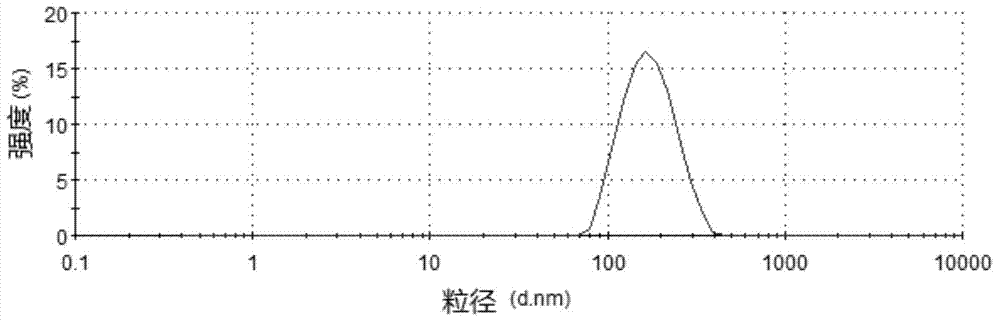
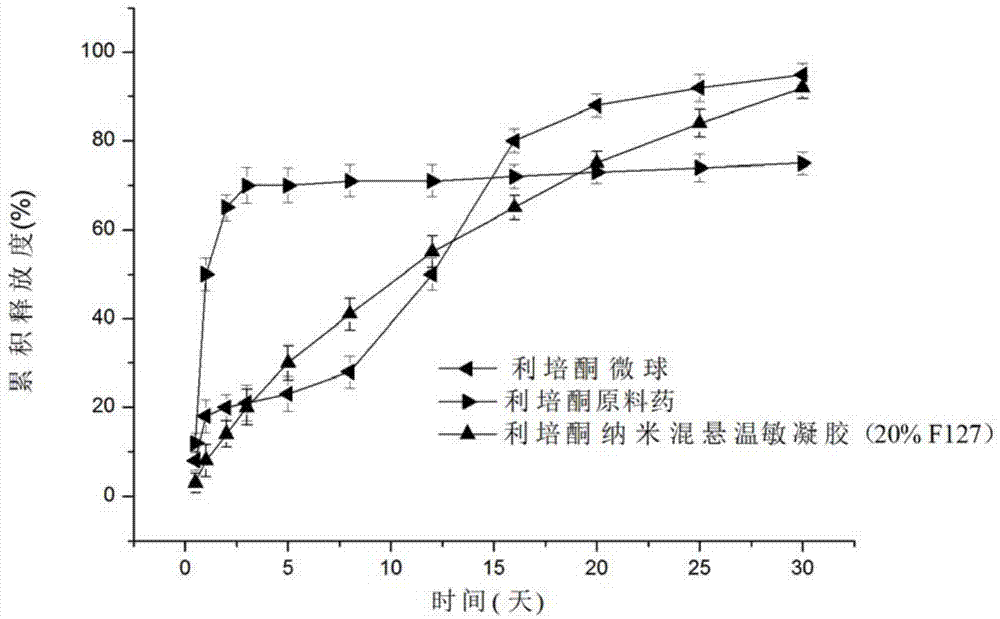
Risperidone nano-suspension temperature sensitive gel and its preparation method
A temperature-sensitive gel and nano-suspension technology, which can be used in pharmaceutical formulations, aerosol delivery, and medical preparations containing active ingredients. Achieve broad application prospects, avoid the effects of less drug release and less residue
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] The risperidone nano-suspension thermosensitive gel of this embodiment contains: risperidone 2g, docosahexaenoic acid 0.5g, poloxamer 407 (polyoxyethylene-polyethylene glycol) per 100ml nano-suspension thermosensitive gel Oxypropylene-polyoxyethylene triblock copolymer, F127) 20g, and the balance is water for injection.
[0043] The preparation method of the risperidone nano-suspension thermosensitive gel of the present embodiment comprises the following steps:
[0044]1) Dissolve 2g of risperidone and 0.5g of docosahexaenoic acid in 10ml of tetrahydrofuran to obtain an organic phase, and take water for injection as the aqueous phase;
[0045] 2) The organic phase obtained in step 1) was rotary evaporated to remove THF under reduced pressure, and dried in a vacuum oven at 35°C for 12 hours, then hydrated with 50ml of water phase, vortexed, and ultrasonically treated (400W, 4min) for dispersion. Obtain nano-suspension;
[0046] 3) Add 20g of poloxamer 407 (polyoxyethyl...
Embodiment 2
[0049] The risperidone nano-suspension thermosensitive gel of this embodiment, every 100ml nano-suspension thermosensitive gel contains: risperidone 1g, povidone 1g, poloxamer 407 (polyoxyethylene-polyoxypropylene-polyoxyethylene Ethylene triblock copolymer, F127) 15g, the balance is distilled water.
[0050] The preparation method of the risperidone nano-suspension thermosensitive gel of the present embodiment comprises the following steps:
[0051] 1) Dissolve 1g of risperidone and 1g of povidone in 10ml of chloroform to obtain an organic phase, and take distilled water as the aqueous phase;
[0052] 2) At room temperature, add the organic phase obtained in step 1) into 50ml of distilled water and stir at high speed (10000rpm, 10min) to disperse evenly to form a slightly light blue opalescent, translucent risperidone emulsion, which is then removed by rotary evaporation chloroform, and dried in a vacuum oven at 35°C for 12 hours to further remove the remaining chloroform to...
Embodiment 3
[0056] The risperidone nano-suspension thermosensitive gel of this embodiment contains: risperidone 0.25g, polyethylene glycol vitamin E succinate 1g, poloxamer 407 (polyoxyethylene) per 100ml nano-suspension thermosensitive gel - Polyoxypropylene-polyoxyethylene triblock copolymer, F127) 10 g, the remainder being distilled water.
[0057] The preparation method of the risperidone nano-suspension thermosensitive gel of the present embodiment comprises the following steps:
[0058] 1) Dissolve 0.25g of risperidone and 1g of polyethylene glycol vitamin E succinate in 50ml of methanol to obtain an organic phase, and take distilled water as the aqueous phase;
[0059] 2) Under stirring conditions at room temperature and a rotating speed of 10,000 rpm, add the organic phase obtained in step 1) into 50 ml of water phase and disperse evenly to form a translucent risperidone emulsion with slightly light blue opalescence, then remove methanol by rotary evaporation, and Dry in a vacuum...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com