscible with the drug solvent or a volatile solid-compound, preferably a volatile salt. In a preferred embodiment, spray drying is used to remove the solvents and the pore forming agent. The resulting porous matrix has a faster rate of dissolution following administration to a patient, as compared to non-porous matrix forms of the drug. In a preferred embodiment, microparticles of the porous drug matrix are reconstituted with an aqueous medium and administered parenterally, or processed using standard techniques into tablets or capsules for oral administration.
Owner:ACUSPHERE INC
Porous drug matrices and methods of manufacture thereof
InactiveUS20050058710A1Fast dissolutionExtended half-lifePowder deliveryGranular deliveryDrugs solutionMicroparticle
Drugs, especially low aqueous solubility drugs, are provided in a porous matrix form, preferably microparticles, which enhances dissolution of the drug in aqueous media. The drug matrices preferably are made using a process that includes (i) dissolving a drug, preferably a drug having low aqueous solubility, in a volatile solvent to form a drug solution, (ii) combining at least one pore forming agent with the drug solution to form an emulsion, suspension, or second solution and hydrophilic or hydrophobic excipients that stabilize the drug and inhibit crystallization, and (iii) removing the volatile solvent and pore forming agent from the emulsion, suspension, or second solution to yield the porous matrix of drug. Hydrophobic or hydrophilic excipients may be selected to stabilize the drug in crystalline form by inhibiting crystal growth or to stabilize the drug in amorphous form by preventing crystallization. The pore forming agent can be either a volatile liquid that is immiscible with the drug solvent or a volatile solid compound, preferably a volatile salt. In a preferred embodiment, spray drying is used to remove the solvents and the pore forming agent. The resulting porous matrix has a faster rate of dissolution following administration to a patient, as compared to non-porous matrix forms of the drug. In a preferred embodiment, microparticles of the porous drug matrix are reconstituted with an aqueous medium and administered parenterally, or processed using standard techniques into tablets or capsules for oral administration.
Owner:ACUSPHERE INC
Oral delivery of pharmaceuticals via encapsulation
An alternate drug delivery system for dissolution of pharmaceuticals in the mouth wherein a therapeutically effective amount of a drug is encapsulated using an encapsulation method. Encapsulation reduces the perceived off flavors of drugs, allowing the active components to dissolve pleasantly in the mouth. This allows more rapid absorption of the active compounds through the oral cavity compared to traditional tablets, which require breakdown and absorption in the gastrointestinal tract. The delivery system can be incorporated into a variety of applications, such as breath mint tablets or chewing gum. Benefits of this invention include portability and the ability to take pharmaceuticals without water and without the off taste of chewable tablets, thereby leading to increased patient compliance.
Owner:BATTEY ALYCE S +1
Drug delivery systems comprising weakly basic drugs and organic acids
InactiveUS20070196491A1Increase probabilityAvoid eliminationPowder deliveryNervous disorderParticulatesRegimen
A pharmaceutical dosage form such as a capsule, a conventional or orally disintegrating tablet capable of delivering a nitrogen (N)-containing therapeutic agent having a pKa in the range of from about 5 to 14 into the body in a sustained-released fashion, in order to be suitable for a twice- or once-daily dosing regimen, comprises at least one organic acid, which solubilizes the therapeutic agent the drug prior to releasing it into the hostile intestinal environment wherein said weakly basic drug is practically insoluble. The unit dosage form is composed of a multitude of multicoated particulates (i.e., immediate-release beads, sustained-release beads and / or one or more timed, pulsatile-release bead populations) is designed in such a way that said weakly basic drug and said organic acid do not come into close contact during processing and / or storage for in-situ formation of acid addition compounds while ensuring that the acid is not depleted prior to completion of the drug release.
Owner:ADARE PHARM INC
Sustained-release drug delivery compositions and methods
InactiveUS20100092562A1Improve stabilityReduce molecular weightPowder deliveryOrganic active ingredientsImmediate releaseDecongestant
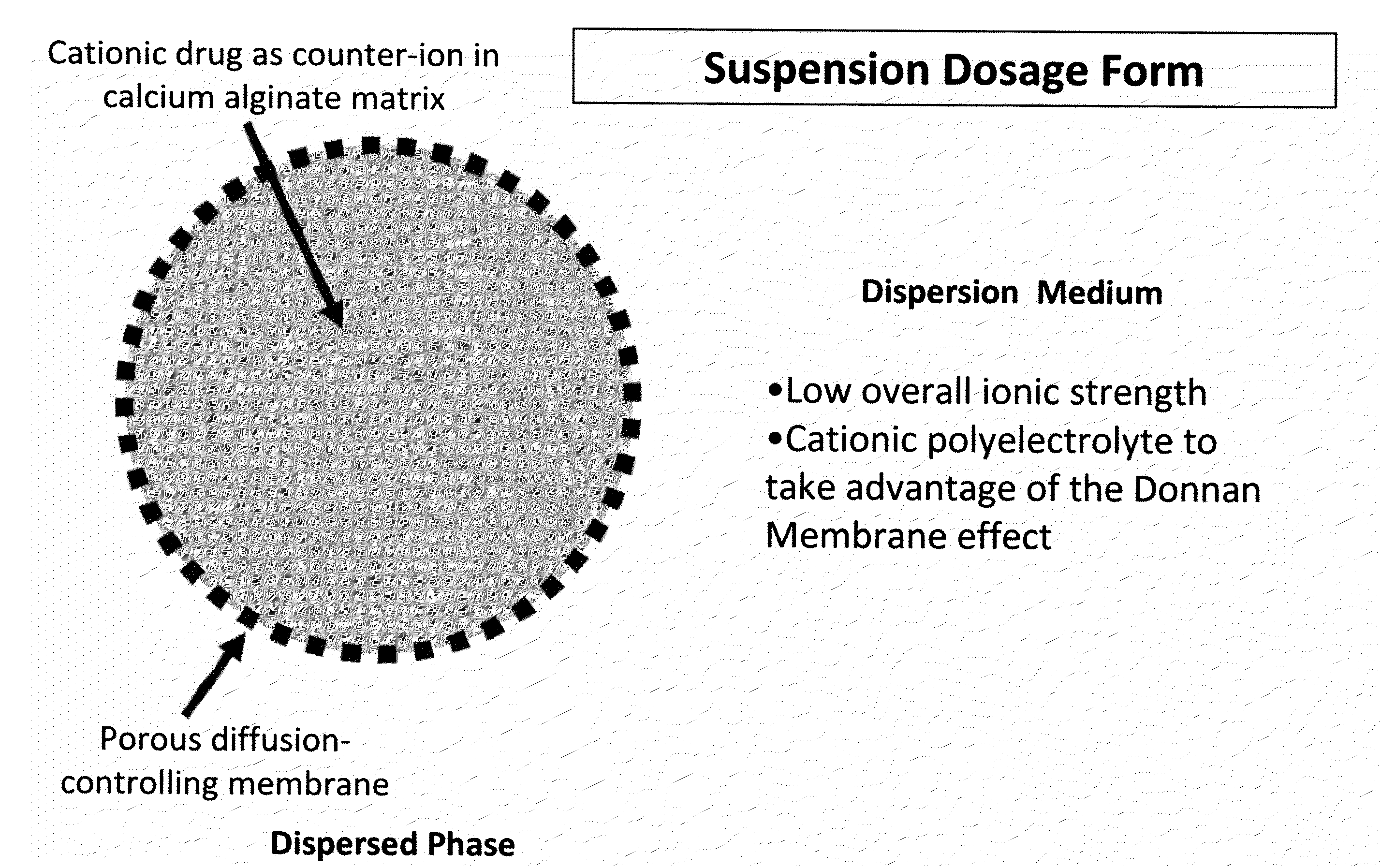
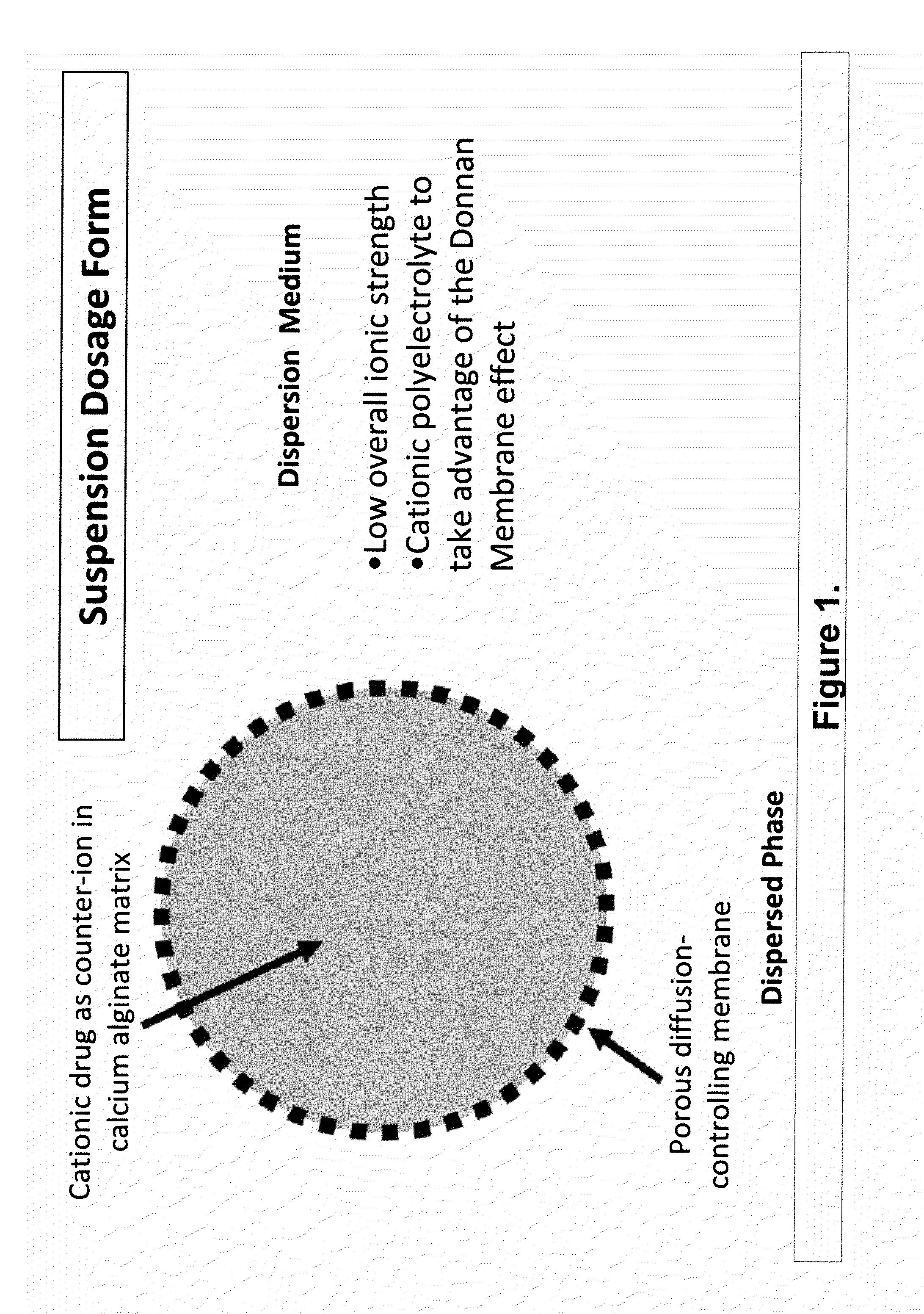
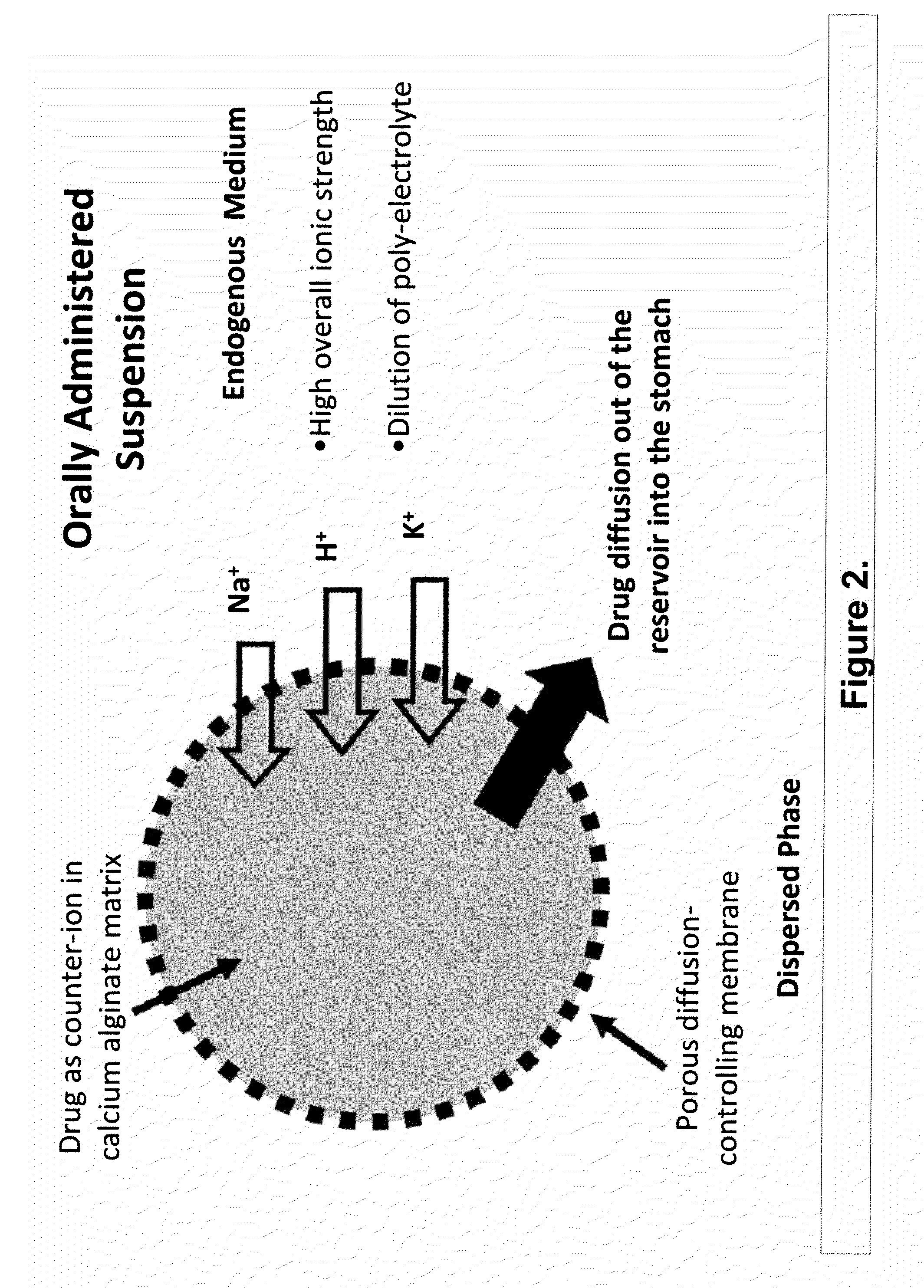
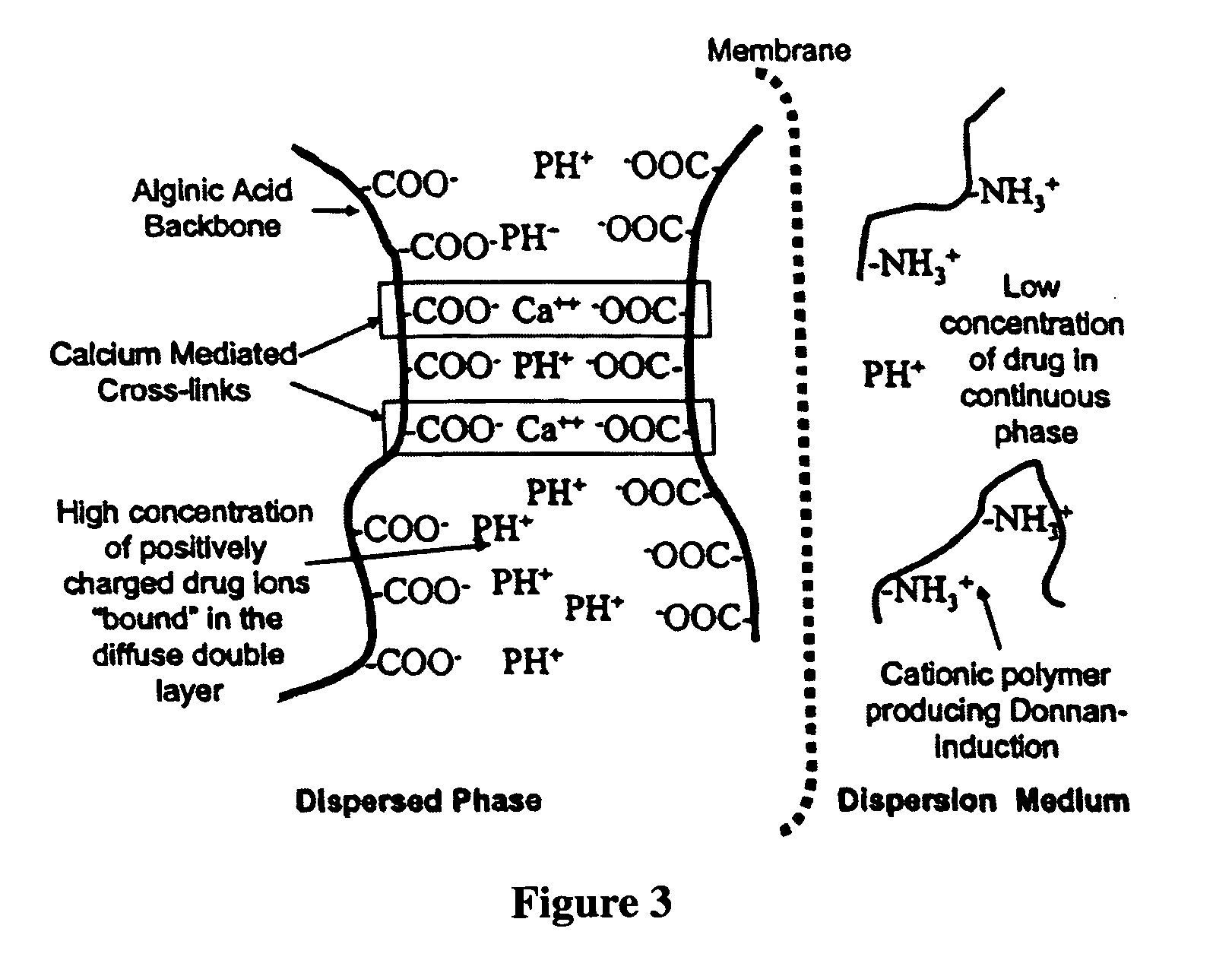
The present invention relates to liquid sustained release suspension dosage forms. In particular, the invention encompasses sustained release compositions comprising a dispersed phase, which contains an ion-exchange matrix drug complex, a diffusion controlling membrane coating and a dispersion medium comprising an excipient capable of impeding water activity such that drug dissolution is inhibited prior to administration. Further, the invention provides for compositions wherein several active ingredients associate in a single bead in the dispersed phase, such that the abuse potential of such active ingredients is reduced. The invention also encompasses sustained release formulations of combination drugs comprising an extended release phase and an immediate release phase. The formulations of the invention may be used to treat a variety of conditions and symptoms, including those that require administration of several drugs, such as cold and allergy symptoms. In one of the embodiments, the sustained release composition combines an antihistamine, an antitussive and a decongestant. The invention further provides for methods of making and using such formulations.
Owner:UPM PHARMA
Composition and method for controlling drug delivery from silicone adhesive blends
InactiveUS20050019385A1Reduce concentrationPrevent/inhibit crystallizationSheet deliveryBandagesControlled drugsSolubility
Compositions and methods for controlling transdermal drug delivery, particularly of amine-functional and basic drugs, comprising a blend of a first silicone-based polymer having a reduced silanol concentration and a second silicone-based polymer have a substantial or high silanol concentration. The blend of such silicone-based polymers, particularly pressure-sensitive silicone adhesives, provides sufficient drug solubility and reduced initial drug delivery onset to permit a prolonged delivery duration at a substantially zero-order rate of delivery.
Owner:NOVEN PHARMA
Aqueous sustained-release drug delivery system for highly water-soluble electrolytic drugs
InactiveUS20060134148A1Reduce molecular weightQuick releasePowder deliveryPharmaceutical non-active ingredientsElectrolysisIon exchange
Owner:HOLLENBECK R GARY
Transdermal drug patch
InactiveUS20010033858A1Sufficient transdermal permeabilityConstant concentrationNervous disorderSheet deliverySolubilityTransdermal patch
The present invention is directed toward a formulation for supplying additional drug for delivery in a transdermal drug delivery device. The invention comprises a drug, such as fentanyl that is capable of transdermal delivery, and a solution having a pre-designed solubility for the drug. The solution dissolves only a portion of said drug and allows a significant portion of the drug to remain undissolved in solution, thus providing extra drug to be delivered at a consistent, controlled delivery rate. The invention may used in conjunction with controlled heat.
Owner:ZARS INC
Ophthalmic Lipophilic and Hydrophilic Drug Delivery Vehicle Formulations
InactiveUS20140378401A1Increase shear forcePhase-low viscosityBiocideSenses disorderSolubilitySide effect
The ophthalmic drug delivery vehicles provide comfort and compliance; drug solubility, residence time and permeability; and reduce side effects. In addition, the delivery vehicle can be slightly modified to provide an artificial tear formulation.
Owner:PS THERAPIES LTD
Oral delivery system comprising a drug/polymer complex
InactiveUS20050287212A1High low solubility drug loadingIncrease volumePowder deliveryPill deliverySolubilityHydrophilic polymers
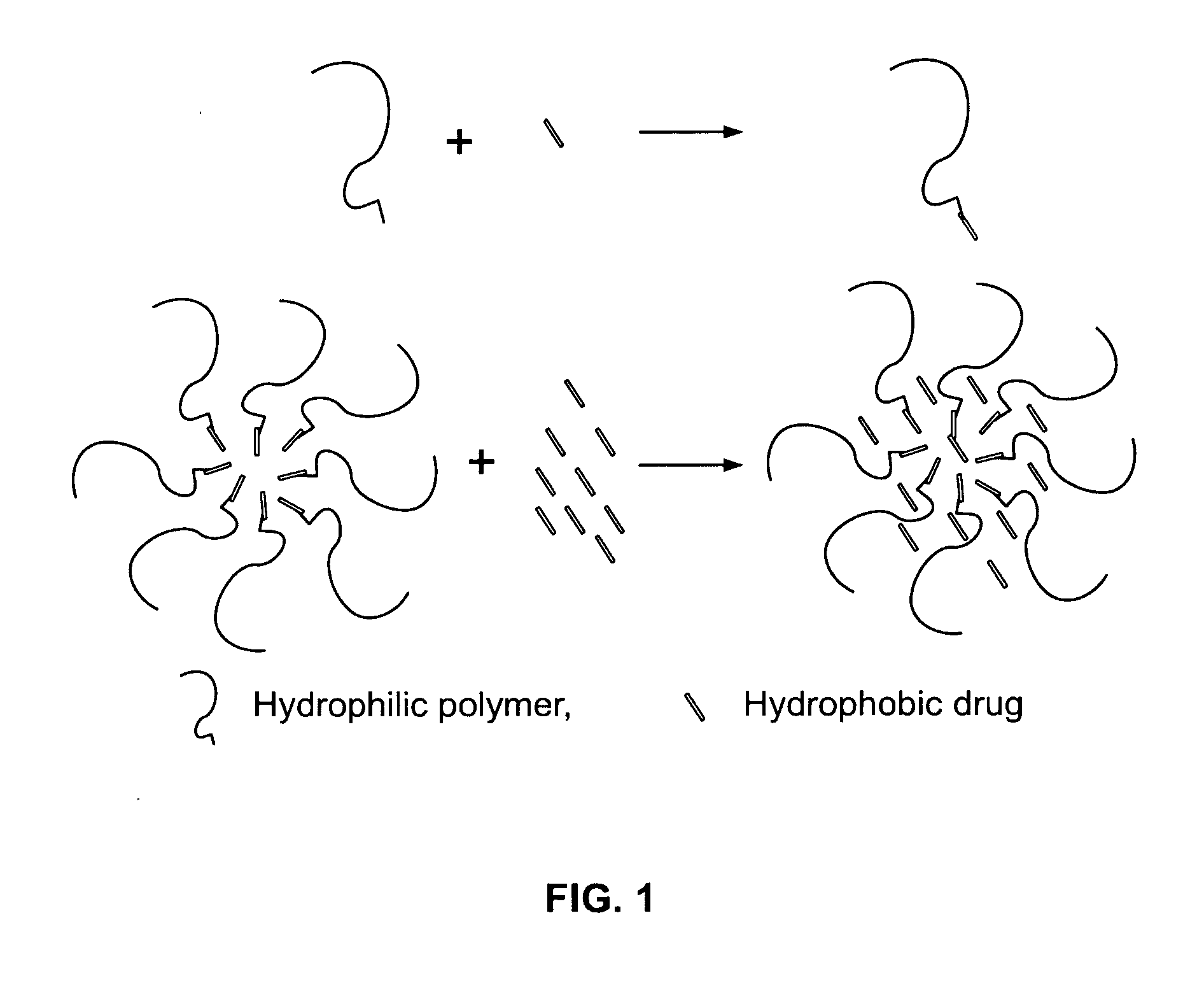
This invention pertains to the enhanced delivery of orally administered pharmaceutical agents and methods, dosage forms and devices thereof. In particular, the invention is directed to methods including providing a low solubility drug having a pKa between about 6 and about 9; dissolving the low solubility drug in an aqueous solution, wherein a pH of the aqueous solution is less than about 6.0; dissolving a hydrophilic polymer in the aqueous solution, wherein the weight ratio of the hydrophilic polymer to the low solubility drug is less than or equal to about 0.15; lyophilizing the aqueous solution to obtain a lyophilized powder. Also disclosed are drug formulations made according to the method, and dosage forms that include the drug formulations.
Owner:ALZA CORP
Coated fine particles containing drug for intrabuccally fast disintegrating tablet
The present invention makes it possible to provide drug-containing coated microparticles for quick-disintegrating oral tablets wherein microparticles containing a drug with an unpleasant taste are coated with a film composed of (1) a pH-independent water-insoluble polymer accounting for 60% or more but less than 80% of the film and (2) a pH-independent water-soluble substance accounting for more than 20% to 40% or less of the film, these microparticles being characterized in that the rate of dissolution of the drug from the drug-containing microparticles is 0% to 10% in one minute and 80% to 100% in 30 minutes, and the average particle diameter is 350 μm or less, in order to realize sufficient control of oral drug dissolution and fast gastrointestinal drug dissolution.
Owner:ASTELLAS PHARMA INC
Process for producing a pharmaceutical solid preparation containing a poorly soluble drug
InactiveUS20050158386A1High dissolution rateEasy to prepareOrganic active ingredientsPowder deliveryMedicineBiochemical engineering
Among the conventional processes for producing solid dispersion, the solid dispersion obtained by a solvent method is excellent in terms of solubility and bioavailability of a poorly soluble drug. However, due to frequent uses of organic solvents in the solvent method, problems have arisen such as organic solvent residue in products, environmental pollution and operational safety as well as corporate problems such as capital investment and the like required to avoid such events. The present invention provides a process for preparing pharmaceutical solid preparations without use of organic solvents frequently used in conventional solvent methods. Specifically, the present invention provides a process for producing a pharmaceutical solid preparation, which is formulated by spraying a solution wherein a poorly soluble drug is dissolved in a plasticizer, and an aqueous solution and / or water dispersion of a water-soluble polymer from nozzle outlets simultaneously and separately in the process for producing the pharmaceutical solid preparation.
Owner:SHIN ETSU CHEM IND CO LTD
Lurasidone hydrochloride orally-disintegrating tablet preparation and preparation method thereof
InactiveCN103054824AOrganic active ingredientsNervous disorderLurasidone HydrochlorideOrally disintegrating tablet
The invention belongs to the technical field of medicine, and relates to a lurasidone hydrochloride orally-disintegrating tablet preparation and a preparation method thereof. The orally-disintegrating tablet comprises the following components in mass percent: 5-60 percent of lurasidone hydrochloride, 25-80 percent of filling material, 5-20 percent of disintegrating agent, 0.02-0.15 percent of wetting agent, 0.2-5 percent of flavoring agent and 0.5-2 percent of lubricating agent. The invention aims to provide a lurasidone hydrochloride orally-disintegrating tablet with simple preparation process, low cost, convenience in use and quick action to the indications; and compared with the conventional preparation for oral use, the lurasidone hydrochloride orally-disintegrating tablet preparation can reduce the difficulty in swallowing, improve the compliance, is suitable for special populations such as the elderly, children, patients with difficulty in swallowing and mental patients, can be fully disintegrated within 60 s, can be disintegrated into extremely fine powder, is higher in drug dissolution rate and quick in absorption, and can improve the bioavailability of insoluble drugs, for example, lurasidone hydrochloride.
Owner:BEIJING VENTUREPHARM BIOTECH
Dirithromycin enteric-coated formulation
The invention relates to a dirithromycin (DRM) enteric preparation with hydroxypropyl methylcellulose phthalate (HPMCP) being the framework material of drug-containing core and the coating material of an enteric coating layer. The enteric preparation has the advantages that the drug content is higher, the drug dissolution rate is less affected by the coating material, the preparation cost is low and the preparation process is simple.
Owner:SHANDONG INST OF PHARMA IND
Compound alpha-ketoacid chewing tablet and preparation method thereof
InactiveCN101416947AExpand the range of dosage formsExpand the range of dosage forms, with a dispersed stateUrinary disorderPill deliveryDiseaseAdditive ingredient
The invention relates to a drug preparation and a preparation method thereof, in particular to a compound Alpha-ketonic acid chewable tablet used for curing chronic renal failure, and a preparation method thereof. The compound Alpha-ketonic acid chewable tablet comprises the following components: Alpha-ketophenylalanine calcium, Alpha-hydroxymethionine calcium, Alpha-ketoleucine calcium, Alpha-ketoisoleucine calcium, Alpha-ketovaline calcium, tryptophan, histidine, tyrosine, threonine, lysine, acetate, a filling agent, a bonding agent, a taste-masking agent, a lubricant and a coating agent. The compound Alpha-ketonic acid chewable tablet not only widens the dosage form range of the compound Alpha-ketonic acid chewable tablets, but also has the advantages of having good dispersing state, short disintegration time, fast drug dissolution, rapid absorption, high biological availability and convenient taking, being capable of being swallowed, chewed and sucked and being especially suitable for old people, stroke patients, patients with special diseases and patients having swallowing difficulty.
Owner:无锡曙辉药业有限公司
Composition capable of improving solubility and bioavailability of insoluble medicament
InactiveCN103285401APotential application value is goodImprove bioavailabilityOil/fats/waxes non-active ingredientsPharmaceutical drugOil phase
The invention belongs to the technical field of medicaments, and relates to a composition capable of improving the solubility and the bioavailability of an insoluble medicament. The composition consists of an oil phase, an emulsifier and a coemulsifier, wherein the oil phase, the emulsifier and the coemulsifier have the specific composition ratio range of 1: (0.5 to 4): (0.5 to 3). According to the composition, the hydrophobic property of the medicament can be improved, the oral absorbability of the medicament can be improved, and meanwhile, the medicament can be protected by a formed nano shell barrier, so that the medicament is separated from an external environment temporarily, and the degradation and the transformation of the medicament caused by external environment factors are avoided; and therefore, the residence time and the action time of the medicament in vivo can be prolonged, and the bioavailability of the medicament is improved.
Owner:SHENYANG PHARMA UNIVERSITY
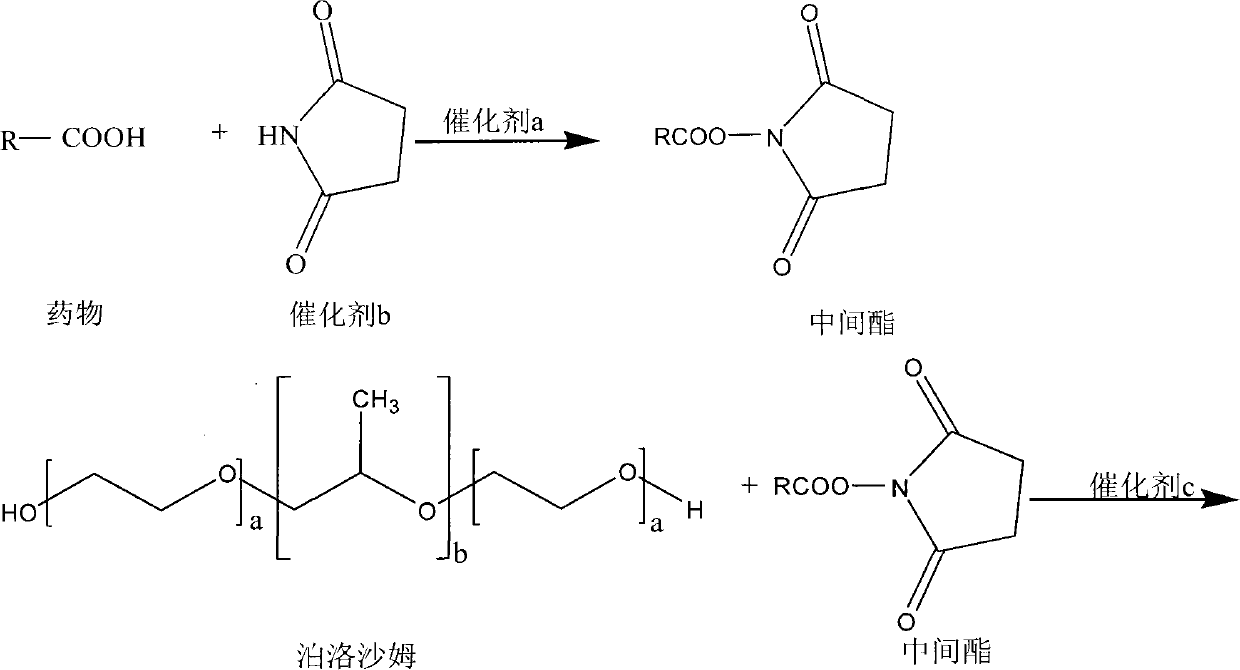
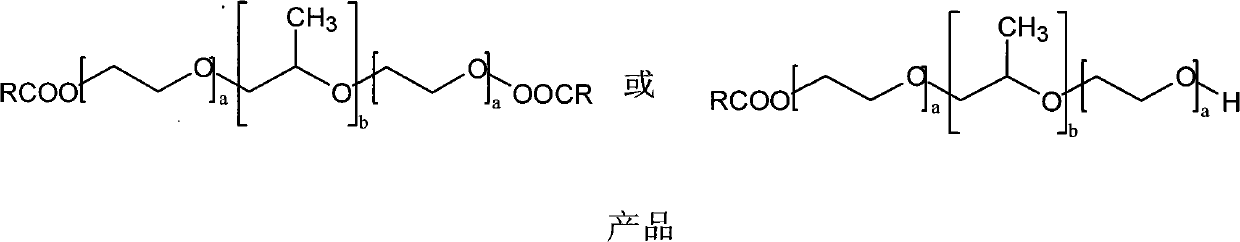
Poloxamer-carboxylic acid drug conjugate and preparation method and application thereof
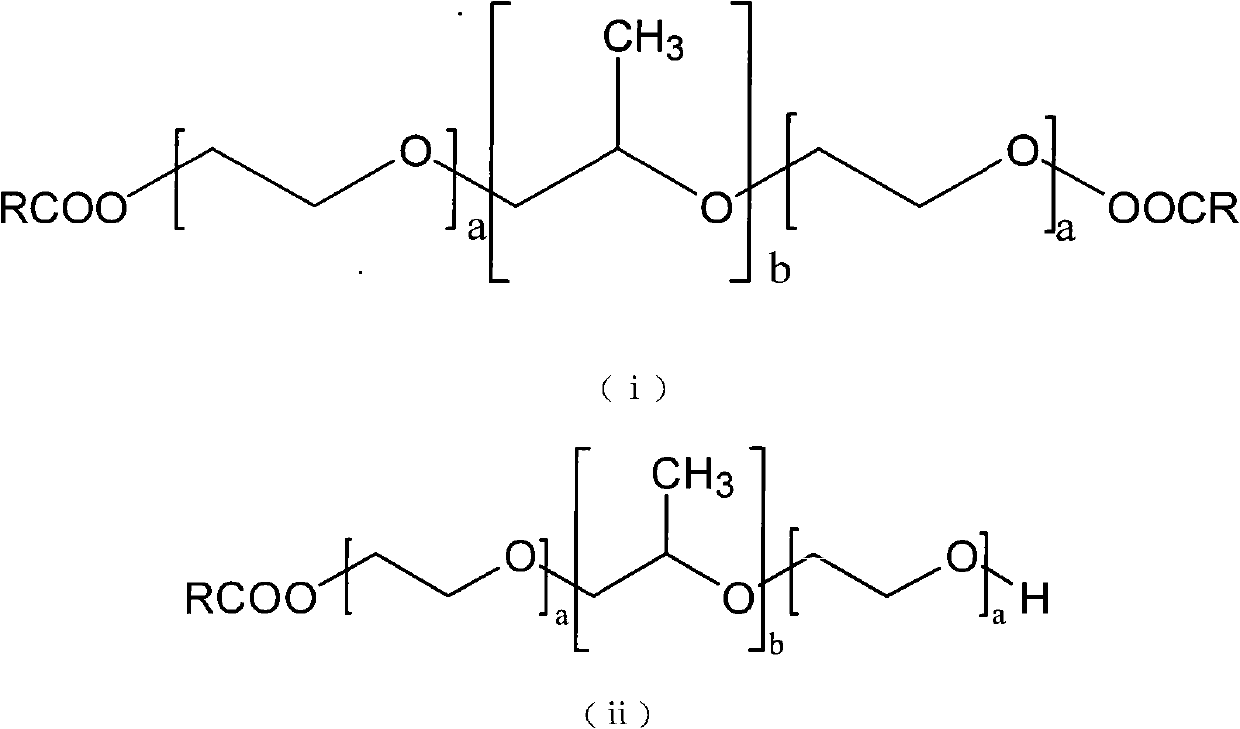
InactiveCN101991860AImprove securityImprove physiological activityPowder deliveryPharmaceutical non-active ingredientsSolubilityDrug carrier
The invention discloses a poloxamer-carboxylic acid drug conjugate and a preparation method and application thereof. The conjugate is formed by directly linking the carboxyl of the carboxylic acid drug with the carboxyl of the poloxamer by the ester bond in the presence of a catalyst. The conjugate is the compound in the general formula (i) or (ii). Compared with the original carboxylic acid drugs, the conjugate has greatly improved drug solubility, enhanced pharmacological effects, reduced adverse reaction and higher safety. By utilizing the hydrophobic carboxylic acid drug, the conjugate can enhance the hydrophobicity of the poloxamer and greatly improve the amphipathy of the poloxamer, thus being capable of forming stable micellar structure under waterborne environment. As the drug carrier, the conjugate can complete further encapsulation of the drugs and can meet different release requirements by controlling the chemical grafted amount and the physical encapsulation amount or realize combined therapy of the drugs by physically embedding other pharmaceutically active drugs. The invention has simple preparation method, mature process and high yield and is suitable for industrialproduction.
Owner:CHINA PHARM UNIV
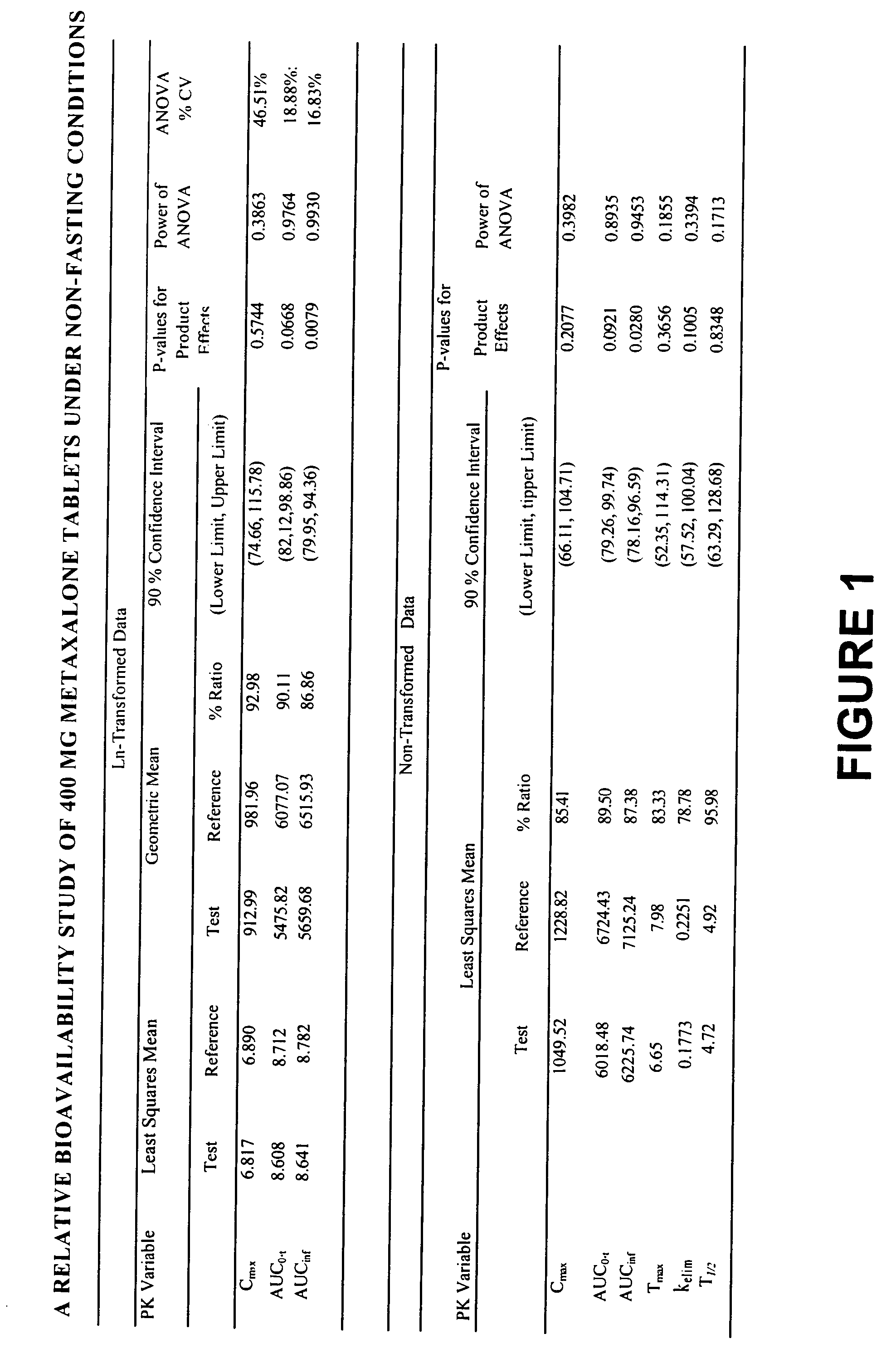
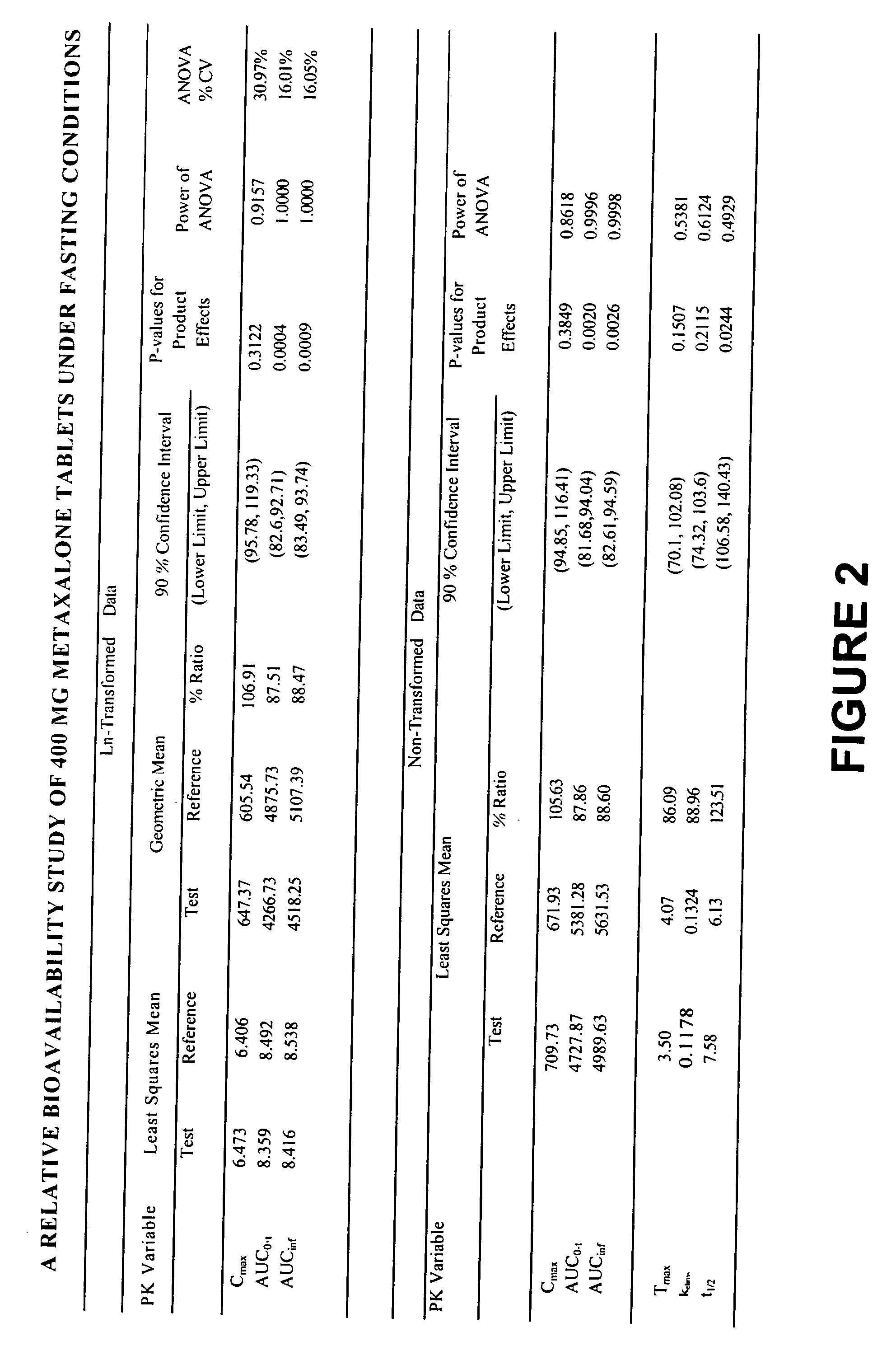
Bioavailable compositions of metaxalone and processes for producing the same
Pharmaceutical compositions comprising metaxalone which demonstrate improved dissolution and bioavailability characteristics compared to the commercially available product, and methods of producing them are provided. In a preferred embodiment, a dosage form comprising metaxalone and at least one inactive powder excipient is bioequivalent to its commercially available counterpart (Skelaxin® 400-mg tablets) after oral administration to fasting or non-fasting human subjects, while at the same time displaying faster drug dissolution rates than the Skelaxin® tablets as demonstrated from three different dissolution tests. In another preferred embodiment, a dosage form comprising metaxalone, at least one inactive powder excipient and a nonvolatile liquid is significantly more bioavailable than the commercially available Skelaxin® 400-mg tablets after oral administration to fasting human subjects.
Owner:SPIREAS SPIRIDON
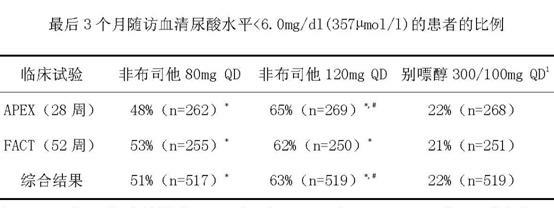
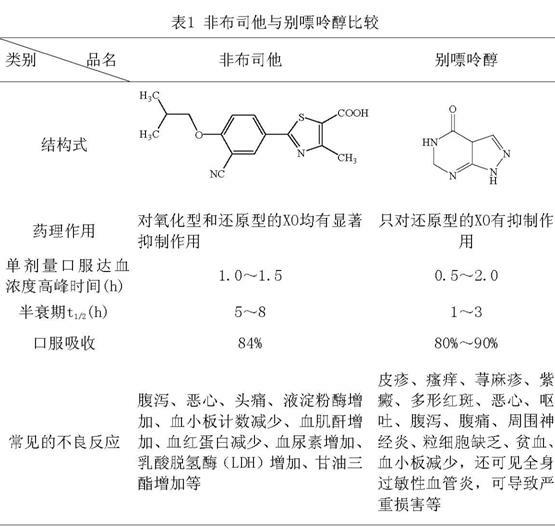
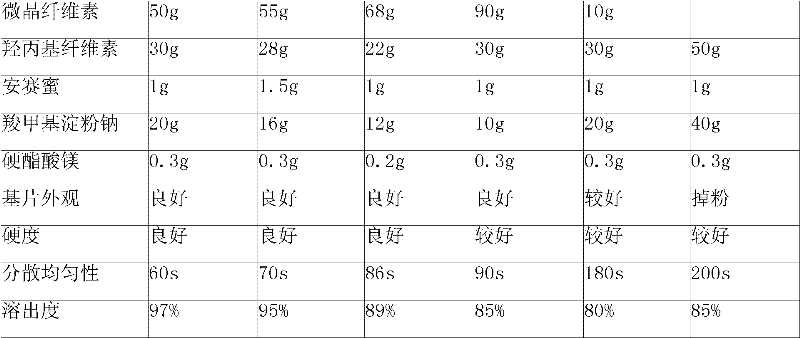
Febuxostat dispersible tablet and preparation method thereof
InactiveCN101966163ADissolution is rapid and completeImprove bioavailabilityOrganic active ingredientsSkeletal disorderCurative effectMagnesium stearate
The invention relates to a febuxostat dispersible tablet and a preparation method thereof. The febuxostat dispersible tablet comprises febuxostat as a main material and a diluent, a disintegrant, a binder, a flow aid, a lubricant and other assistants as auxiliary materials, wherein the flow aid is silicone dioxide, and the lubricant is magnesium stearate. The febuxostat dispersible tablet is prepared from the following components in percentage by weight: 5-30% of the febuxostat, 20-80% of the diluent, 5-30% of the disintegrant, 0.1-5% of the binder, 1-10% of the silicone dioxide, 0.2-5% of the magnesium stearate and 2-8% of other assistants. The febuxostat dispersible tablet prepared by adding a proper amount of auxiliary materials into crushed febuxostat has the advantages of rapid and complete drug dissolution and stable quality, is suitable for long-term storage and convenient to use, and the bioavailability of human bodies on drugs is increased so as to increase curative effect.
Owner:JIANGSU WANBANG BIOPHARMLS +1
Erdosteine composition and preparation method thereof
ActiveCN101606931BImprove dispersion uniformityImprove dissolution efficiencyOrganic active ingredientsPharmaceutical product form changeCarboxymethyl starchAlcohol
The invention provides an Erdosteine composition which consists of the following components by weight portions: 130 to 170 portions of Erdosteine, 35 to 65 portions of lactose, 30 to 70 portions of microcrystalline cellulose, 20 to 40 portions of low substituted hydroxypropyl cellulose, 1 to 5 portions of acesulfame, 10 to 30 portions of sodium carboxymethyl starch and 0.1 to 0.5 portions of magnesium stearate; the invention also provides a preparation method of the Erdosteine composition, comprising the following steps of: preparing materials and pelleting; mixing the Erdosteine and the low substituted hydroxypropyl cellulose evenly, then grinding, adding the sodium carboxymethyl starch, adding absolute ethyl alcohol, wet-mixing, cutting, drying, granulation and tabletting; mixing granules and microcrystalline cellulose evenly, adding the lactose, the acesulfame and the magnesium stearate for pelleting, subpackaging and obtaining the Erdosteine composition. The Erdosteine compositionhas good dispersible uniformity and high drug dissolution efficiency.
Owner:SHANDONG LUOXIN PHARMA GRP CO LTD
Azilsartan tablet
ActiveCN104523632ADissolution rate is fastSimple processOrganic active ingredientsPill deliveryCelluloseDiethylene glycol monoethyl ether
The invention belongs to the technical field of medicines, and particularly relates to an azilsartan tablet. The azilsartan tablet contains azilsartan, hydroxy propyl cellulose and fumed silica, and is prepared by the following steps: dissolving the azilsartan and the hydroxy propyl cellulose in diethylene glycol monoethyl ether, adding the fumed silica to adsorb, uniformly mixing with pharmaceutically acceptable auxiliary materials and pressing by a direct tableting process. Compared with the prior art, the azilsartan tablet is high in drug dissolution speed and simple in process.
Owner:SHANDONG NEWTIME PHARMA
Valsartan dispersible tablet and preparation method thereof
ActiveCN101167723AGood curative effectLittle side effectsPill deliveryMacromolecular non-active ingredientsValsartanMedicine
The invention provides valsartan dispersible tablets and a preparation method thereof, which contain effective doses of valsartan and pharmaceutical adjuvants, which include disintegrants, diluents, binders, lubricants, glidants and surface Active agent, based on 100 parts of valsartan, the dosage of disintegrating agent is 2-50 parts, the dosage of diluent is 10-150 parts, the dosage of binder is 2-25 parts, and the dosage of lubricant is 0.5-20 parts. The dosage of the liquid agent is 0.2-10 parts, and the surfactant is 0.1-2.5 parts; the present invention also provides the preparation method of the valsartan dispersible tablet. Compared with other dosage forms, the valsartan dispersible tablet has a good dispersion state , Short disintegration time, rapid drug dissolution, convenient administration, low production cost, no need for special equipment, convenient and stable carrying and transportation, etc.
Owner:HAINAN HUALON PHARM
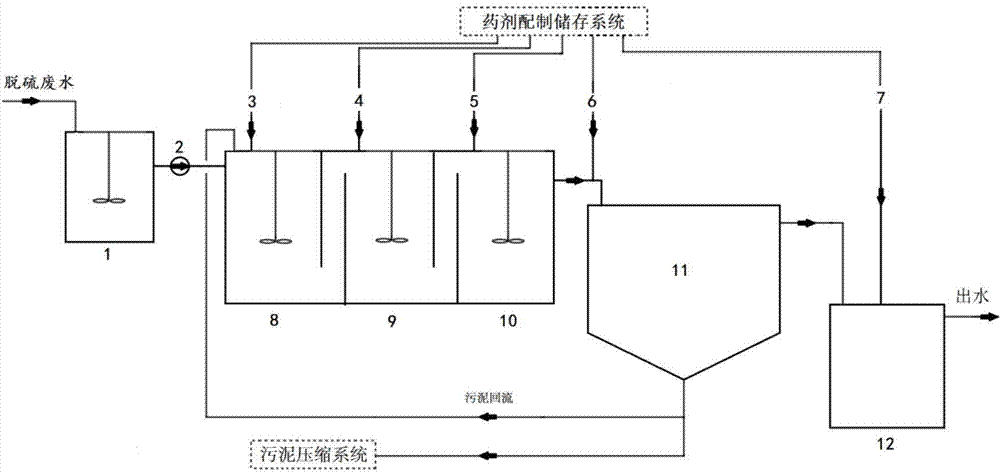
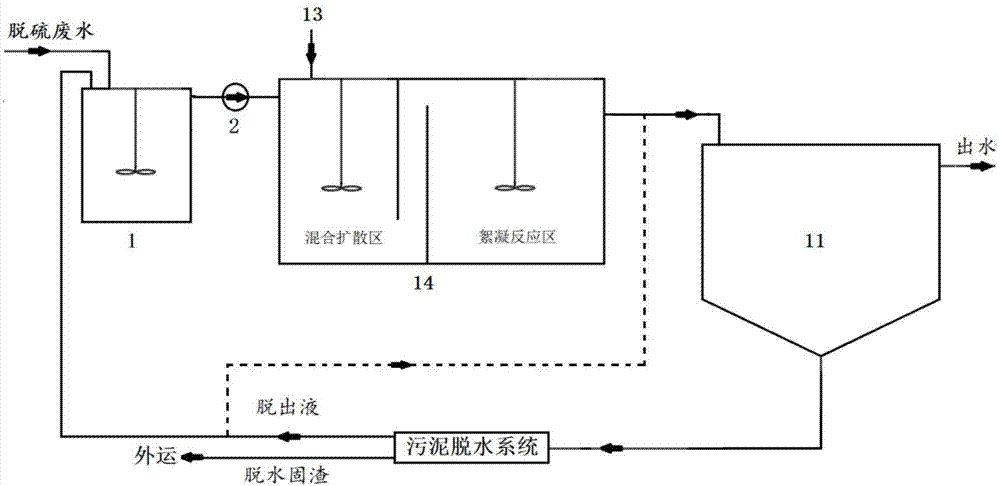
Flue gas desulfurization wastewater treatment process for coal-fired boiler
InactiveCN104761079ASimple processSimple control systemWater treatment compoundsSpecific water treatment objectivesFiltrationWater quality
The invention discloses a flue gas desulfurization wastewater treatment process for a coal-fired boiler. The process comprises the steps of:mixing and collecting the overflow obtained by treating desulfurization wastewater with a hydrocyclone and an underflow filtration squeezing filtrate in a wastewater buffer tank, and introducing the mixture to a flocculation reactor to complete a dosing and flocculation reaction process; precipitating the wastewater after flocculation reaction into a clarification concentration tank for a period of time, and using a supernatant as desulfurization process water or sending the supernatant into a factory public drainage system; dewatering the sludge at the bottom of the clarification concentration tank, introducing the dewatering liquid into a waste water buffer tank or returning to a water inlet pipe of the clarification concentration tank, and treating the dewatering solid residue and solid residue from underflow dewatering by hydrocyclone. The outlet water quality from the process meets the requirements of DL / T997-2006 standard. The process only needs to add a solid powder treatment agent, and doesn't need drug dissolution preparation; the pH value of the outlet water doesn't need to be adjusted; and the process has the advantages of simple process flow, less equipment investment, simple control system, and great improvement on the operation stability of the desulfurization wastewater treatment system.
Owner:TIANJIN UNIV
Process for enhancing the solubility of poorly soluble drugs
InactiveUS7607596B1Enhance dissolution rate/solubilityPoorly soluble drugsPowder deliveryGranulation by material expressionSolubilityMedicine
The solubility / dissolution rate of a poorly soluble drug is enhanced by a process that utilizes a twin-screw extruder containing (i) a feed zone containing a first liquid and powder feed stations; (ii) a grinding / mixing zone containing a second liquid feed port located at an upstream portion of such zone; (iii) a granulation zone containing a second powder feed station located at an upstream portion of such zone and a third liquid feed port located at a downstream portion of such zone; and (iv) a wet milling zone.
Owner:NEUROCRINE BIOSCI INC +1
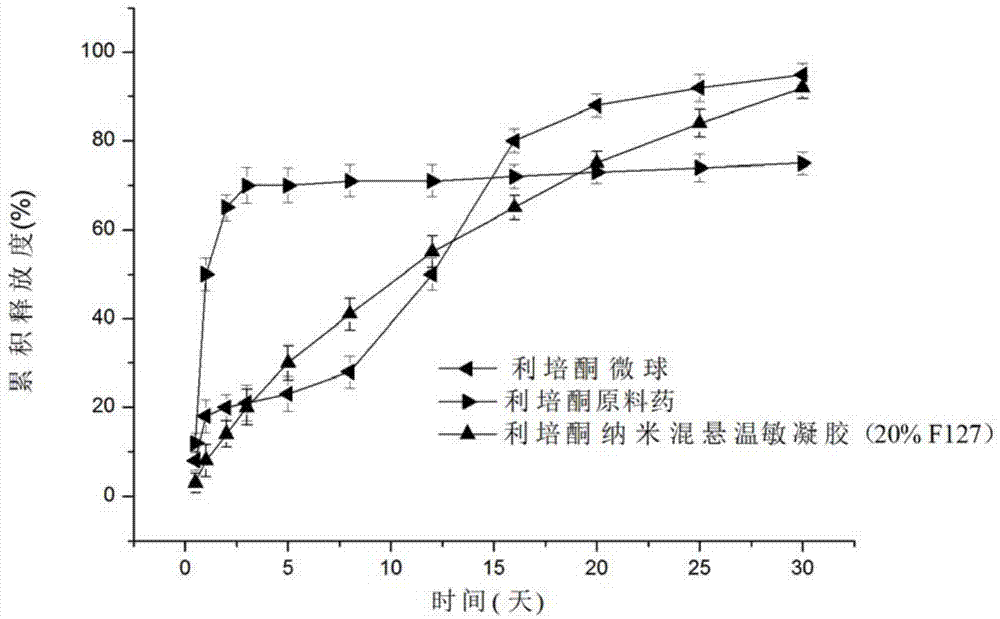
Risperidone nano-suspension temperature sensitive gel and its preparation method
InactiveCN104288091AImprove complianceIncrease dissolution rateOrganic active ingredientsNervous disorderWater basedPatient compliance
The invention discloses a risperidone nano-suspension temperature sensitive gel and its preparation method. Each 100ml of the nanosuspension temperature sensitive gel contains 0.1-10g of risperidone, 0.1-5g of a stabilizer, 1-50g of a temperature sensitive gel material, 0-5g of an additive, and the balance of a water-based solvent. The risperidone nano-suspension temperature sensitive gel is a temperature sensitive controlled-release in situ gel. The above dosage form makes risperidone highly dispersed, so the gel has the advantages of good drug load and good stability; after the gel is administrated in a liquid form, phase transition occurs in applied sites, and the liquid becomes non-chemically-crosslinked semisolid gel, so the gel has good histocompatibility and improves the drug dissolution rate; and the administrated gel has a long retention time in the applied sites as a drug reservoir, so the gel has a slow release effect, prolongs the drug release time, improves the drug bioavailability, reduces the administration frequency and improves the drug effect and the patient compliance.
Owner:HENAN UNIV OF SCI & TECH
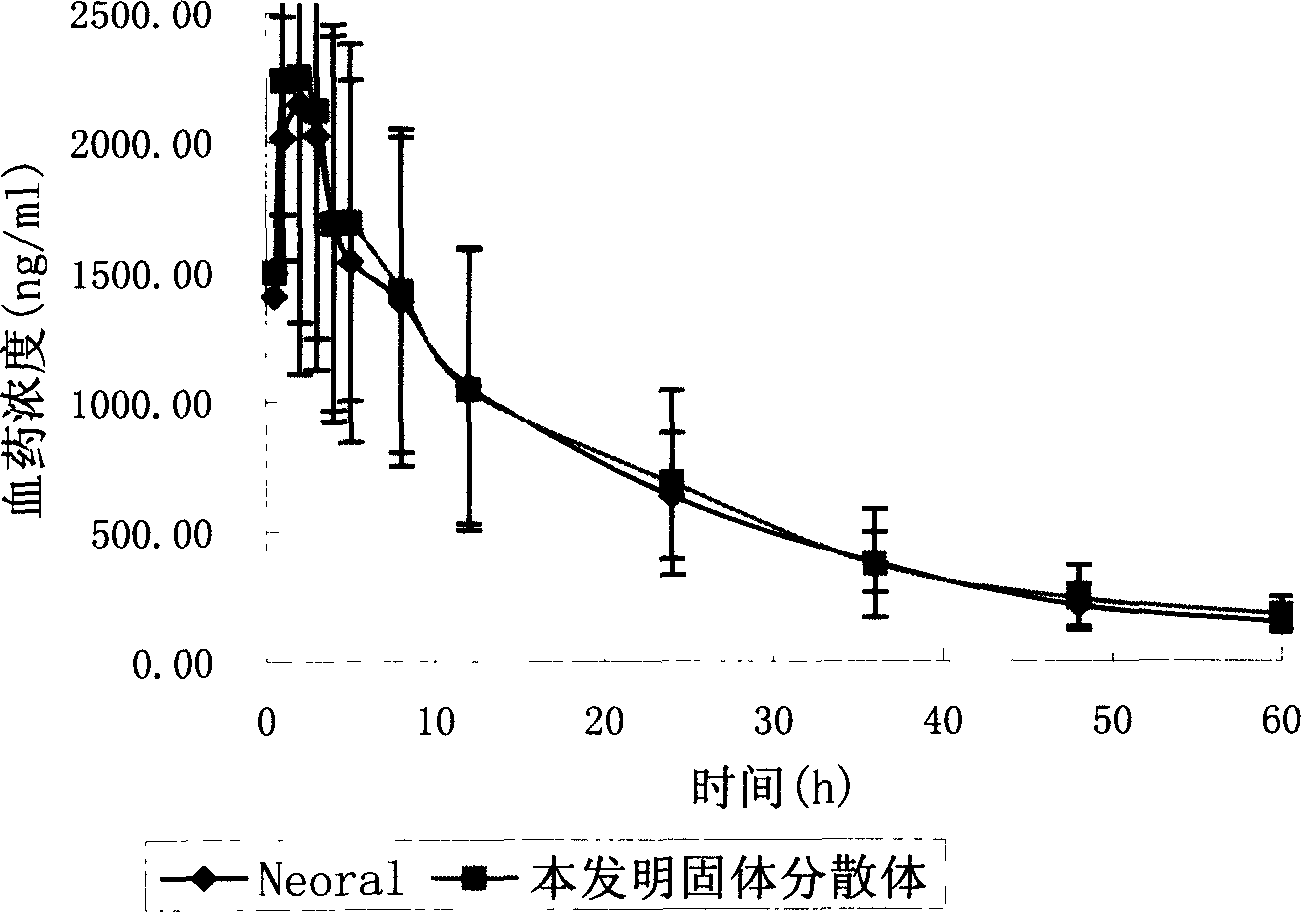
Cyclosporin A dispersion solid and its preparation method
InactiveCN1559606AIncrease dissolution ratePowder deliverySuppositories deliverySolubilityCaplet Dosage Form
A dispersing solid (capsule, tablet, particle, suppository and dripping pill) of cyclosporin A is prepared from cyclosporin A and carrier by solvent method, solvent fusion method, etc. It has high solubility of easy absorption.
Owner:FUDAN UNIV +1
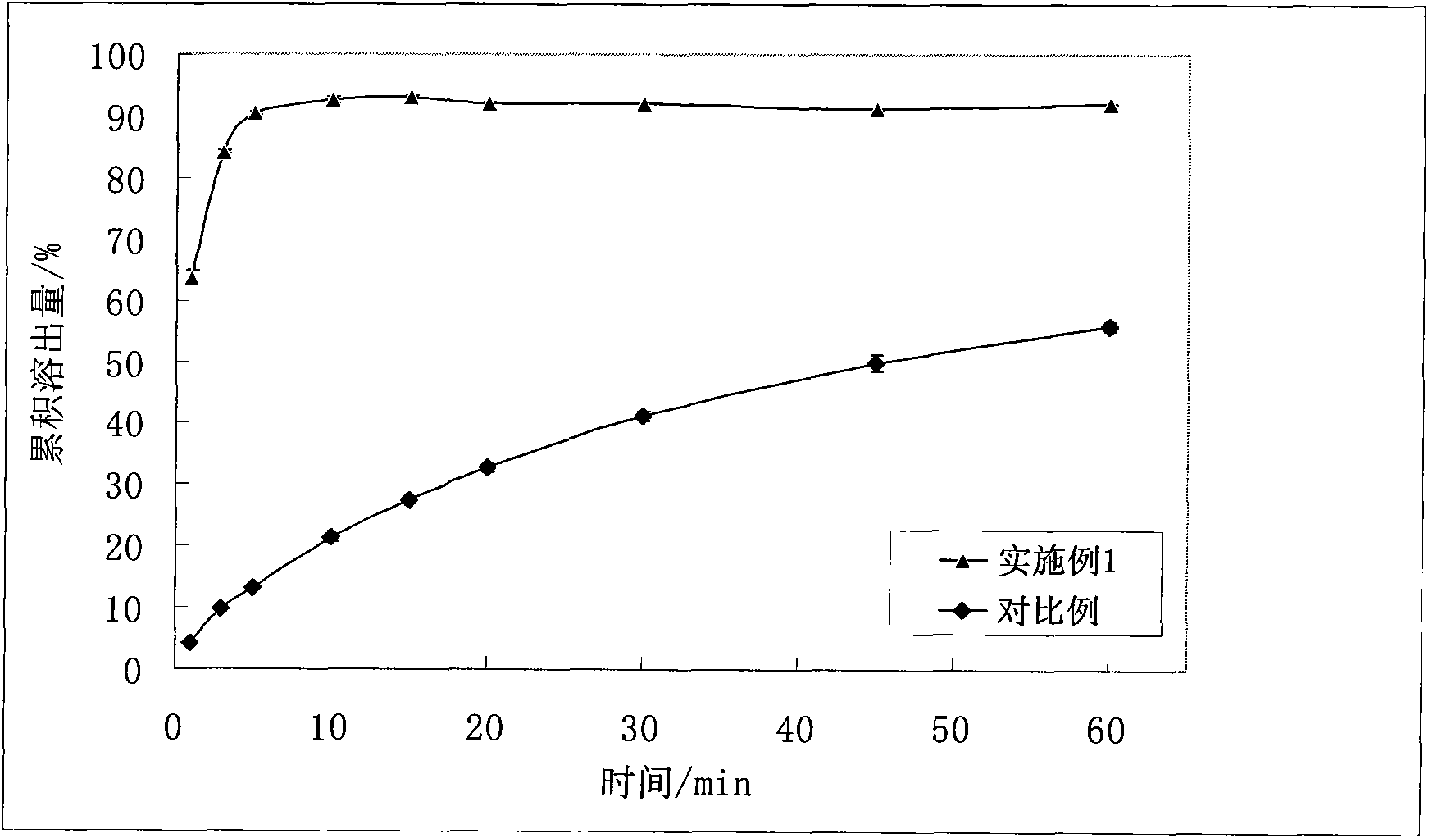
Preparation method of low melting point drug solid dispersion
InactiveCN101961306AReduce usageSmooth releasePharmaceutical delivery mechanismMacromolecular non-active ingredientsAdhesiveSolvent
The invention belongs to the pharmaceutical field and relates to a preparation method of a low melting point drug solid dispersion. The preparation method comprises the following steps: crushing pharmaceutical raw materials and auxiliary materials, passing through a 60-120-mesh sieve, then uniformly mixing, sealing, placing at the constant temperature of 45-140 DEG C for 0.5-4h, cooling to room temperature and obtaining the solid dispersion, wherein the pharmaceutical raw materials are insoluble drugs with the melting points in the range of 45-130 DEG C; and the auxiliary materials comprise a filling agent, a disintegrant, a dry adhesive, a lubricating agent, a glidant and a surfactant. The preparation method has the following advantages: simple operation, low equipment requirements and no solvent residue; the prepared solid dispersion is easy to crush and can be prepared into quick-release tablets, capsules, power or slow-release tablets; a quick-release preparation prepared through the preparation method has high drug loading, fast drug dissolution speed and high dissolution rate; and the prepared slow-release tablets have good uniformity and stable drug release.
Owner:BEIJING UNIV OF CHEM TECH
Vildagliptin composition
ActiveCN104161752AImprove stabilityExcellent Tablet PropertiesMetabolism disorderPharmaceutical non-active ingredientsMagnesium saltDiluent
The invention relates to a vildagliptin composition. Specifically the composition comprises vildagliptin, (a) at least one medicinal diluent, (b) at least one disintegrating agent, and (c) lubricant, optionally comprises other pharmaceutical adjuvant, and does not use fatty acid magnesium salts as the lubricant. The composition has a better stability and drug dissolution.
Owner:JIANGSU HANSOH PHARMA CO LTD
Pharmaceutical composition
The present invention provides a solid pharmaceutical composition superior in the stability and dissolution property, wherein the drug dissolution property of a solid dosage form containing a fat and oil-like substance having a low melting point is improved.The present invention provides a solid pharmaceutical composition containing an active ingredient, a fat and oil-like substance having a low melting point and a low viscosity binder, and a method of improving dissolution of an active ingredient from a solid pharmaceutical composition containing the active ingredient and a fat and oil-like substance having a low melting point, which includes using a low viscosity binder.
Owner:TAKEDA PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com