Dirithromycin enteric-coated formulation
A technology of dirithromycin and enteric-coated preparations, which is applied to medical preparations with non-active ingredients, medical preparations containing active ingredients, organic active ingredients, etc., which can solve the problem of drug dissolution rate decline, increased process difficulty, and damaged process. issues of continuity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
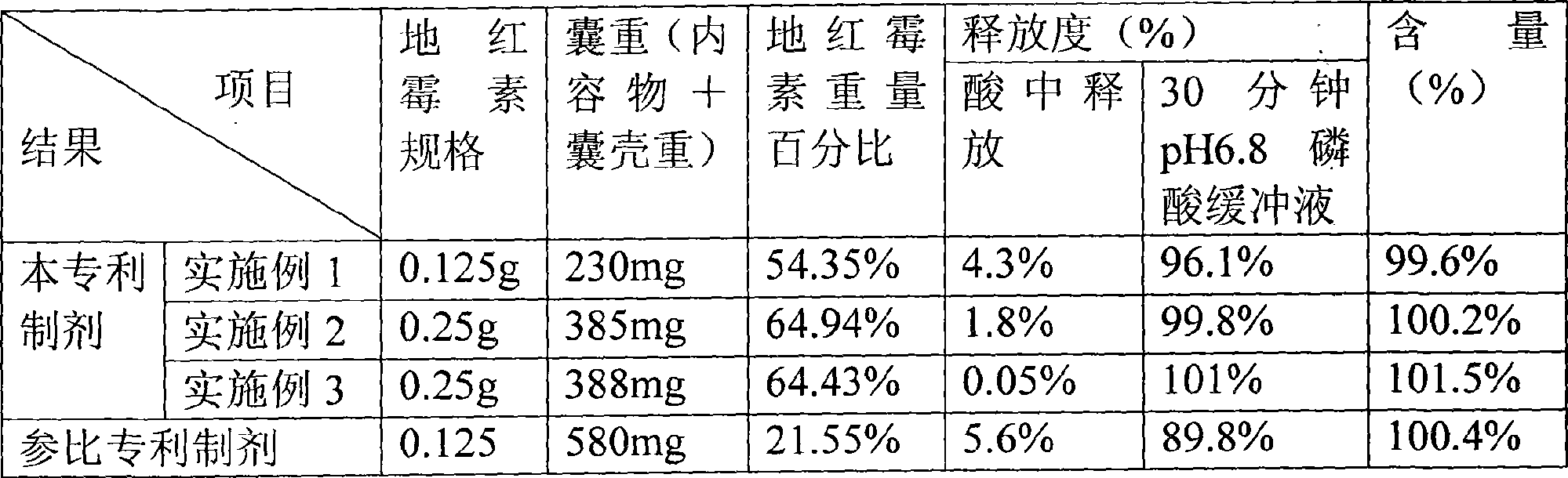
Embodiment 1
[0029] Dirithromycin core prescription:
[0030] Raw material name dosage
[0031] Dirithromycin 125g
[0032] HPMCP 5g
[0033] 80% ethanol 100ml
[0034] Gown Prescription:
[0035] HPMC (3cap) 2.5g
[0036] Triethyl citrate 0.25g
[0037] 80% ethanol 100ml
[0038] Enteric coating prescription:
[0039] HPMCP 7.5g
[0040] Diethyl phthalate 0.75g
[0041] 80% ethanol 125ml
[0042] The preparation method of dirithromycin enteric-coated pellets is as follows:
[0043] Preparation of drug-containing pellet cores:
[0044] Slowly add HPMCP into 100ml of 80% ethanol with a stirring device, and keep stirring until it dissolves into a clear solution as a binder.
[0045] Grind and sieve 125 g of dirithromycin, and use the solution as a binder to make a suitable soft material. Start the extrusion and spheronization equipment, select the aperture of the sieve plate to be 0.8 mm, and rotate at 4000 rpm, extrude and spheronize, and dry the obtained pellets. Pass the obt...
Embodiment 2
[0052] Dirithromycin core prescription:
[0053] Name Dosage
[0054] Dirithromycin 250g
[0055] HPMCP 8g
[0056] 80% ethanol 160ml
[0057] Gown Prescription:
[0058] HPMC (5cap) 2.5g
[0059] PEG400 2.5g
[0061] 80% ethanol 250ml
[0062] Enteric coating prescription:
[0063] HPMCP 20g
[0064] Triethyl citrate 2g
[0065] 80% ethanol 330ml
[0066] The preparation method of dirithromycin enteric-coated pellets is as follows:
[0067] Preparation of drug-containing pellet cores:
[0068] Take 8g of HPMCP and slowly add it into 160ml of 80% ethanol with a stirring device, and keep stirring until it dissolves into a clear solution, as a binder.
[0069] 250g of dirithromycin was crushed and sieved, and the solution was used as a binder to make a suitable soft material. Start the extrusion and spheronization equipment, select the aperture of the sieve plate to be 1mm, and the rotating speed is 4000 rpm, extrude and spheronize, and dry ...
Embodiment 3
[0075] Dirithromycin core prescription:
[0076] Raw material name dosage
[0077] Dirithromycin 250g
[0078] HPMCP 8g
[0079] 80% ethanol 150ml
[0080] Gown Prescription:
[0081] HPMC (3cap) 2.5g
[0082] Propylene glycol 2.5g
[0083] 80% ethanol 100ml
[0084] Enteric coating prescription:
[0085] HPMCP 25g
[0086] Diethyl phthalate 2.5g
[0087] 80% ethanol 416ml
[0088] The preparation method of dirithromycin enteric-coated pellets is as follows:
[0089] Preparation of drug-containing pellet cores:
[0090] Take 8g of HPMCP and slowly add it into 150ml of 80% ethanol with a stirring device, and keep stirring until it dissolves into a clear solution, as a binder.
[0091] 250g of dirithromycin was crushed and sieved, and the solution was used as a binder to make a suitable soft material. Start the extrusion and spheronization equipment, select the aperture of the sieve plate to be 0.8 mm, and rotate at 4000 rpm, extrude and spheronize, and dry the obta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com