Patents
Literature
88 results about "Fexofenadine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Fexofenadine is an antihistamine used to relieve allergy symptoms such as watery eyes, runny nose, itching eyes/nose, sneezing, hives, and itching.
Method of enhancing bioavailability of fexofenadine and its derivatives
The present invention relates to a method of enhancing the bioavailability of a piperidinoalkanol antihistamine in a patient which comprises co-administering to said patient an effective antihistaminic amount of said piperidinoalkanol and an effective p-glycoprotein inhibiting amount of a p-glycoprotein inhibitor.
Owner:AVENTISUB II INC +1
Drug delivery device containing neuraminidase inhibitor and an H1 antagonist
The present invention provides a dual release solid dosage form containing a first composition that releases a neuraminidase inhibitor, such as oseltamivir, zanamivir, or peramivir, in a controlled manner and a second composition that releases an H1 antagonist in a rapid and / or immediate manner. A wide range of H1 antagonist antihistamines, especially fexofenadine and loratadine, can be used in this device. Particular embodiments of the invention provide osmotic devices having predetermined release profiles. The device is useful for the treatment of respiratory congestion and other viral infection associated symptoms.
Owner:ACELLA HLDG LLC +1
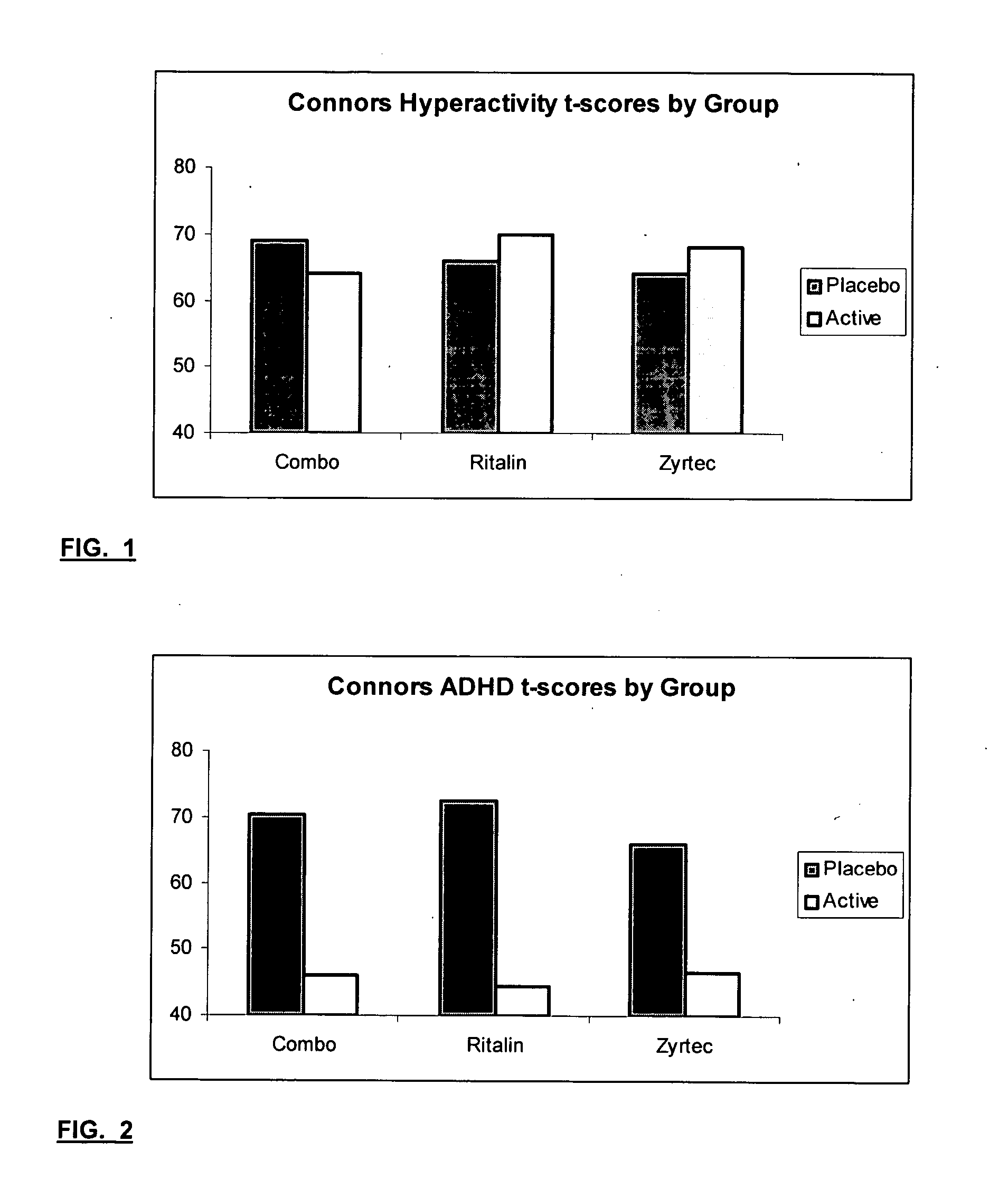
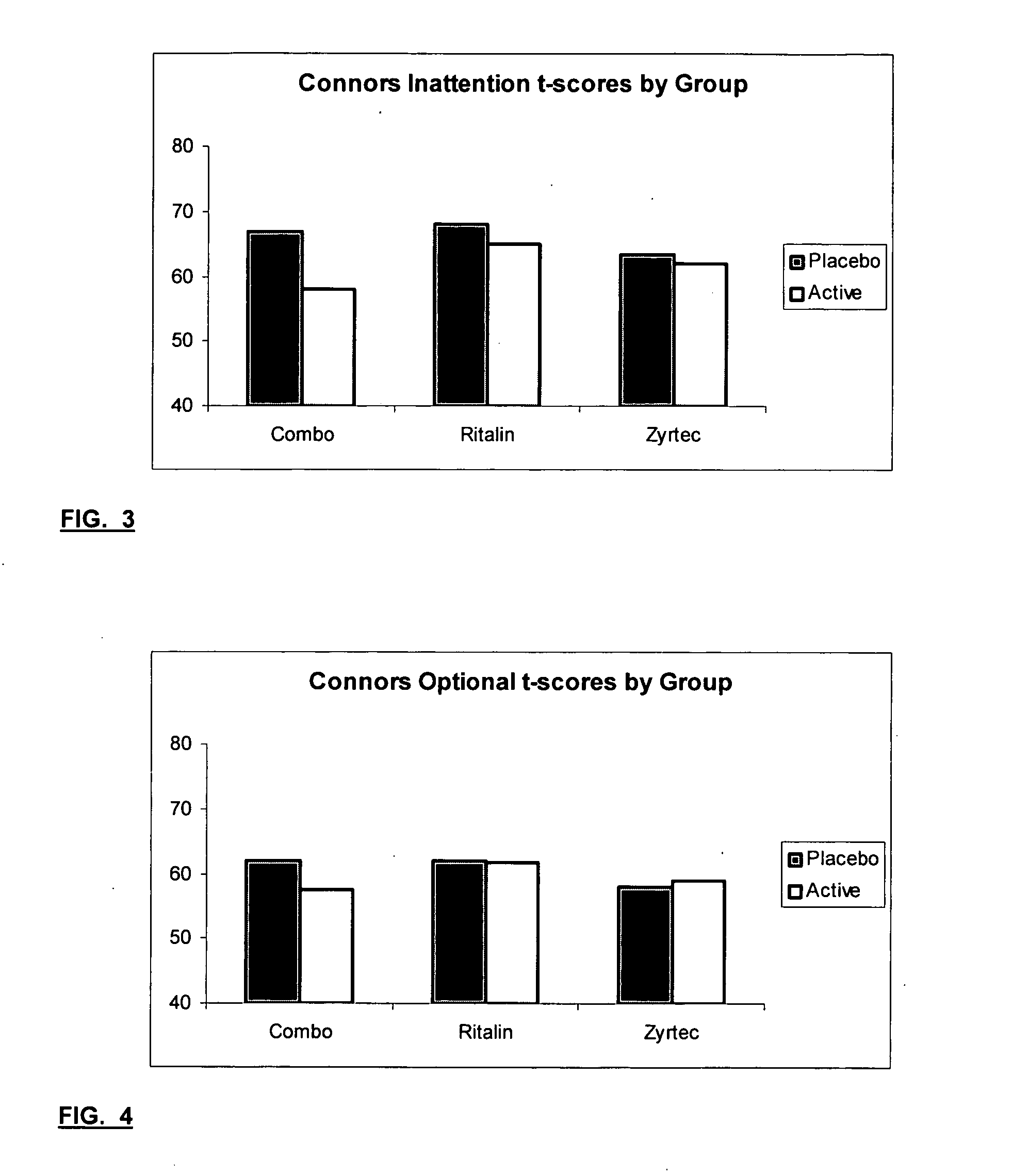
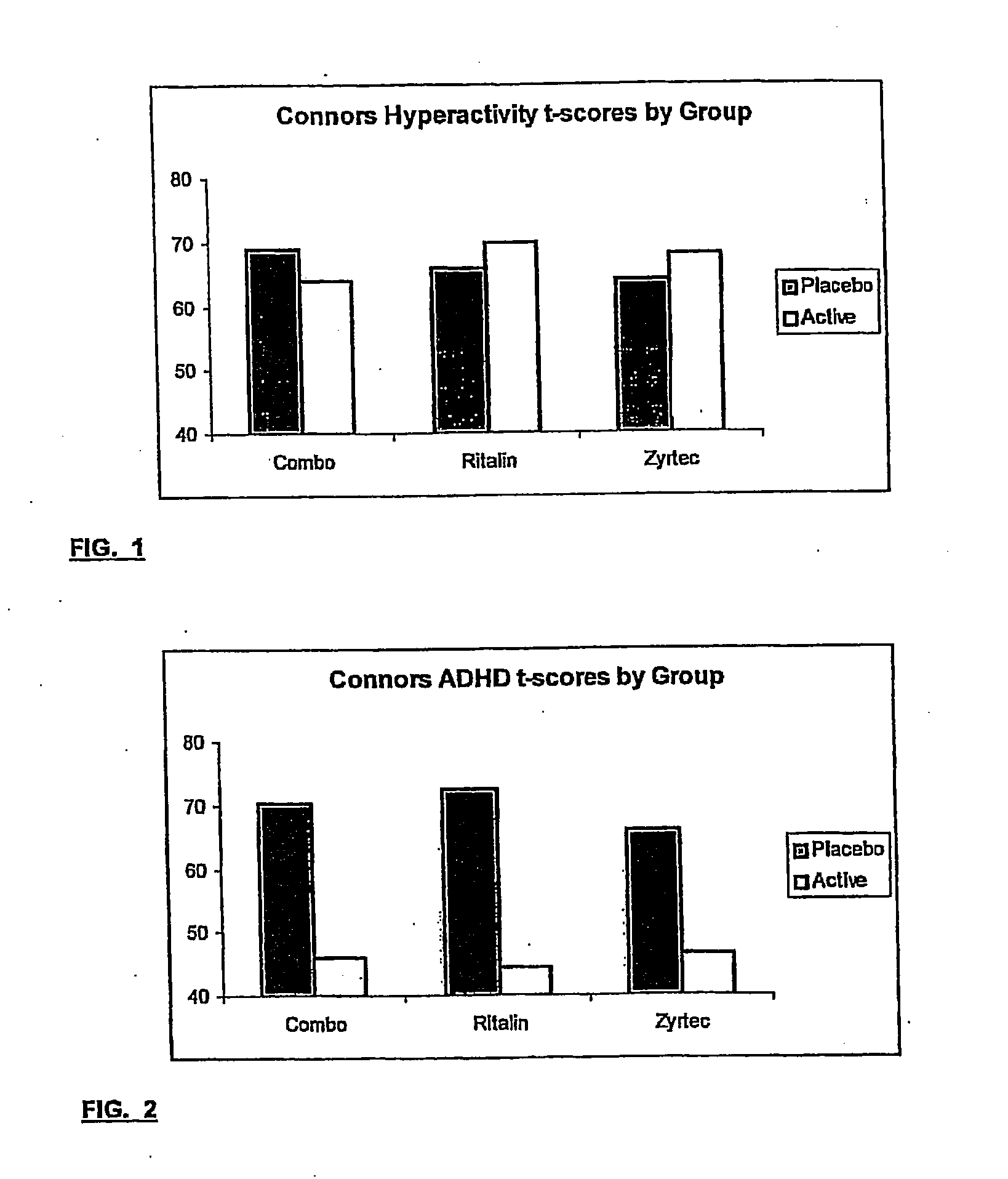
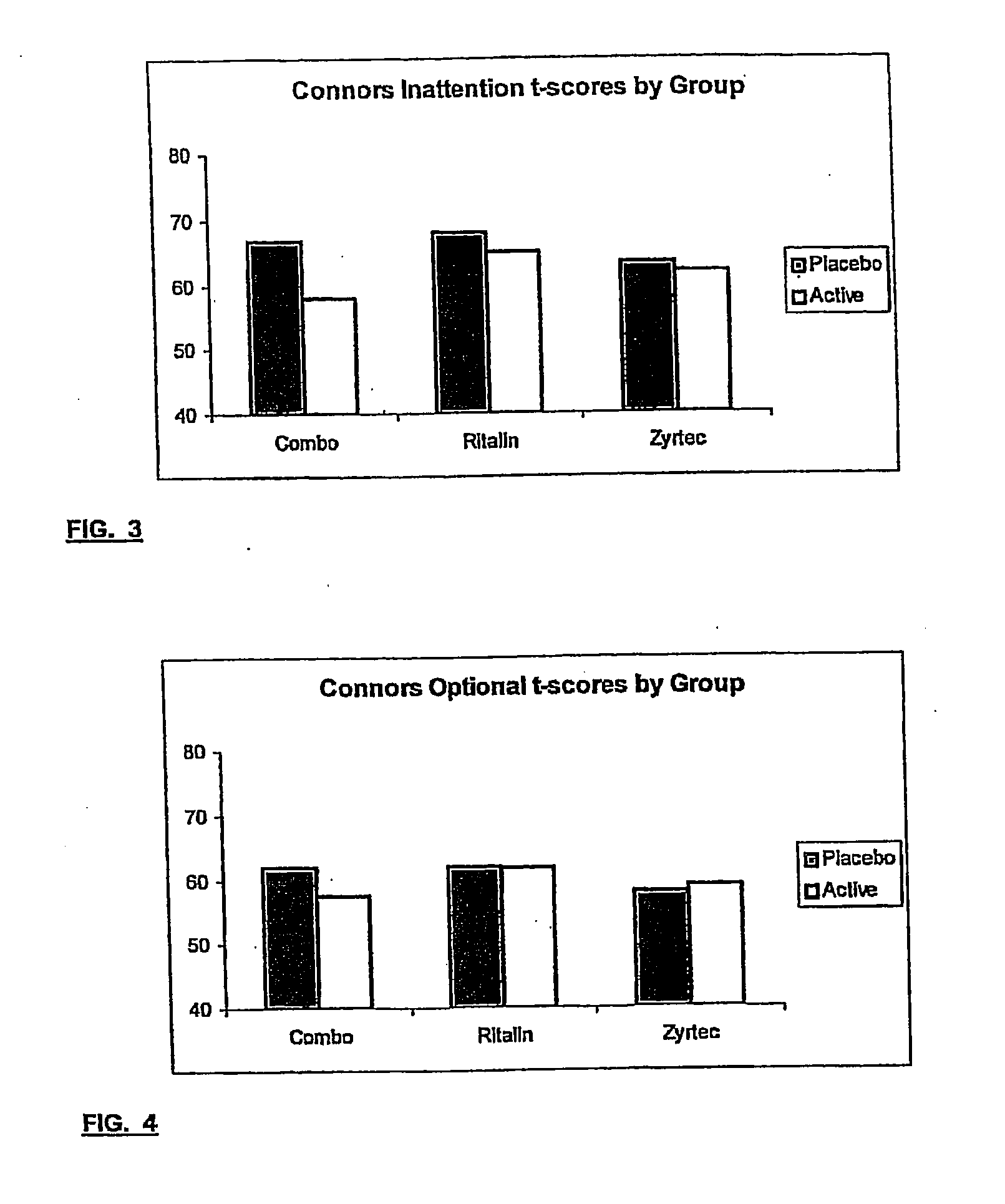
Treatment of behavioral disorders
InactiveUS20050192290A1Ameliorate behavioral disorderSufficient amountBiocideNervous disorderTherapeutic ACTHFexofenadine
The present invention relates to a method for treating a behavior disorder comprising the administration of a therapeutically effective amount of antihistamine, such as ceterizine, fexofenadine; loratadine, and desloratadine. The behavioral disorders may include ADHD, anxieity, depression, and autism. The method may include the administration of the antihistamine in combination with a stimulant medication, such as methylphenidate, thereby to achieve a synergistic effect. In any event, the amount of antihistamine and / or stimulant is effective to downregulate neurotrophic factors such as nerve growth factor or CD40. The invention is also directed to a method of preventing the onset of behavior disorders in patients presenting with symptoms of allergic rhinitis.
Owner:MELAMED ISAAC
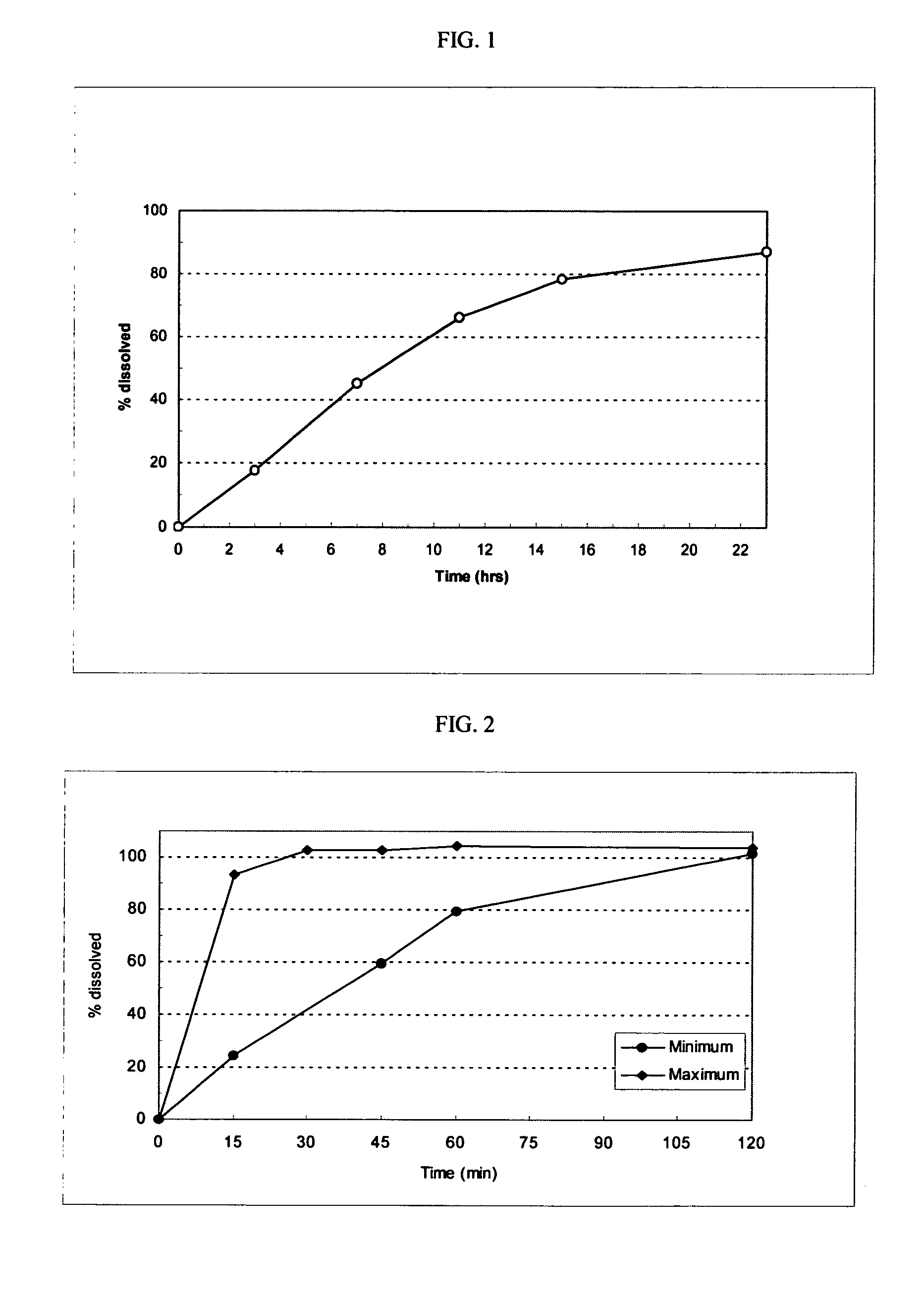
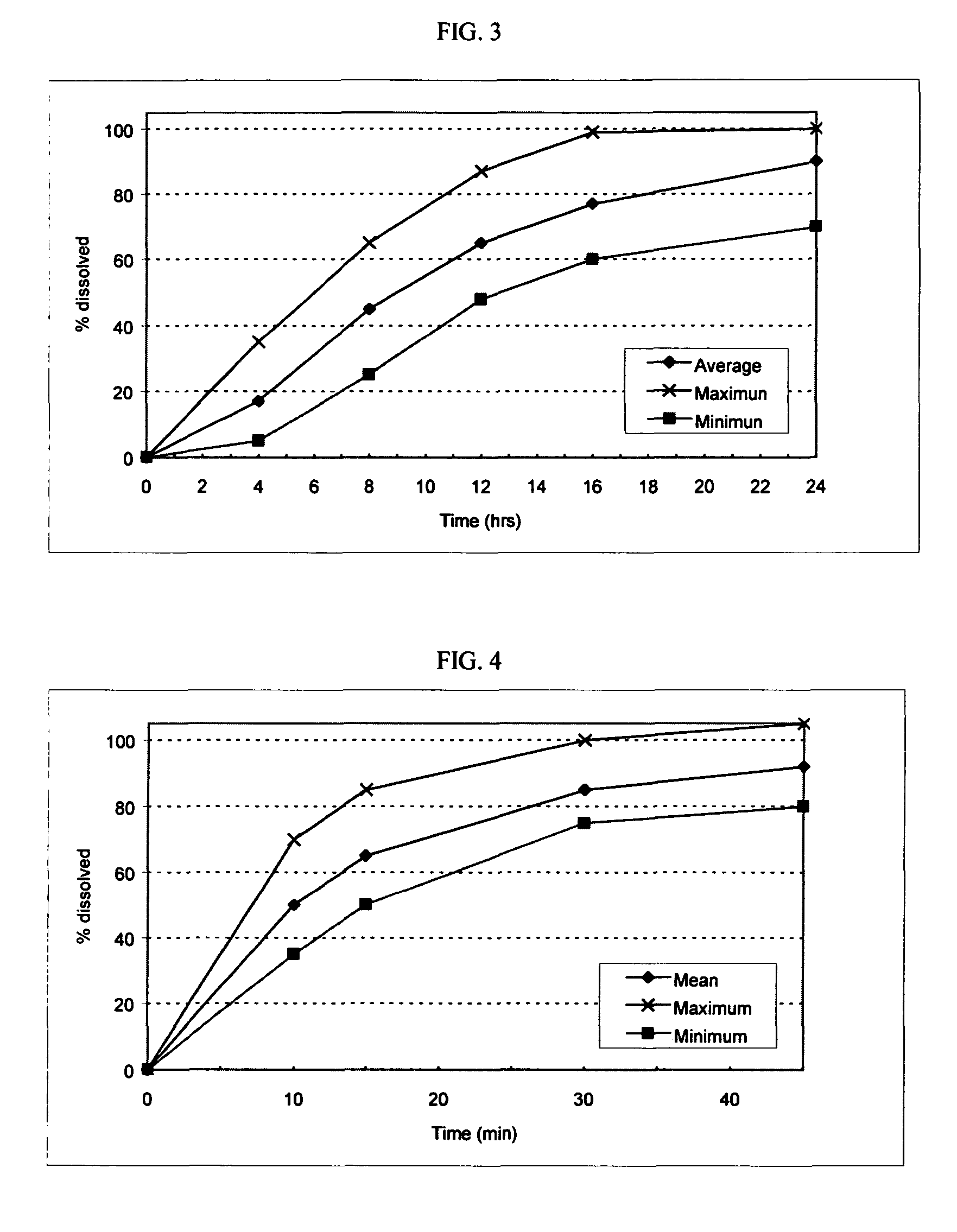
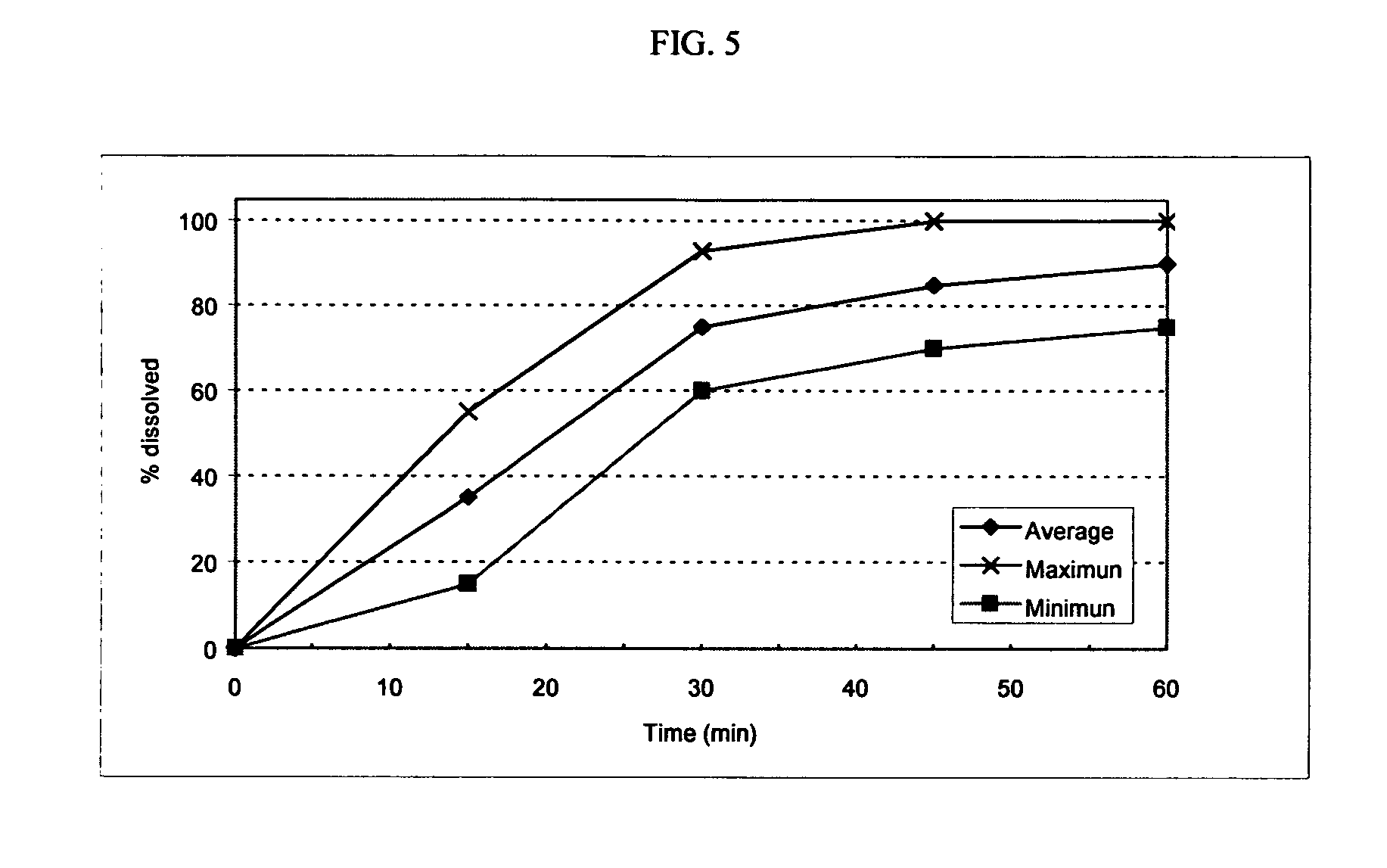
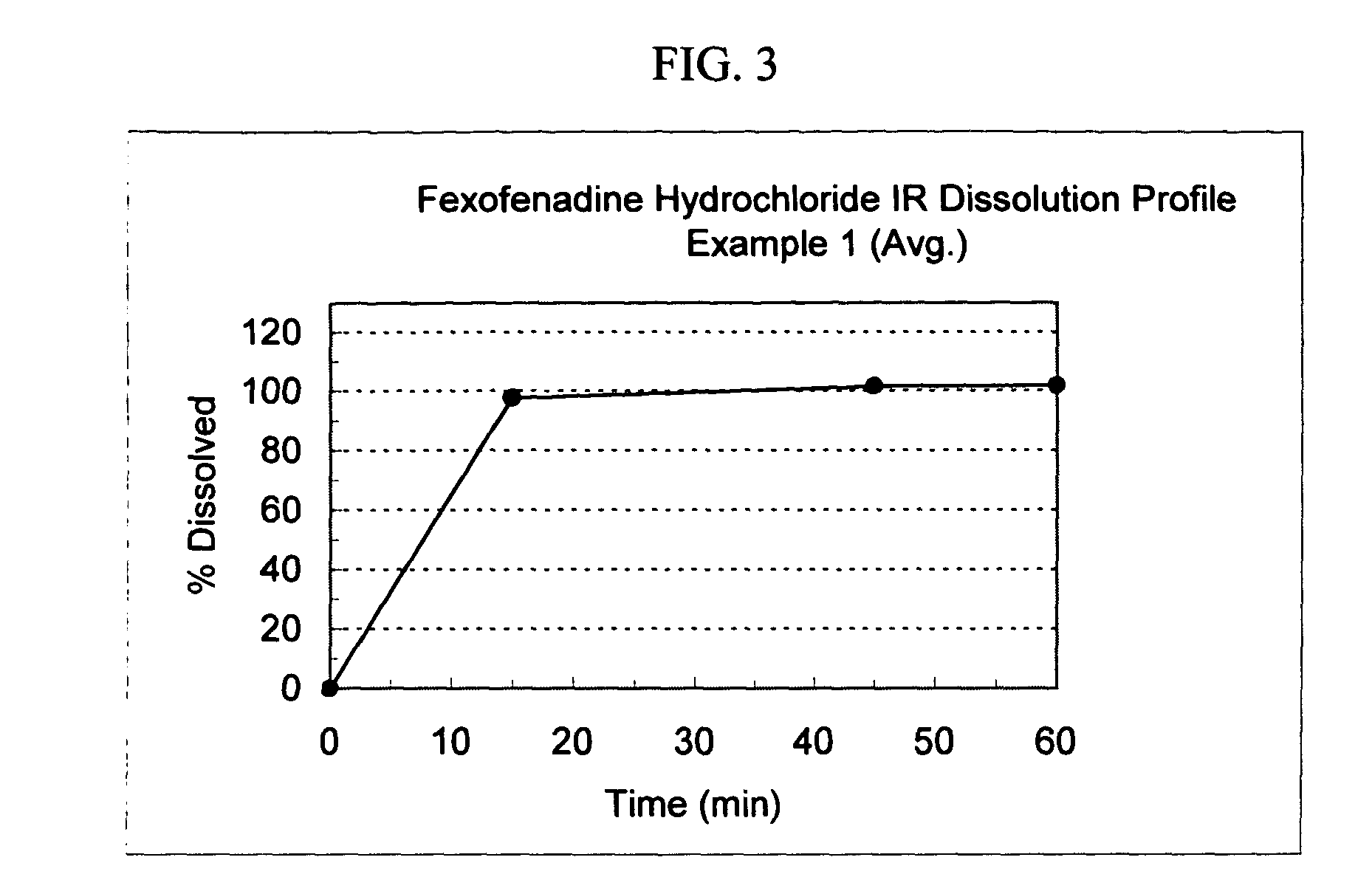
Fexofenadine Microcapsules and Compositions Containing Them
ActiveUS20110250281A1Suitable drug contentFast dissolutionBiocideAntipyreticFexofenadineDrug content
The present invention provides a pharmaceutical composition comprising taste-masked immediate release microcapsules which comprise fexofenadine and a water-insoluble polymer coating. These microcapsules and the pharmaceutical compositions comprising them have suitable drug content and desirable pharmaceutical properties, including a quick dissolution rate of fexofenadine combined with a taste masking effect.
Owner:ADARE PHARM SRL
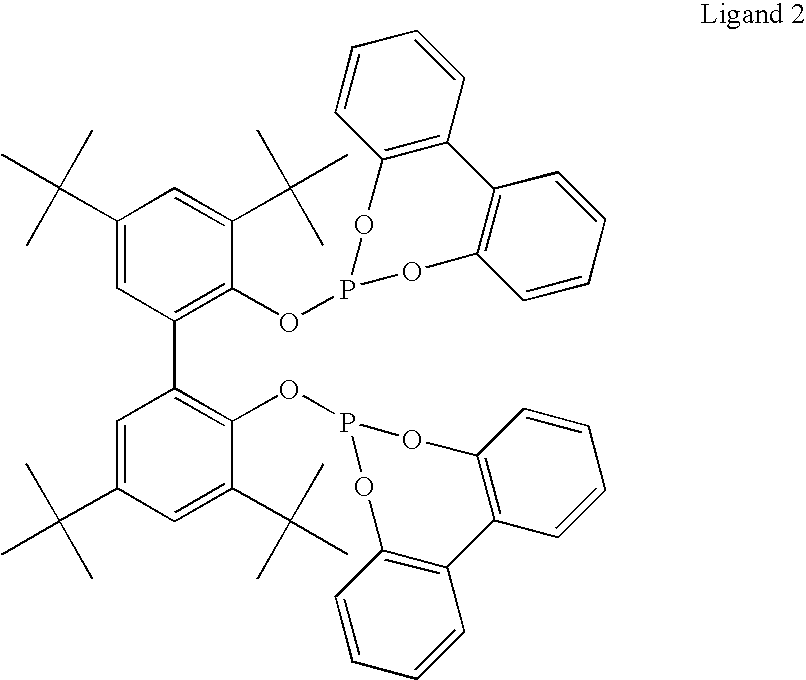
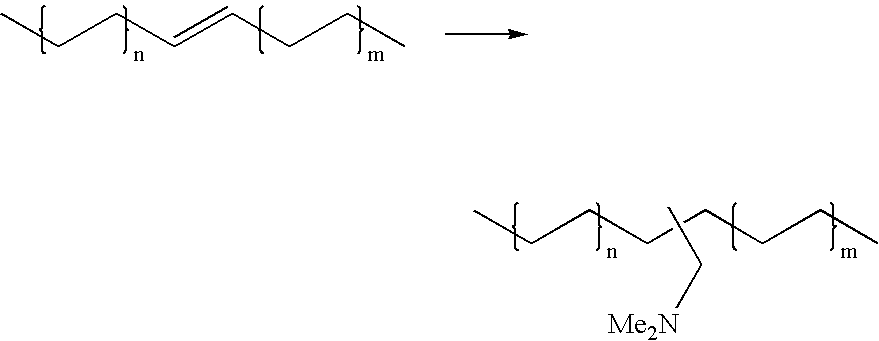
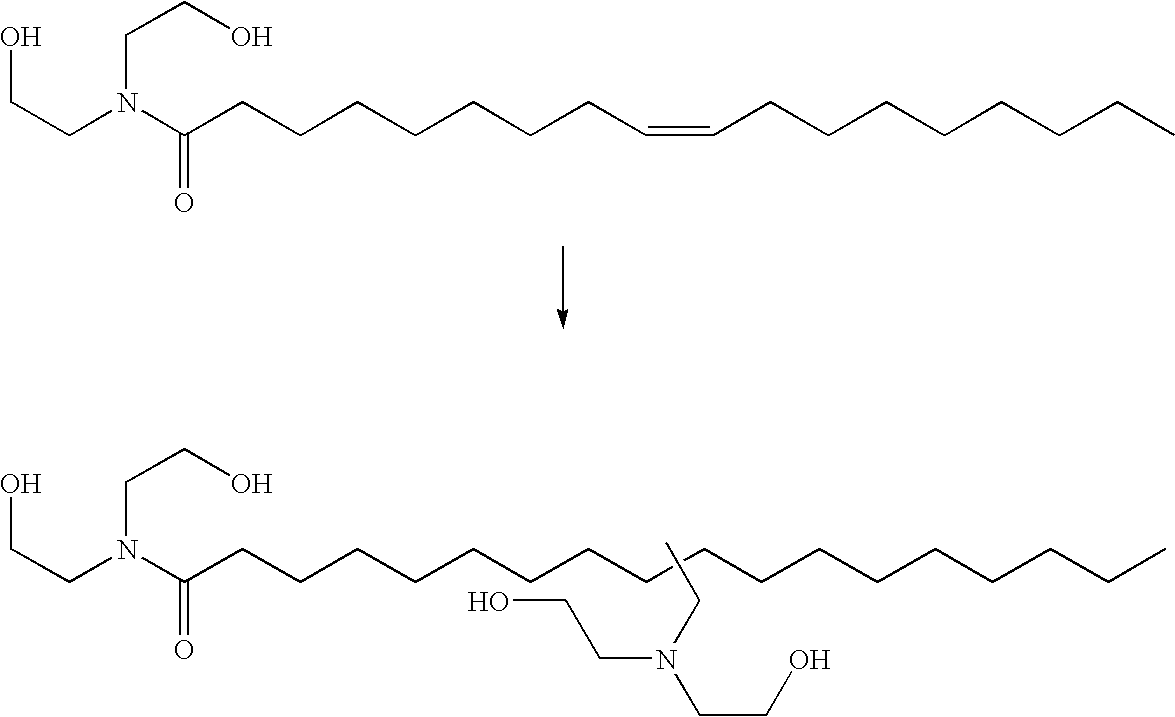
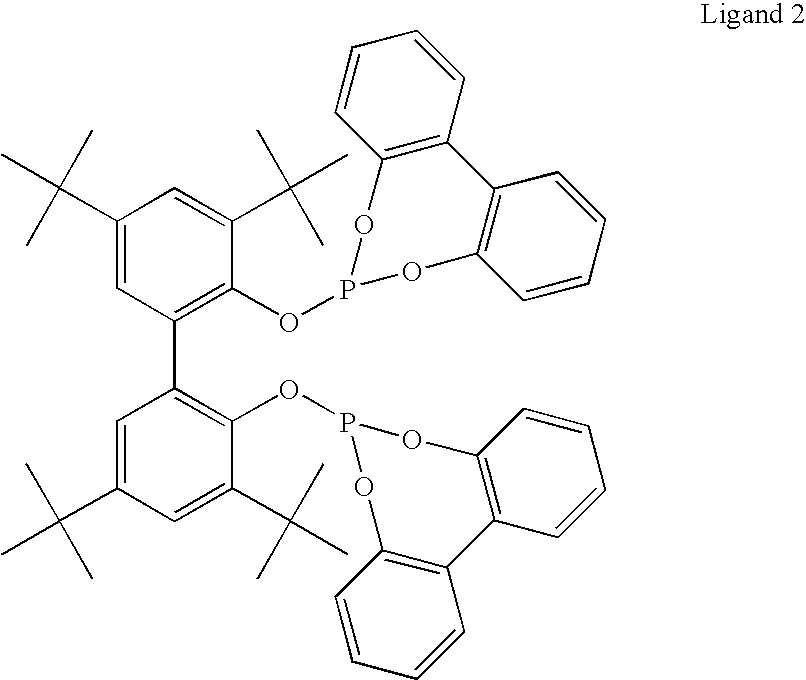
Hydroaminomethylation of olefins
ActiveUS20050215825A1Organic compound preparationCarboxylic acid amides preparationSyngasFexofenadine
The present invention relates to a method comprising the step of contacting under hydroaminomethylation conditions, an olefin, an amine, a rhodium-phosphorous ligand, and synthesis gas (syngas). In particular, it has been discovered that, under some circumstances, a neutral rhodium-monodentate phosphite ligand is prescribed. The invention provides a simple way of making, in high yields and regiospecificity, a variety of products, including pharmacologically active products such as ibutilide, terfenadine, and fexofenadine, and derivatives thereof.
Owner:UNION CARBIDE CORP
Hydroaminomethylation of olefins
The present invention relates to a method comprising the step of contacting under hydroaminomethylation conditions, an olefin, an amine, a rhodium-phosphorous ligand, and synthesis gas (syngas). In particular, it has been discovered that, under some circumstances, a neutral rhodium-monodentate phosphite ligand is prescribed. The invention provides a simple way of making, in high yields and regiospecificity, a variety of products, including pharmacologically active products such as ibutilide, terfenadine, and fexofenadine, and derivatives thereof.
Owner:UNION CARBIDE CORP
Treatment of Behavioral Disorders
The present invention relates to a method for treating a behavior disorder comprising the administration of a therapeutically effective amount of antihistamine, such as cetirizine, fexofenadine; loratadine, and desloratadine. The behavioral disorders may include ADHD, anxiety, depression, and autism. The method may include the administration of the antihistamine in combination with a stimulant medication, such as methylphenidate, thereby to achieve a synergistic effect. In any event, the amount of antihistamine and / or stimulant is effective to downregulate neurotrophic factors such as nerve growth factor or CD40. The invention is also directed to a method of preventing the onset of behavior disorders in patients presenting with symptoms of allergic rhinitis.
Owner:MELAMED ISAAC
Orodispersible tablets containing fexofenadine
The present invention concerns orodispersible tablets, which are able to disintegrate in the buccal cavity upon contact with saliva by formation of an easy-to-swallow suspension, in less than 60 seconds, preferably in less than 40 seconds, containing fexofenadine in the form of coated granules, and a mixture of excipients comprising at least one disintegrating agent, a soluble diluent agent, a lubricant and optionally a swelling agent, a permeabilising agent, sweeteners, flavoring agents and colors; the process for obtaining such orodispersible tablets and the coated granules incorporated therein and the use of said orodispersible tablets in the treatment of seasonal allergic rhinitis.
Owner:ETHYPHARM SA
Orally disintegrating tablet containing fexofenadine or salts of fexofenadine, and preparation method thereof
InactiveCN101836965ADelayed disintegration rateResolve bitternessPharmaceutical non-active ingredientsPill deliveryFexofenadineOrally disintegrating tablet
The invention provides an orally disintegrating tablet containing fexofenadine or salts of fexofenadine, which is prepared by the inclusion technology. When main drugs and inclusions exist at a specific ratio, the tablet can effectively solve the problems of the taste and disintegration of the main drugs, is convenient for operating, transporting and storing, has wide application range and is suitable for large-scale production.
Owner:北京万全阳光医药科技有限公司
Fexofenadine suspension formulation
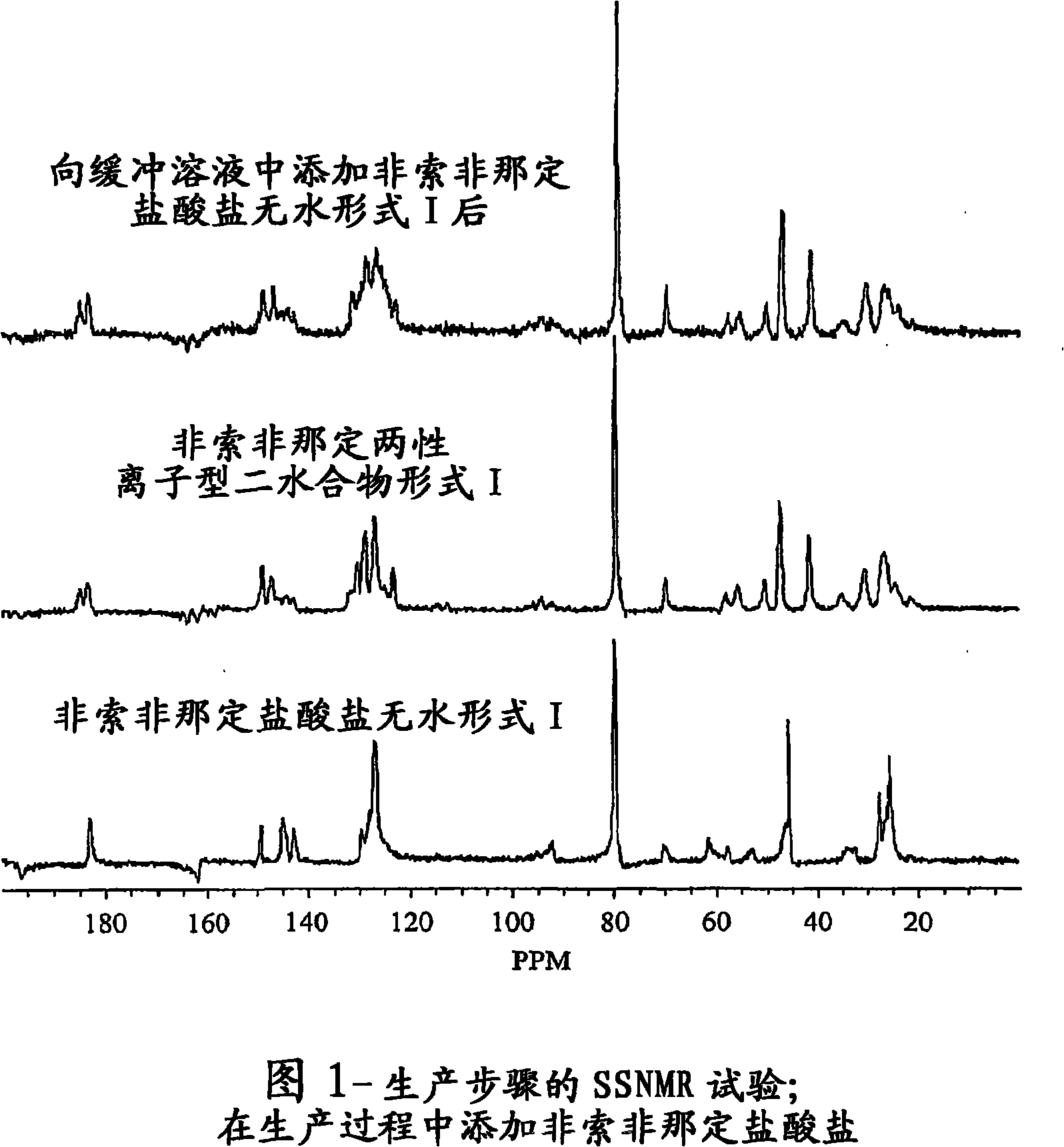
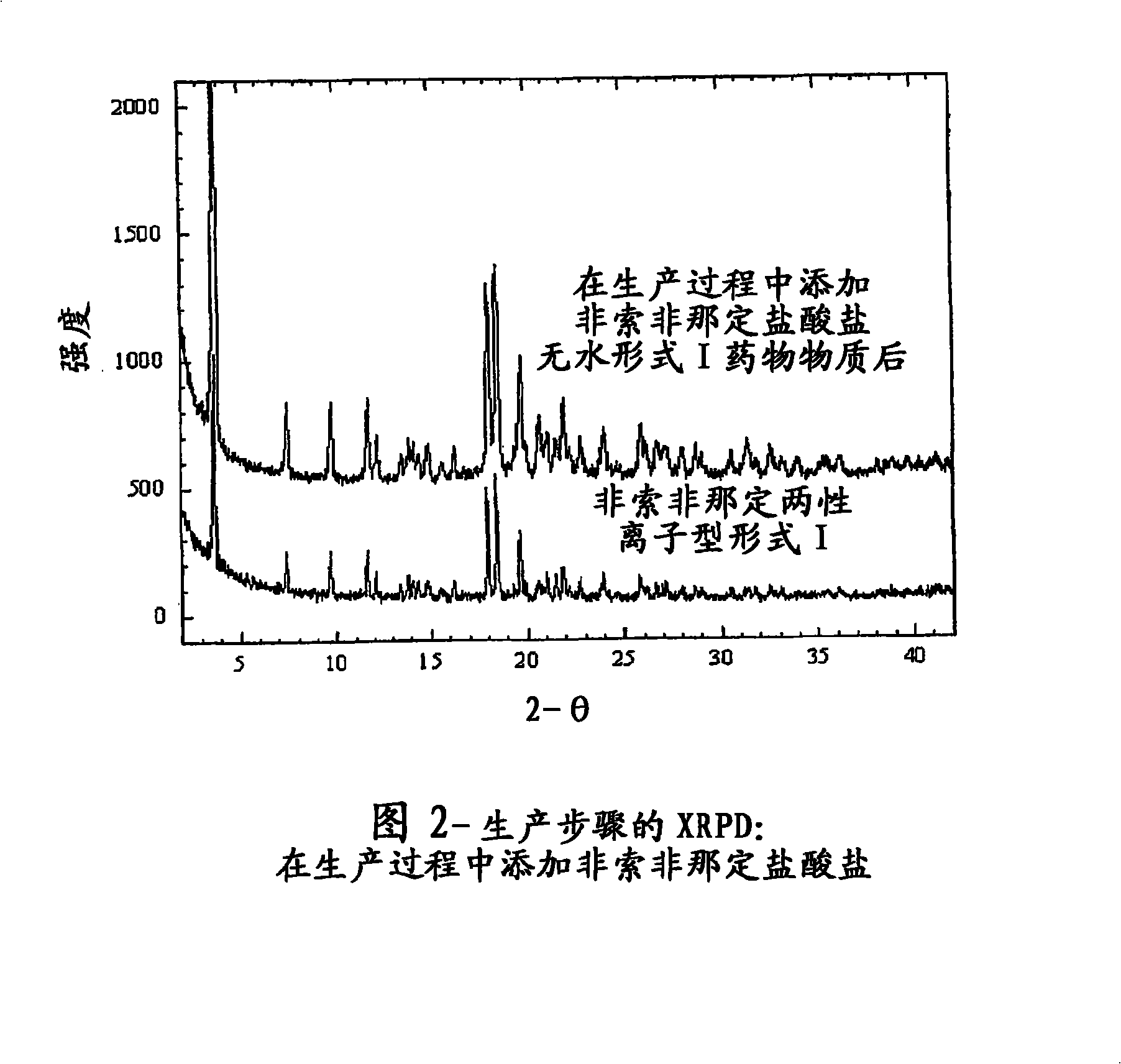
The present invention is directed to an aqueous pharmaceutical suspension of fexofenadine zwitterionic dihydrate Form I.
Owner:SANOFI AVENTIS US LLC
H1-receptor-antagonist-containing inhalation preparation
The invention relates to an H1-receptor-antagonist-containing inhalation preparation which contains an H1 receptor antagonist and one or more pharmaceutical auxiliary materials suitable for inhalation administration. The H1 receptor antagonist is one or more of loratadine, desloratadine, cetirizine, levocetirizine, astemizole, ketotifen, ebastine, fexofenadine, avastin, mequitazine, mizolastine and salts thereof, and preferably one or more of loratadine, desloratadine, cetirizine, levocetirizine, ebastine, mizolastine, avastin, mequitazine, ketotifen and hydrochlorides or fumarates thereof.
Owner:TIANJIN JINYAO GRP
Hydroaminomethylation of olefins
InactiveCN1918110APreparation by reductive alkylationAmino compound preparation by condensation/addition reactionsSyngasFexofenadine
The present invention relates to a method comprising the step of contacting under hydroaminomethylation conditions, an olefin, an amine, a rhodium-phosphorous ligand, and synthesis gas (syngas). In particular, it has been discovered that, under some circumstances, a neutral rhodium-monodentate phosphite ligand is prescribed. The invention provides a simple way of making, in high yields and regiospecificity, a variety of products, including pharmacologically active products such as ibutilide, terfenadine, and fexofenadine, and derivatives thereof.
Owner:UNION CARBIDE CHEM & PLASTICS TECH CORP
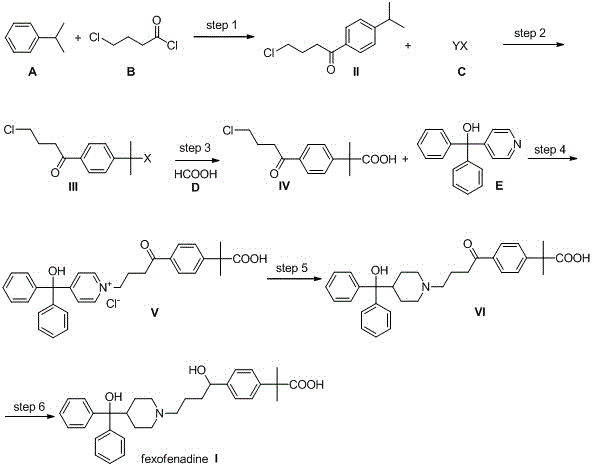
Novel synthetic method of high-purity fexofenadine and intermediate
The invention relates to a simple and efficient synthetic method of fexofenadine (chemical name: 4-{1-hydroxyl-4-[4-(hydroxyl benzhydryl)-1-piperidyl]-butyl}-alpha, alpha-dimethyl-phenylacetic acid (I)) and its intermediate. According to the method, isopropyl benzene is used as a raw material. Through a Friedel-Crafts acylation reaction, a halogenation reaction and a carbonyl insertion reaction, a key intermediate 4-(4-chloro-1-butyryl)-alpha, alpha-dimethyl phenylacetic acid (IV) is obtained; the key intermediate reacts with another raw material dibenzyl-(4-pyridyl)-methanol (E) to obtain a key pyridinium intermediate 4-{4-chloro-[4-hydroxydiphenylmethyl]-1-pyridinium]-1-butyryl}-alpha, alpha-dimethyl phenylacetic acid (V); and through catalytic hydrogenation and metallic hydrogen reduction, high-purity fexofenadine is obtained. The synthetic method provided by the invention has advantages of smooth process, simple reaction, short route, convenient post-treatment, high yield and low cost, and is a very ideal preparation method of fexofenadine and industrialization feasible route.
Owner:CHIZHOU DONGSHENG PHARMA +1
Method for synthesizing intermediate of fexofenadine
InactiveCN101182306AReduce the ratioIncrease steric hindranceOrganic chemistryPhenyl acetic acidFexofenadine
The present invention provides a synthesis method of the intermediates of fexofenadine. The intermediates are 4-[4-[4-(hydroxyl diphenyl methyl)-1-piperidyl]-1-butyryl]-alpha and alpha-dimethyl phenyl acetic acid (v). The method considers methyl allyl alcohol acetic ester and benzene as raw materials to be synthesized as the intermediate (i) by the Friedel-Crafts alkylation reaction, the ortho-para-orientation effect of phenylethyl alcohol and two methyl are used to increase steric hindrance; the intermediate (ii) with the comparatively pure intermediate is obtained by the Friedel-Crafts alkylation reaction; the intermediate (iii) is obtained by alkaline hydrolysis; then the intermediate (iv) is obtained by potassium permanganate oxidation; the intermediate (v) is generated by the condensation under the alkaline condition by the phase transfer catalysis; the v is deoxidized by sodium borohydride as slat to obtain the fexofenadine. The method has smooth technology, short route, high yield, low cost and friendly environment and is a feasible route for the industrialization.
Owner:CHONGQING UNIV
Preparation method of fexofenadine intermediate
The invention provides a preparation method of a fexofenadine intermediate 2-[4-[4-[4-(hydroxy diphenylmethyl)-1-piperidyl]-1-oxobutyl] phenyl]-2,2-dimethyl acetate, and belongs to the technical field of fexofenadine intermediate synthesis. Alpha-alpha-dimethyl phenylacetic acid is used as a raw material in the method; and the intermediate I is synthesized by the steps of esterification, Friedel-Crafts reaction, acid hydrolysis, purification, esterification, condensation and the like. The preparation method has the advantages that: the raw materials and the auxiliary materials are cheap and easily obtained, the process is smooth, the cost is low, the quality is high, the method is suitable for industrial scale production of medicines and the like.
Owner:浙江华纳药业有限公司
Composition for treating respiratory and skin diseases, comprising at least one leukotriene antagonist and at least one antihistamine
A pharmaceutical composition useful in the treatment of sneezing, itching runny nose, nasal congestion, redness of the eye, tearing, itching of the ears or palate, shortness of breath, inflammation of the bronchial mucosa, reduced Forced Expiratory Volume In One Second (FEV1), coughs, rash, itchy skin, headaches, and aches and pains associated with seasonal allergic rhinitis, perennial allergic rhinitis, common colds, otitis, sinusitus, allergy, asthma, allergic asthma and / or inflammation, in a mammalian organism in need of such treatment. The composition comprises: i) an effective amount of at least one leukotriene antagonist selected from a) montelukast, b) 1-(((R)- (3-(2-(6,7- difluoro-2- quinolinyl)ethenyl) phenyl)-3-(2- (2-hydroxy-2- propyl)phenyl) thio)methylcyclopropaneacetic acid; c) 1-(((1(R)-3 (3-(2-(2,3- dichlorothieno[3, 2-b]pyridin-5-yl) -(E)-ethenyl)phenyl) -3-(2-(1-hydroxy-1- methylethyl) phenyl)propyl) thio)methyl) cyclopropaneacetic acid; d) pranlukast; or f) [2-[[2-(4-tert -butyl-2-thiazolyl) -5-benzofuranyl] oxymethyl]phenyl] acetic acid; or a pharmaceutically acceptable salt thereof; in admixture with ii) an effective amount of at least one antihistamine which is descarboethoxyloratidine, cetirizine, fexofenadine, ebastine, astemizole, norastemizole, epinastine, efletirizine or a pharmaceutically acceptable salt thereof.
Owner:SCHERING AG
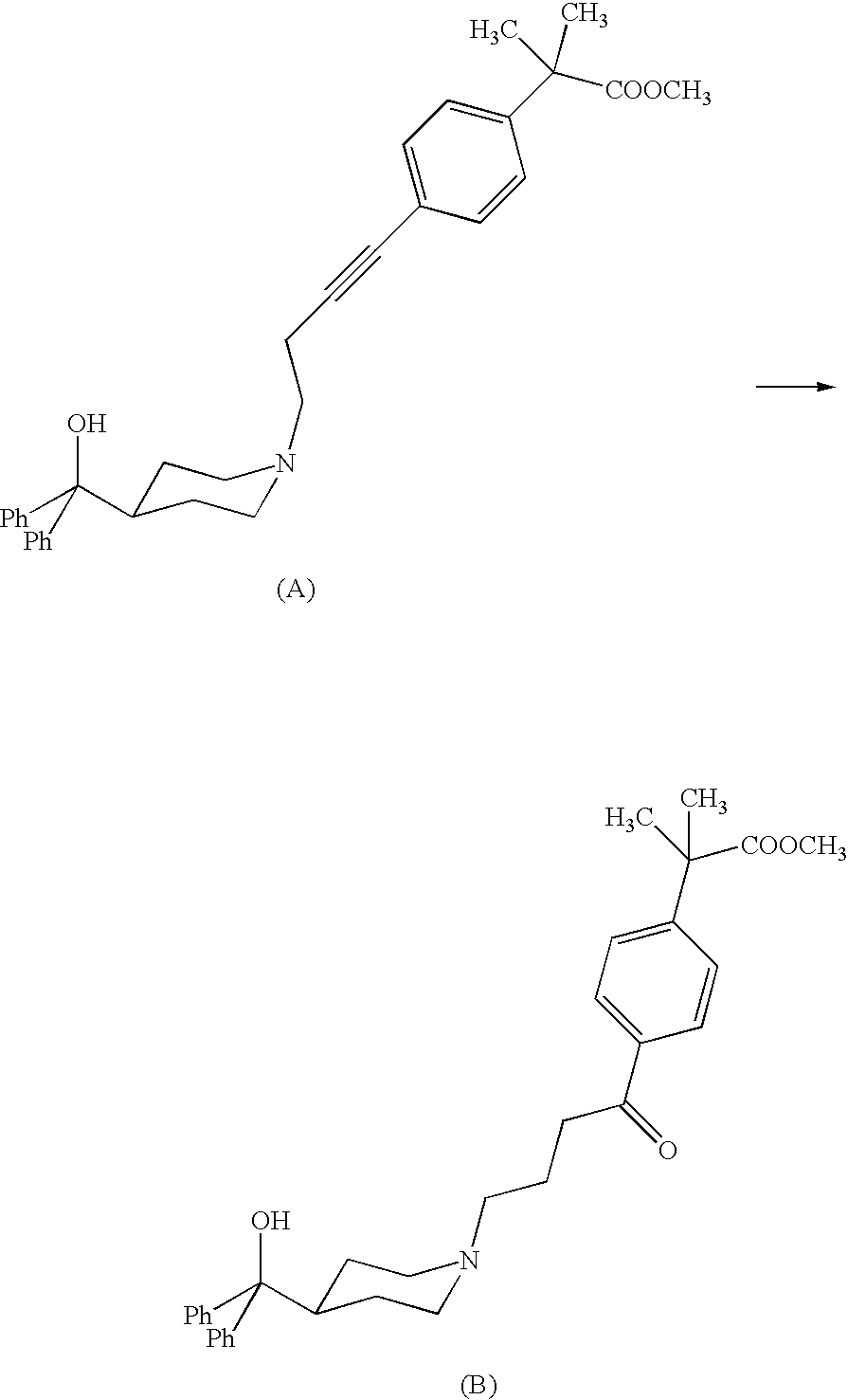
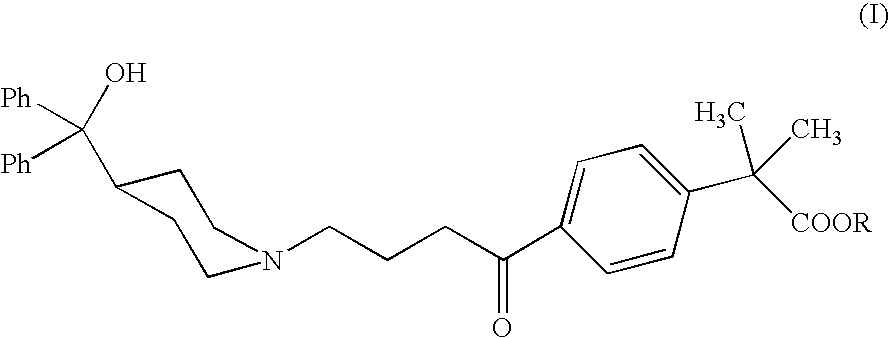
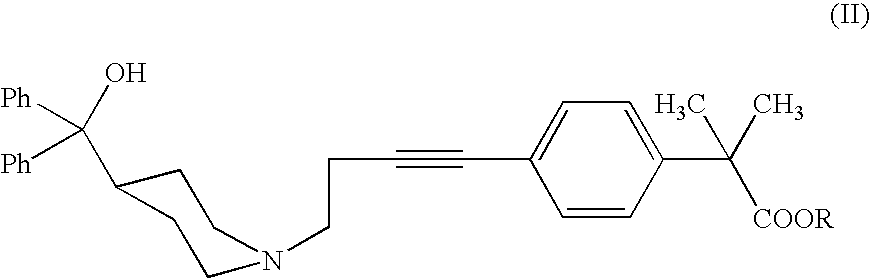
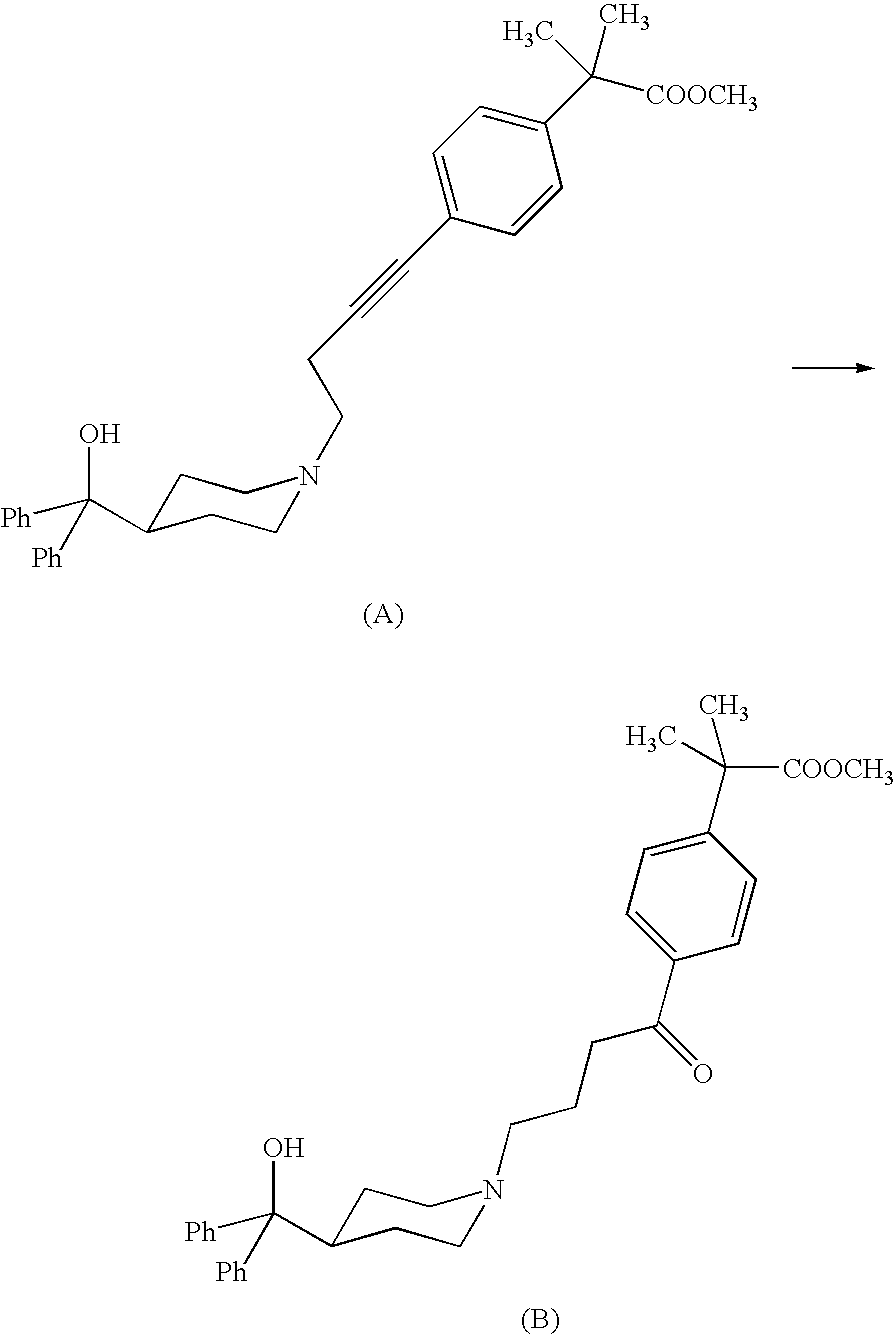
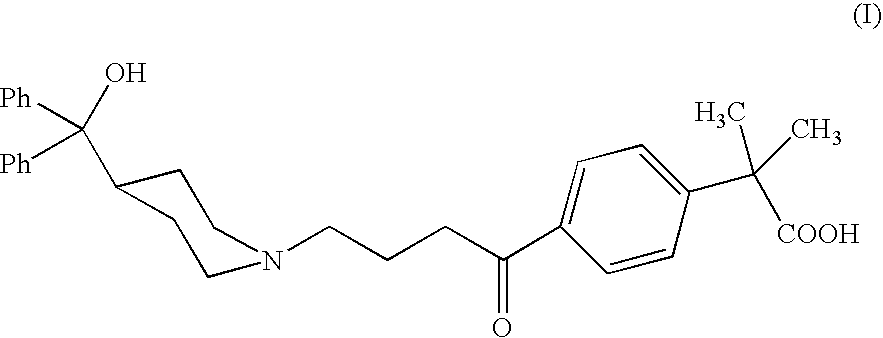
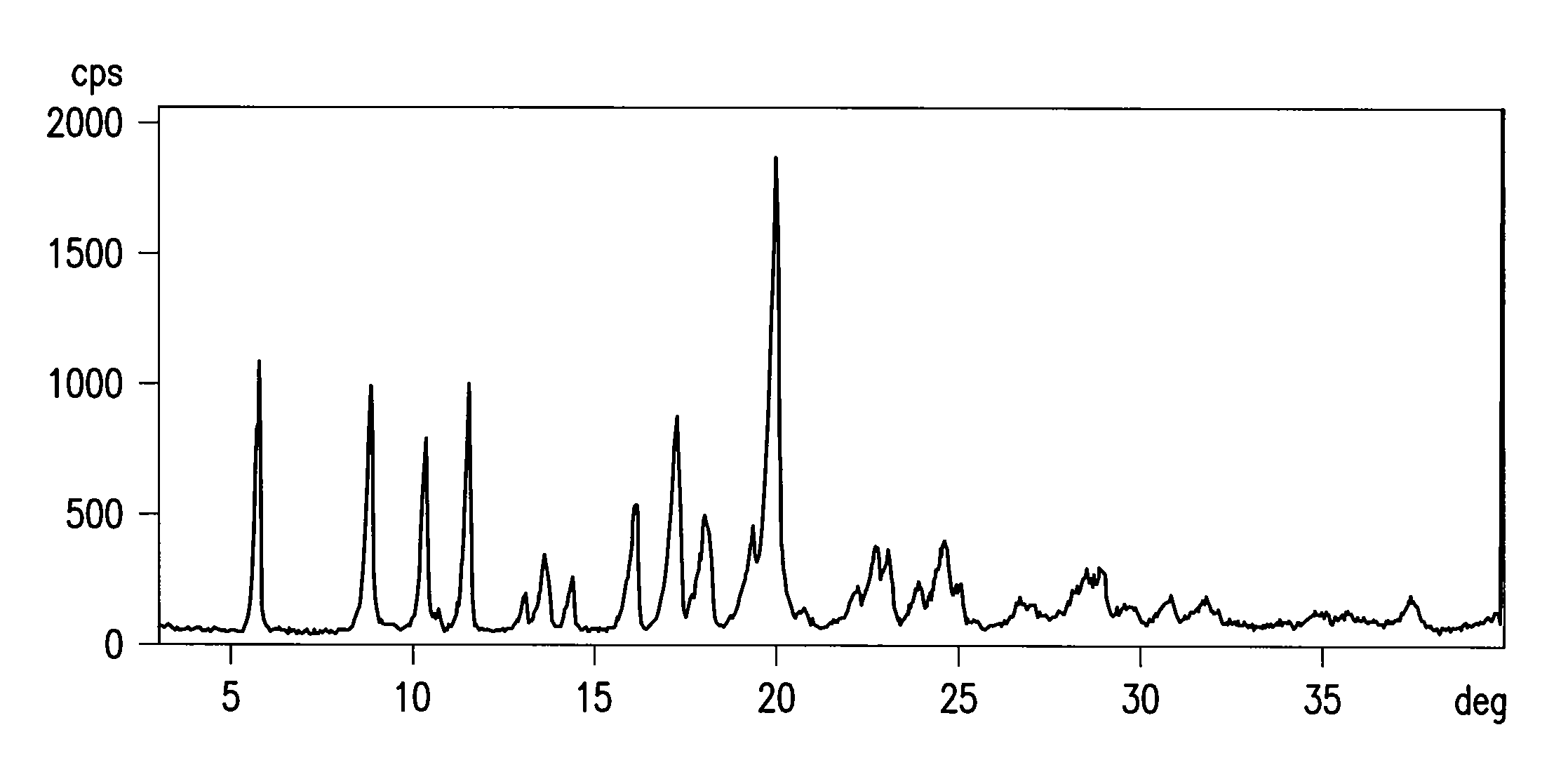
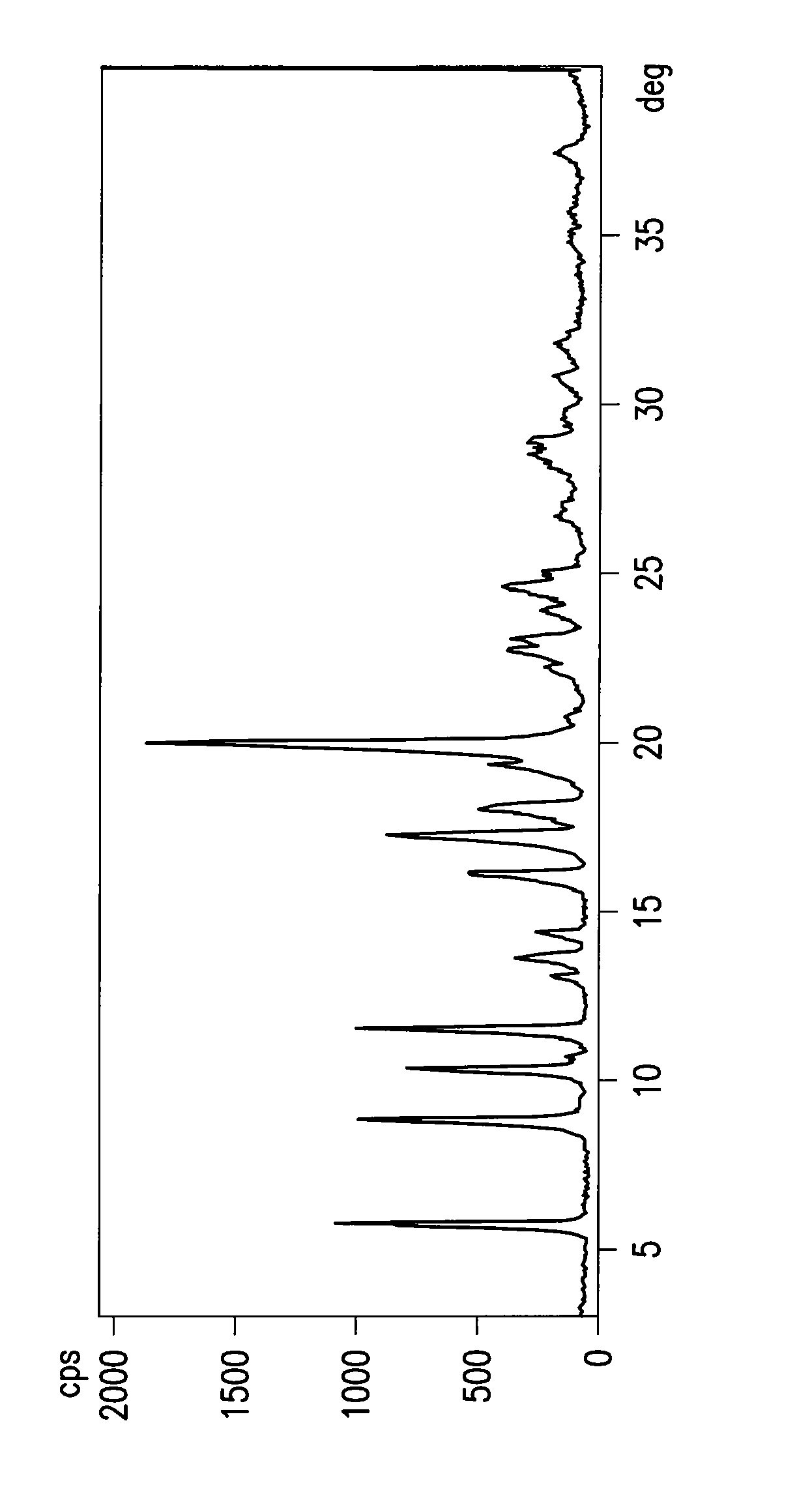
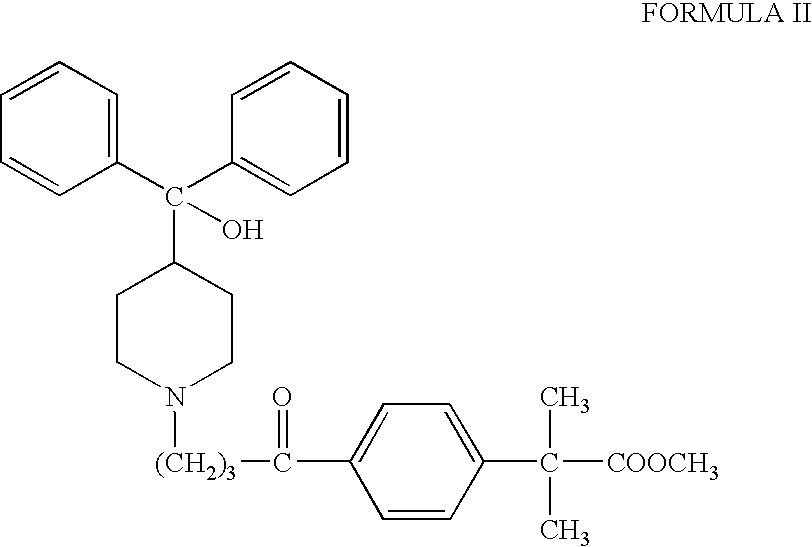
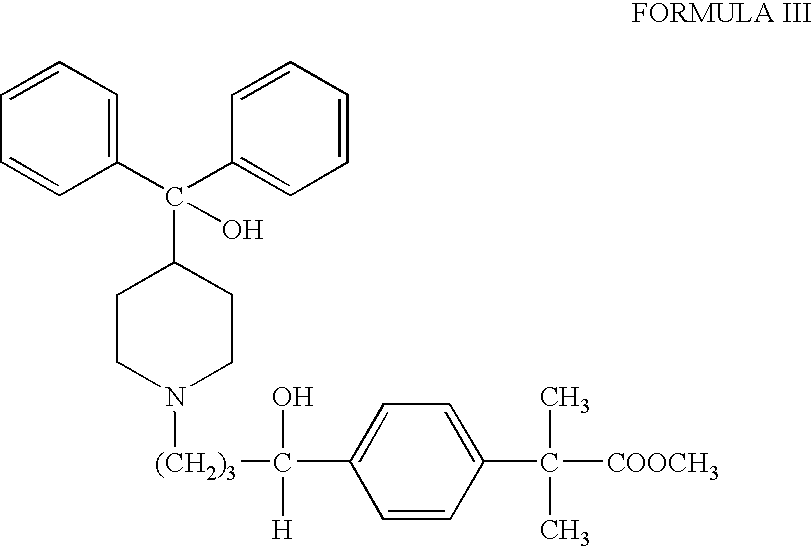
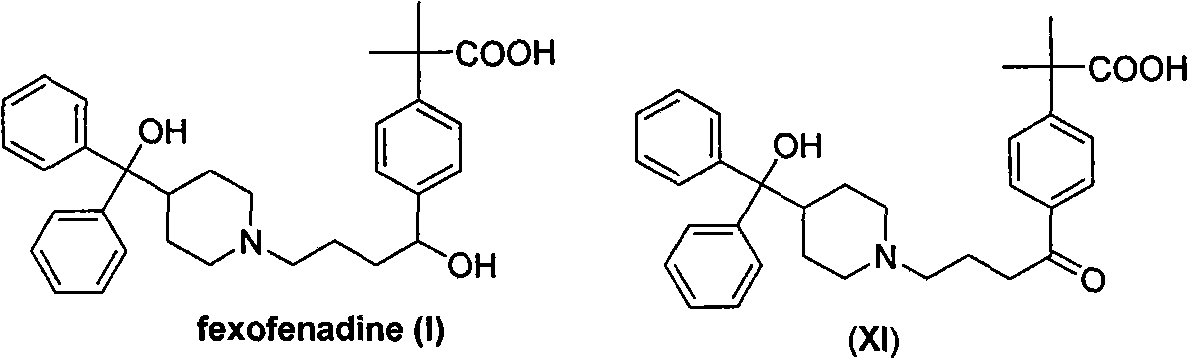
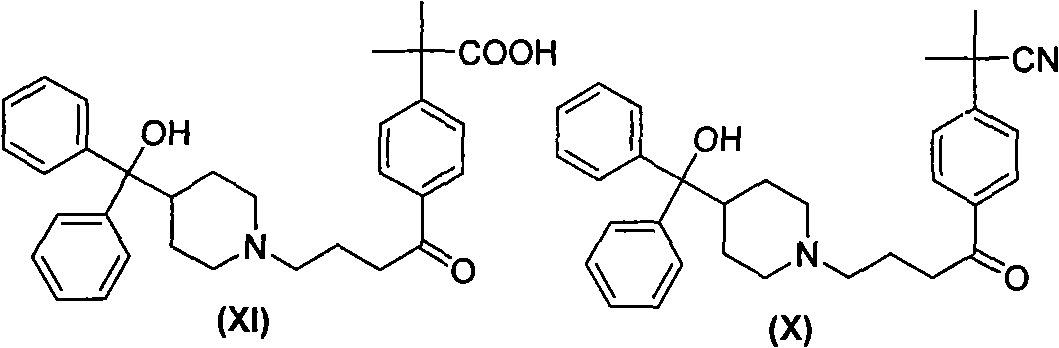
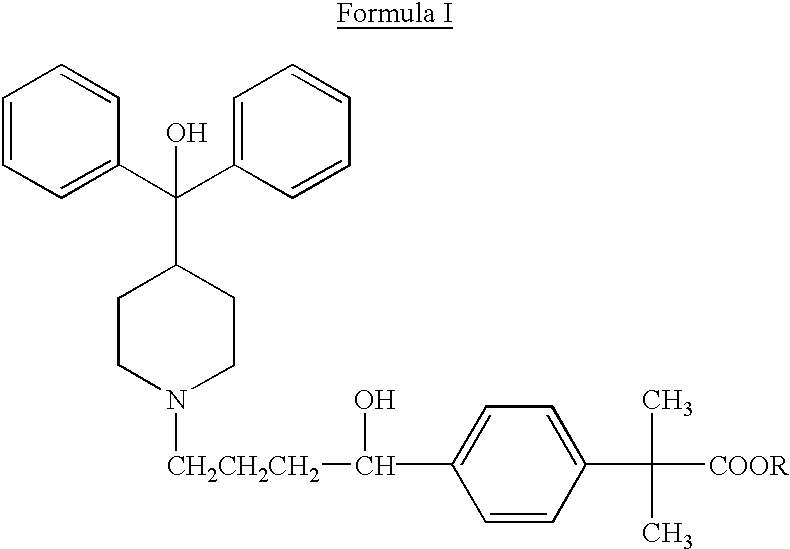
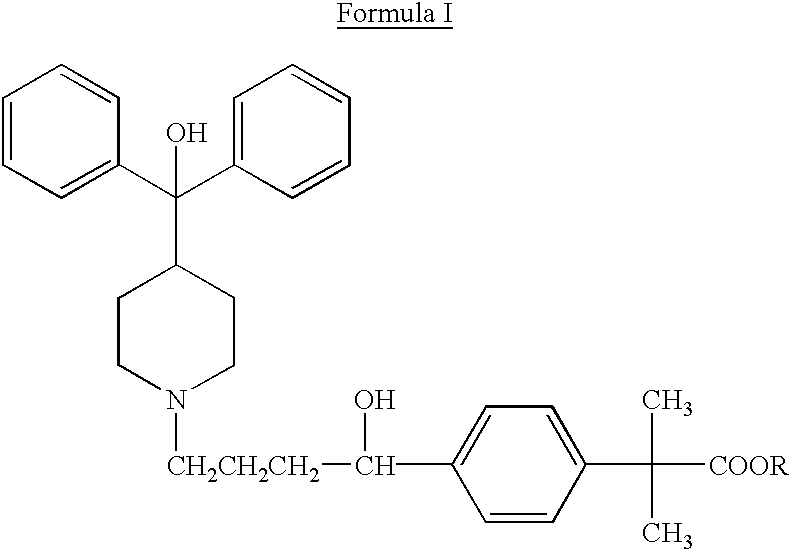
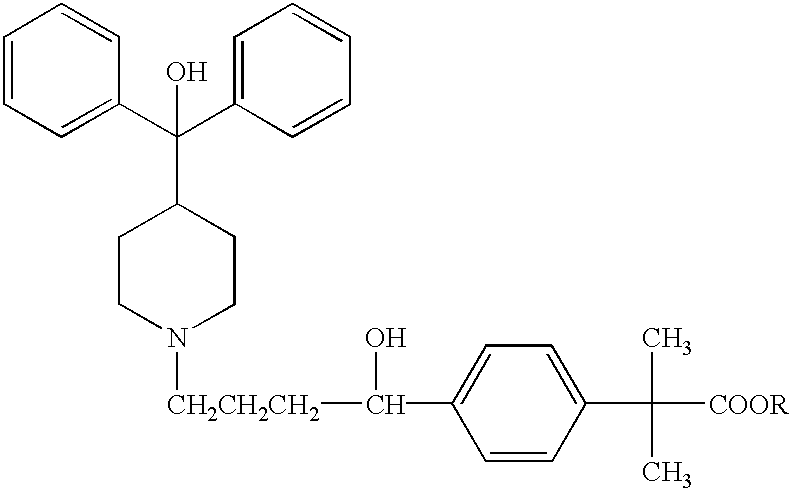
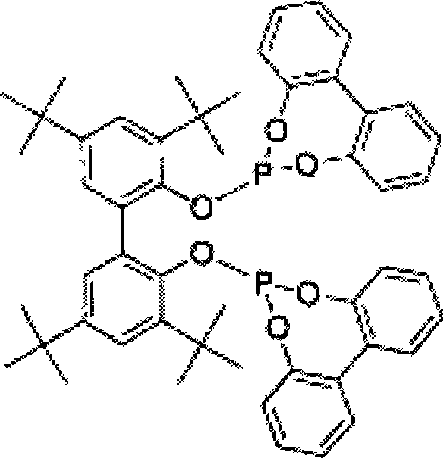
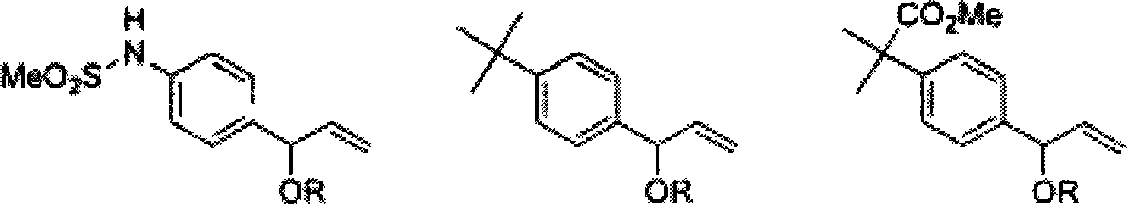
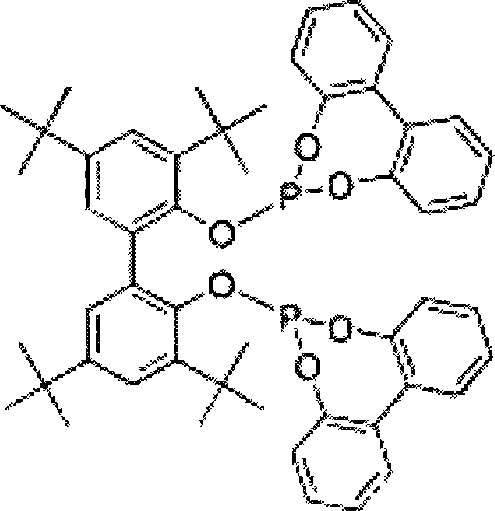
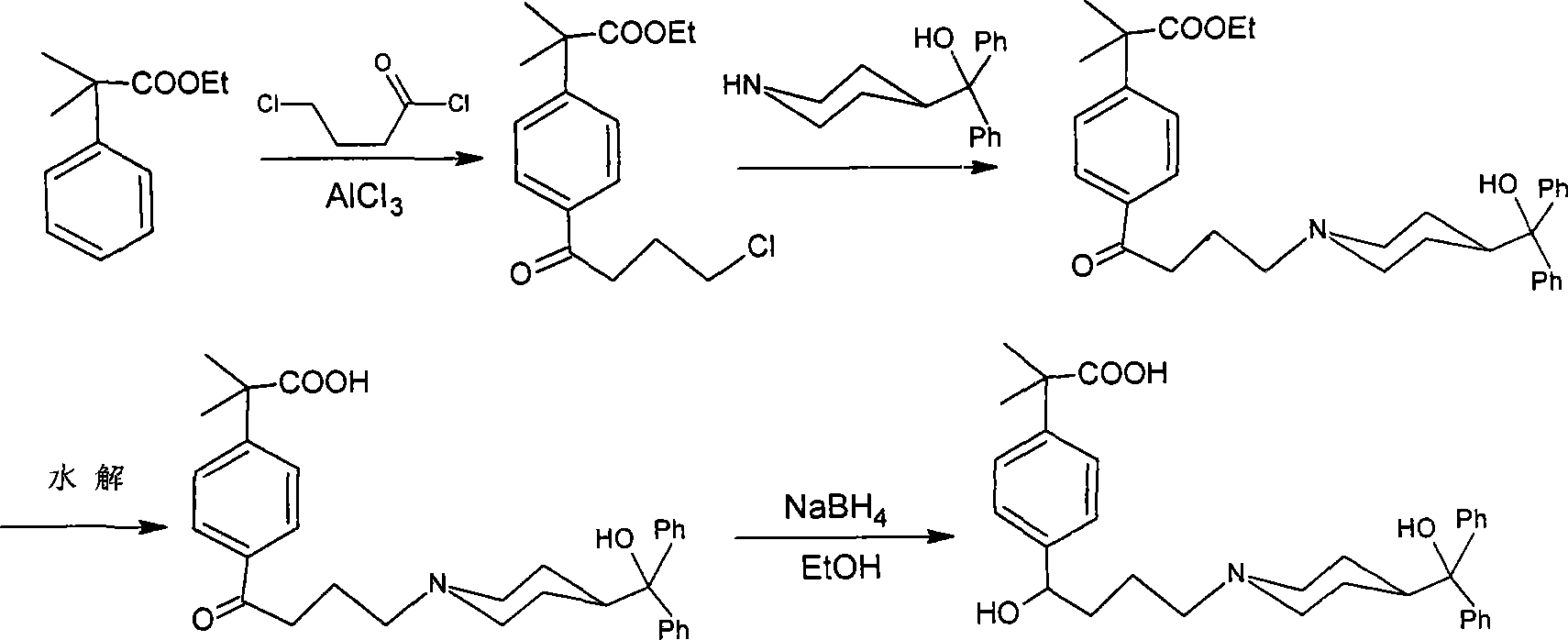
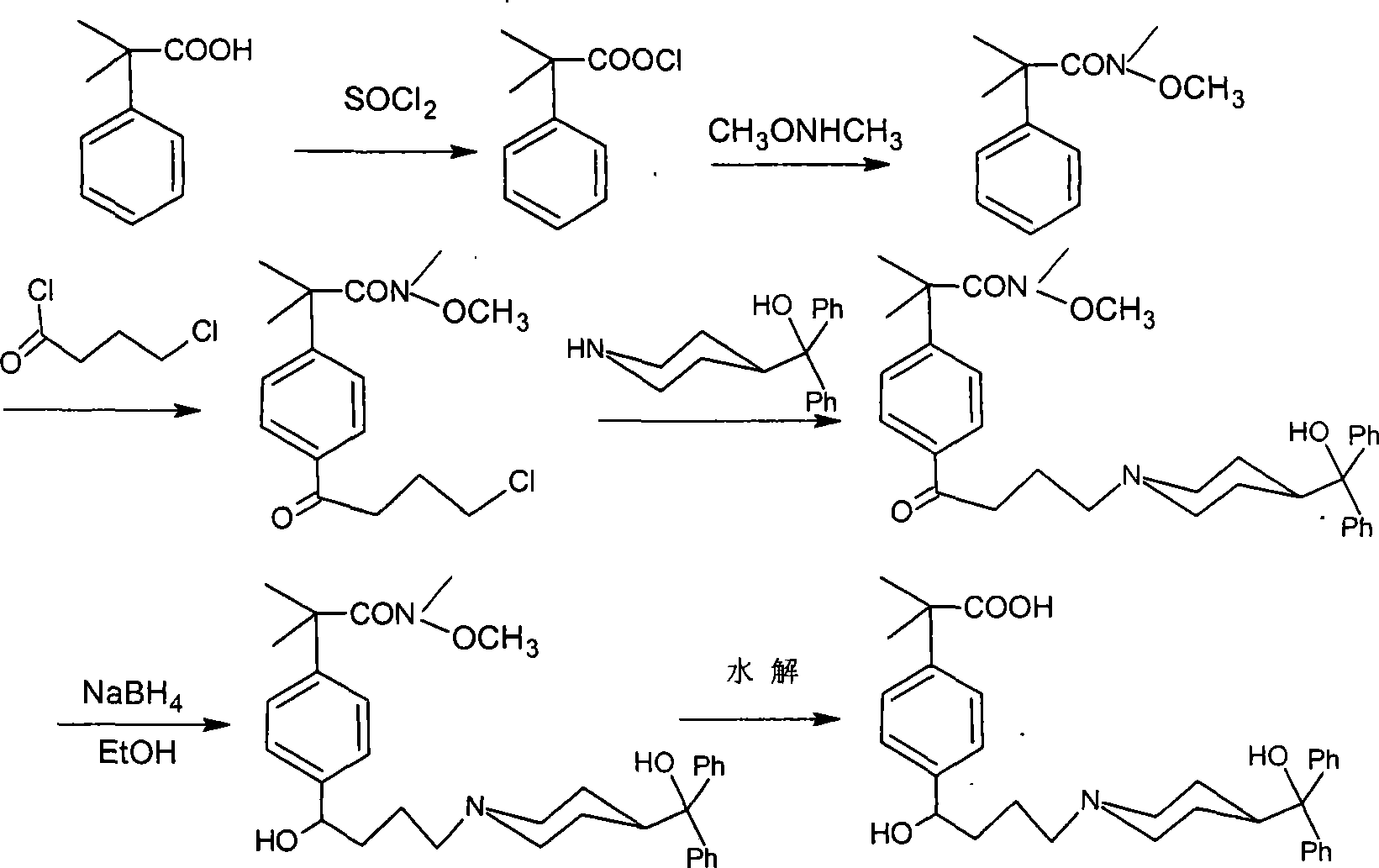
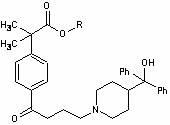
Process for the preparation of 4-[1-hydroxy -4-[4-(hydroxydiphenylmethly)-1-piperidinyl]-butyl]-alpha, alpha-dimethylbenzeneacetic acid
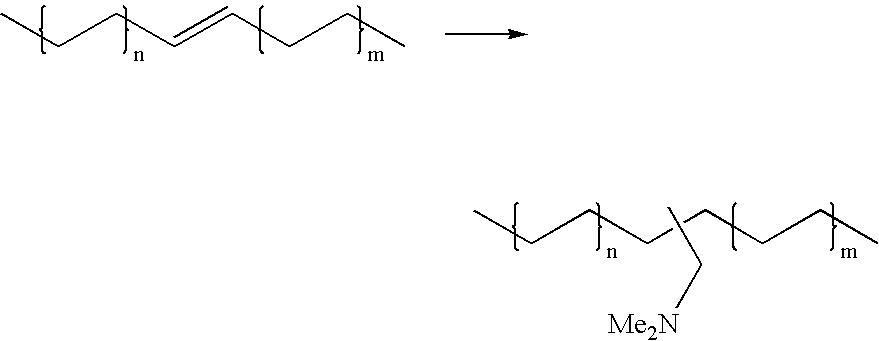
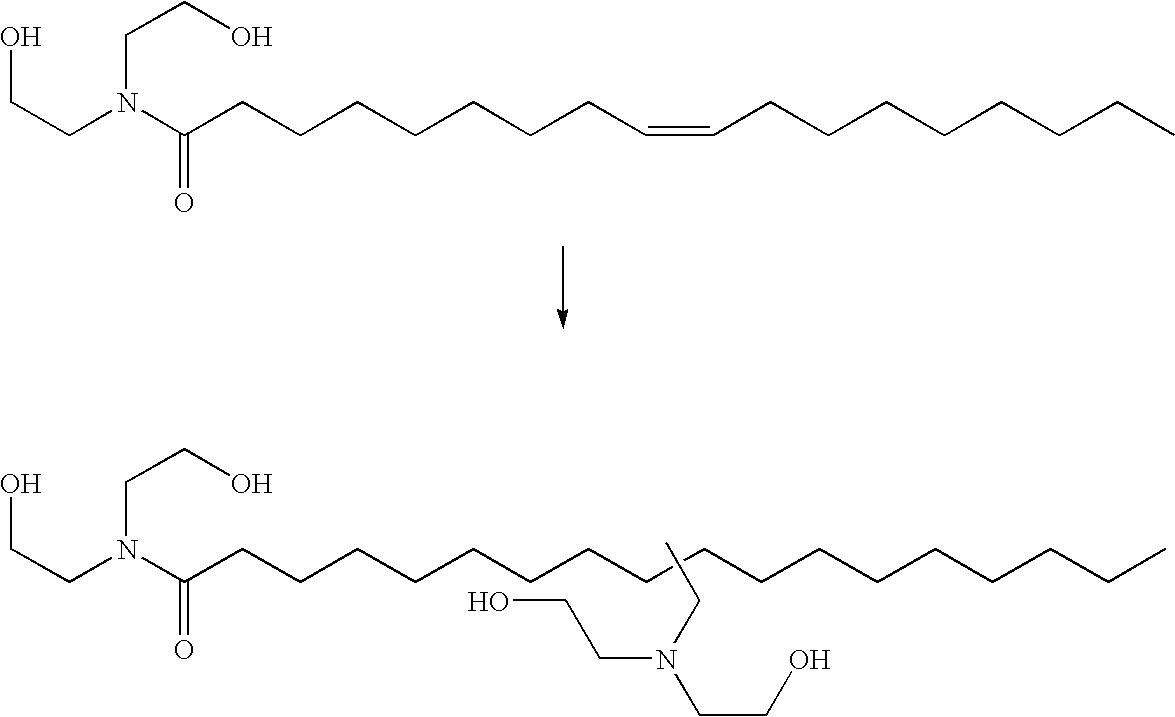
A process for the preparation of 4-[1-hydroxy-4-[4-(hydroxydiphenylmethyl)-1-piperidinyl]-butyl]-alpha,alpha-dimethylbenzeneacetic acid (Fexofenadine) of formula
Owner:DINAMITE DIPHARMA ABBREVIATED DIPHARMA
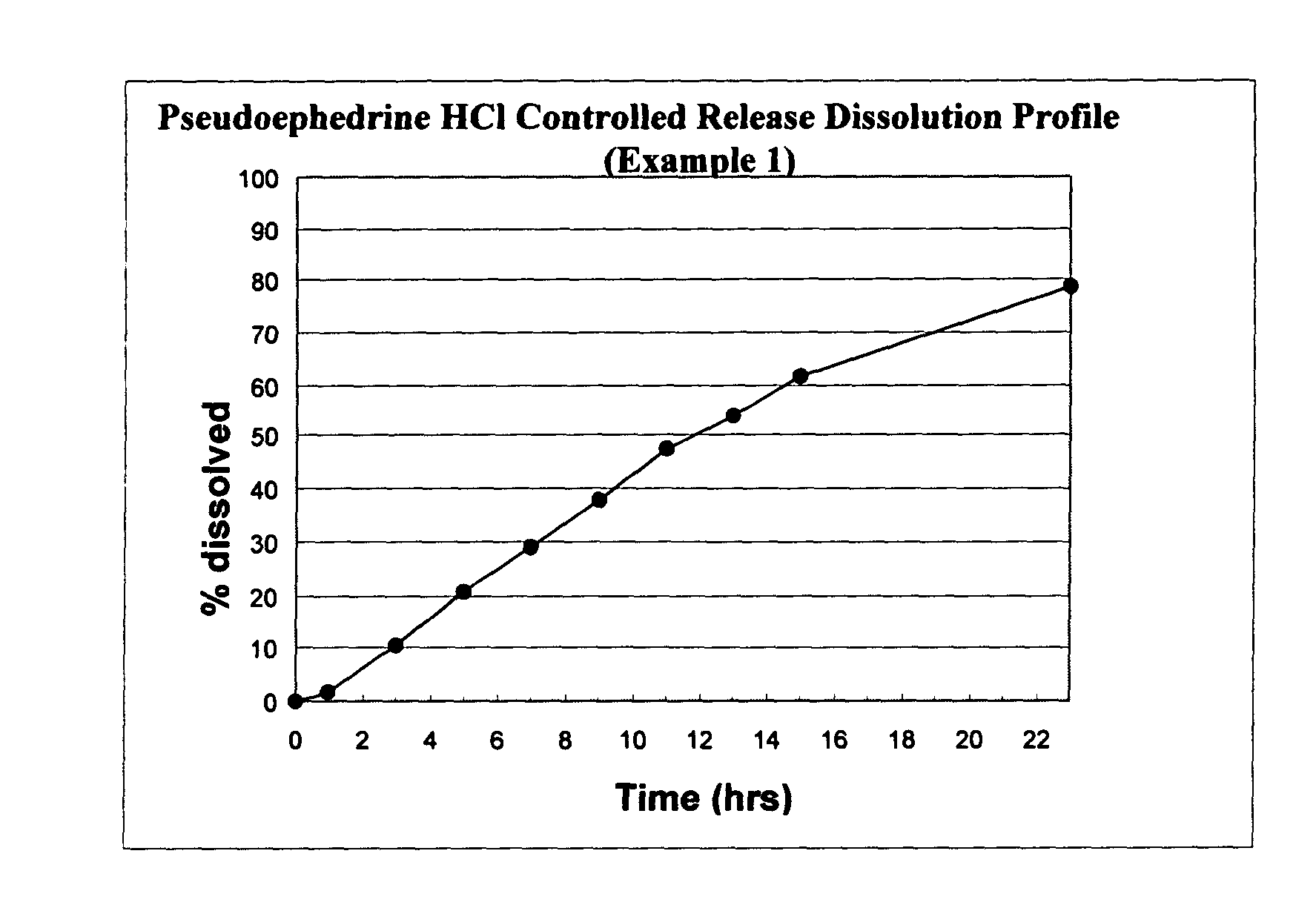
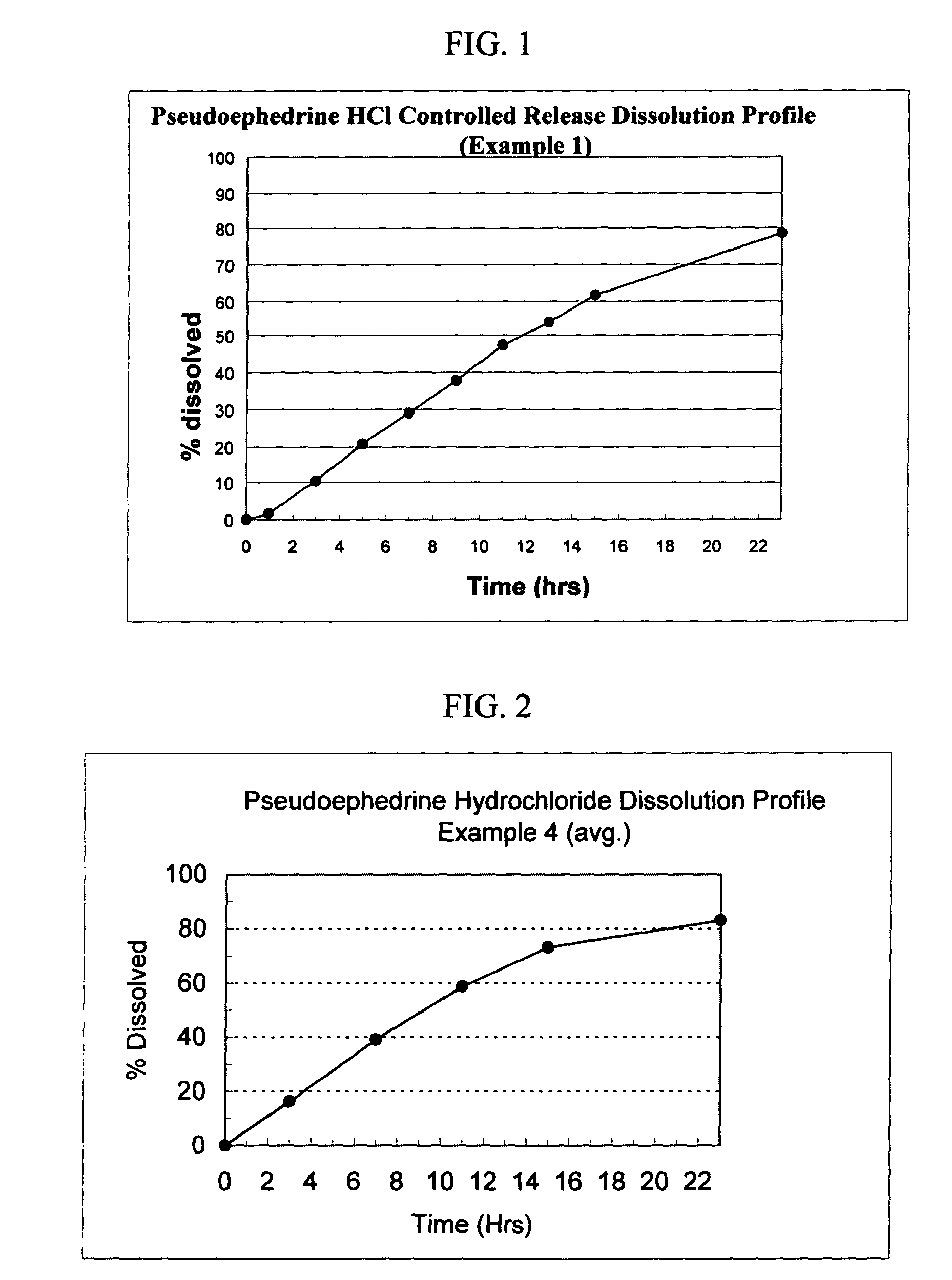
Osmotic device containing pseudoephedrine and an H1 antagonist
InactiveUS7147870B2Faster rateOvercome disadvantagesOsmotic deliveryDrageesDiseaseControlled release
The present invention provides an osmotic device containing controlled release pseudoephedrine in the core in combination with a rapid release H1 antagonist in an external coat. A wide range of H1 antagonist antihistamines, especially fexofenadine, can be used in this device. Particular embodiments of the invention provide osmotic devices having predetermined release profiles. One embodiment of the osmotic device includes an external coat that has been spray coated rather compression coated onto the device. The device with spray coated external core is smaller and easier to swallow than the similar device having a compression coated external coat. The device is useful for the treatment of respiratory congestion related disorders and allergy related disorders. The present devices provide PS and an H1 antagonist according to specific release profiles in combination with specific formulations.
Owner:OSMOTICA KERESKEDELMI & SZOLGALTATO
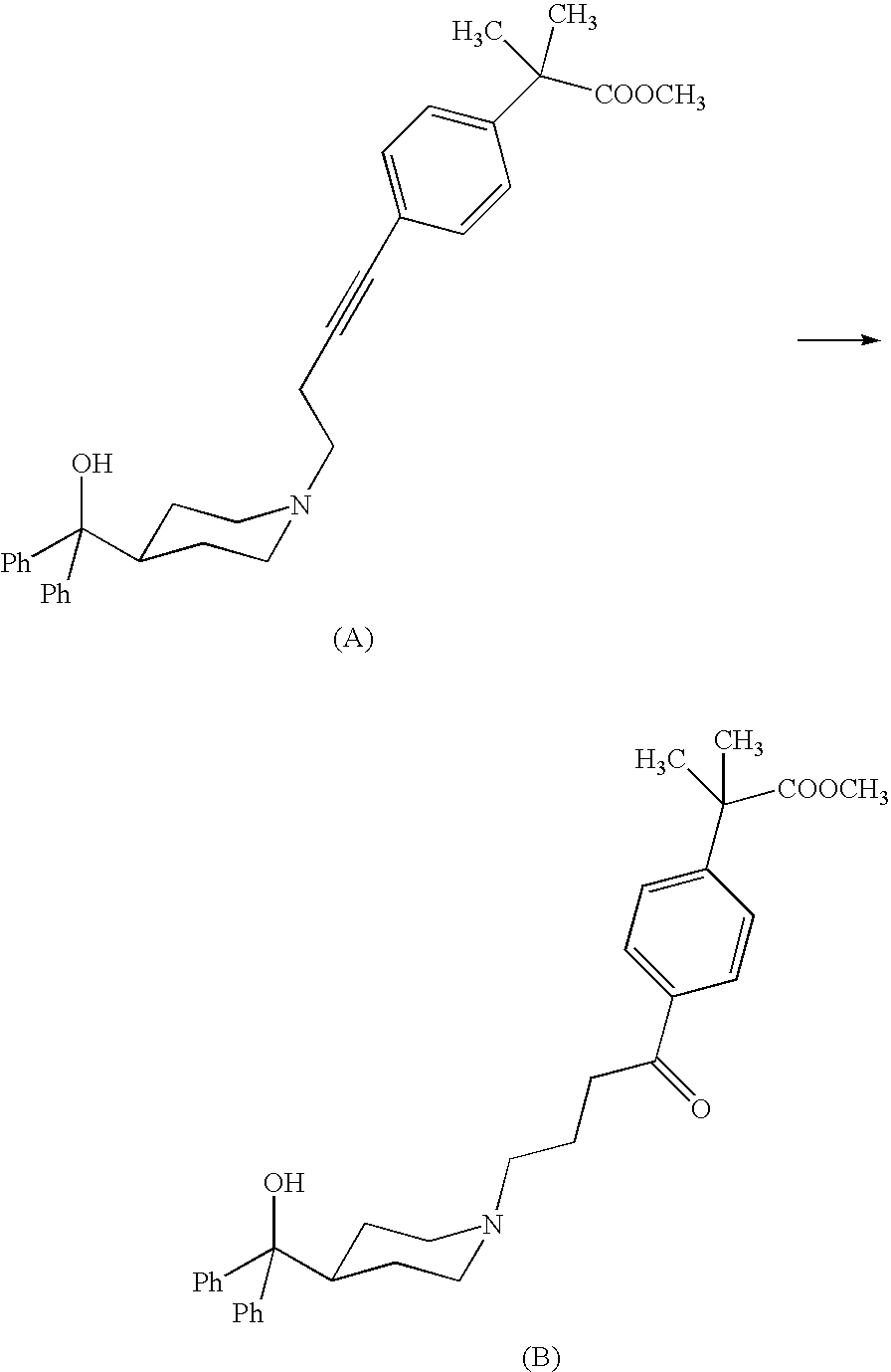
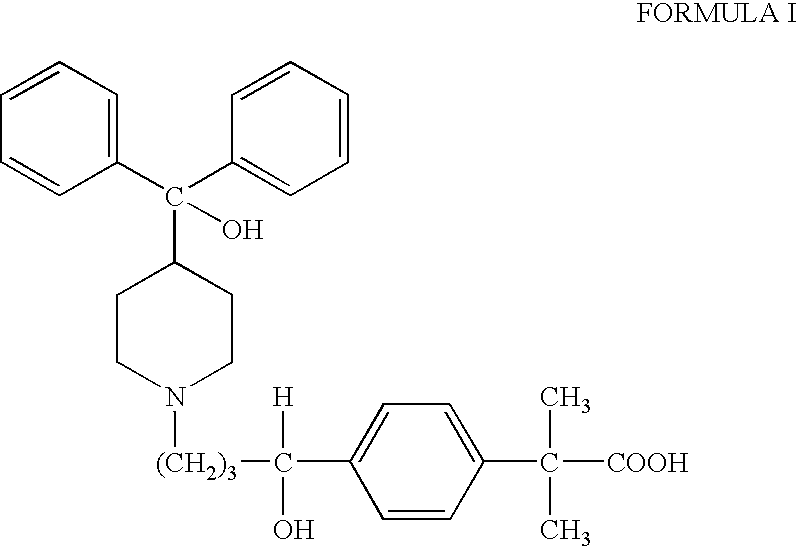
Process for the preparation of 4-[1-hydroxy-4-[4-(hydroxydiphenylmethly)-1-piperidinyl]-butyl]-alpha,alpha-dimethylbenzeneacetic acid
A process for the preparation of 4-[1-hydroxy-4-[4-(hydroxydiphenylmethyl)-1-piperidinyl]-butyl]-alpha,alpha-dimethylbenzeneacetic acid (Fexofenadine) of formula
Owner:DINAMITE DIPHARMA ABBREVIATED DIPHARMA
Process for the preparation of keto intermediates
InactiveUS20100228034A1Small formatYields obtained are surprisingly very highOrganic chemistryFexofenadineAlkyne
Process for the preparation of 4-[1-oxo-4-[4-(hydroxyphenylmethyl)-1-piperidinyl]butyl]-α,α-dimethylbenzenacetic acid, which is an intermediate useful in the preparation of fexofenadine, by hydrating asymmetric alkynes.
Owner:DIPHARMA FRANCIS
External-use formulation of fexofenadine
InactiveCN1813732AEffectively antagonizes sensitizationPromote efficacyAerosol deliveryOintment deliveryDiseaseFexofenadine
The present invention relates to a medicine fexofenadine preparation for external application. The pharmacological experiments show that said fexofenadine can obtain the obvious therapeutic effect for curing dermal allergic diseases.
Owner:LUNAN PHARMA GROUP CORPORATION
Process for the preparation of keto compounds
A process for the preparation of 4-[1-oxo-4-[4-(hydroxydiphenylmethyl)-1-piperidinyl]butyl]-α,α-dimethylbenzeneacetic acid, useful as an intermediate in the preparation of fexofenadine.
Owner:DIPHARMA SPA
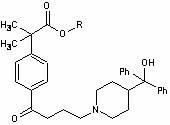
Pharmaceutical compositions of antihistamine and decongestant
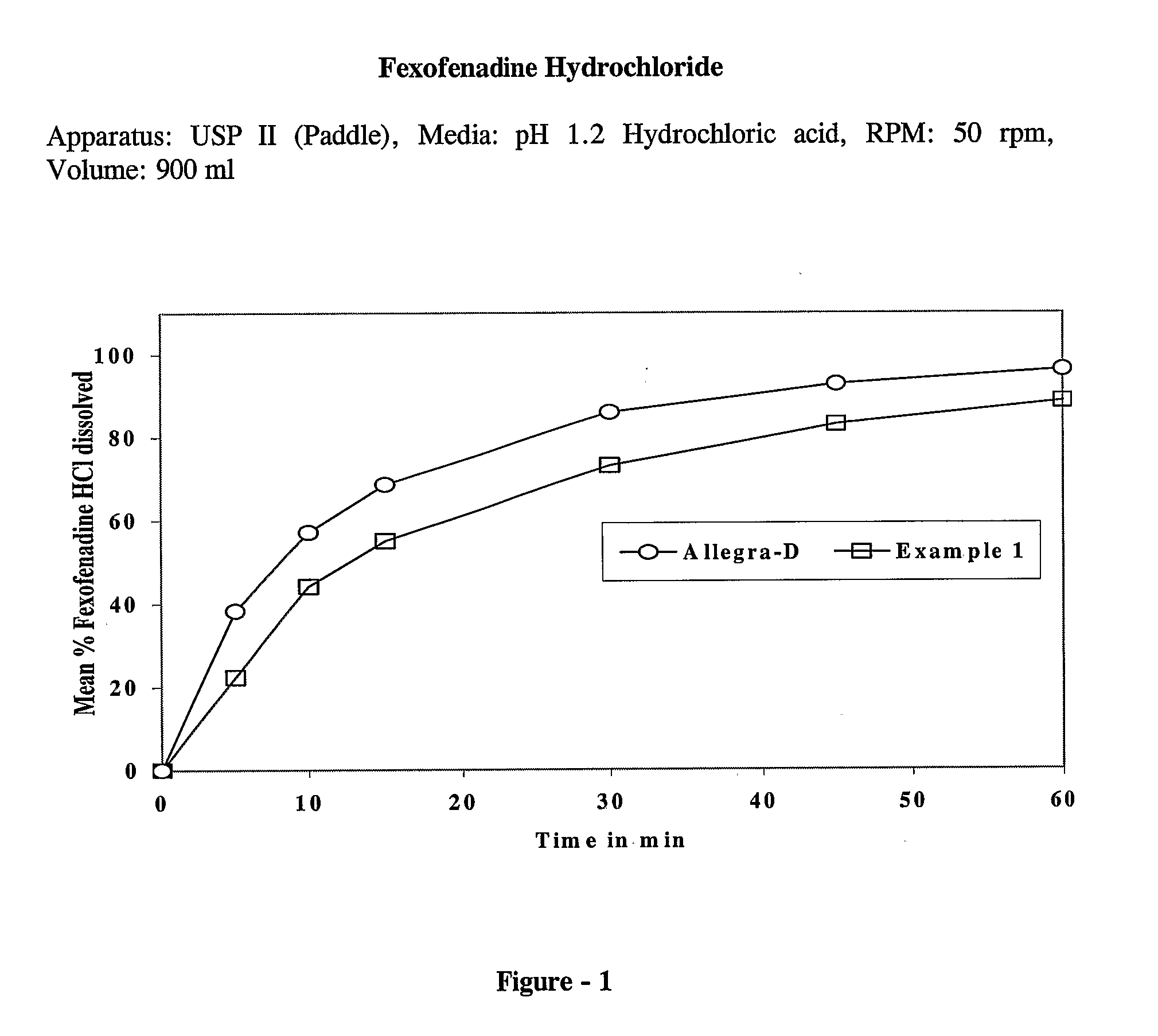
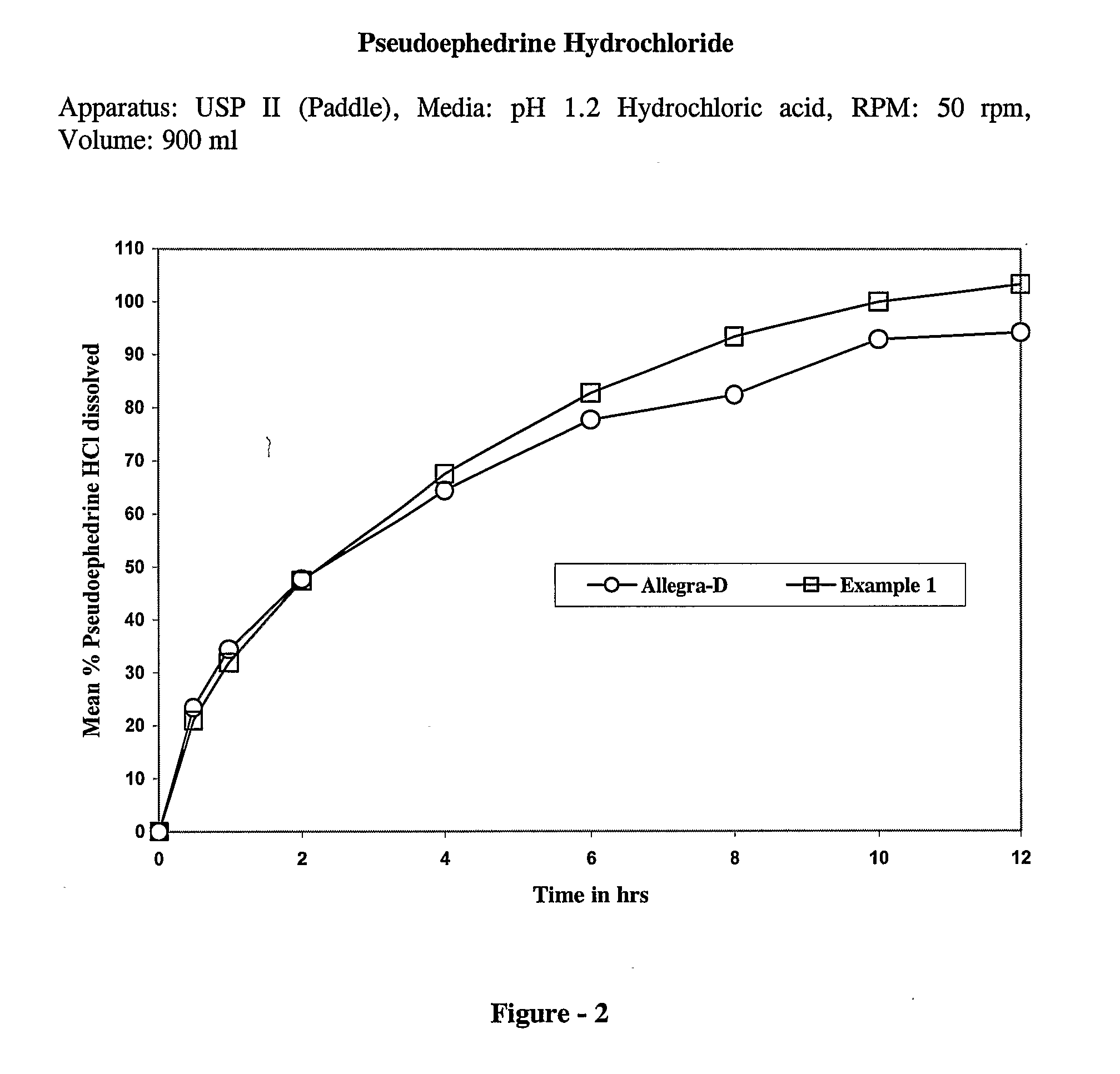
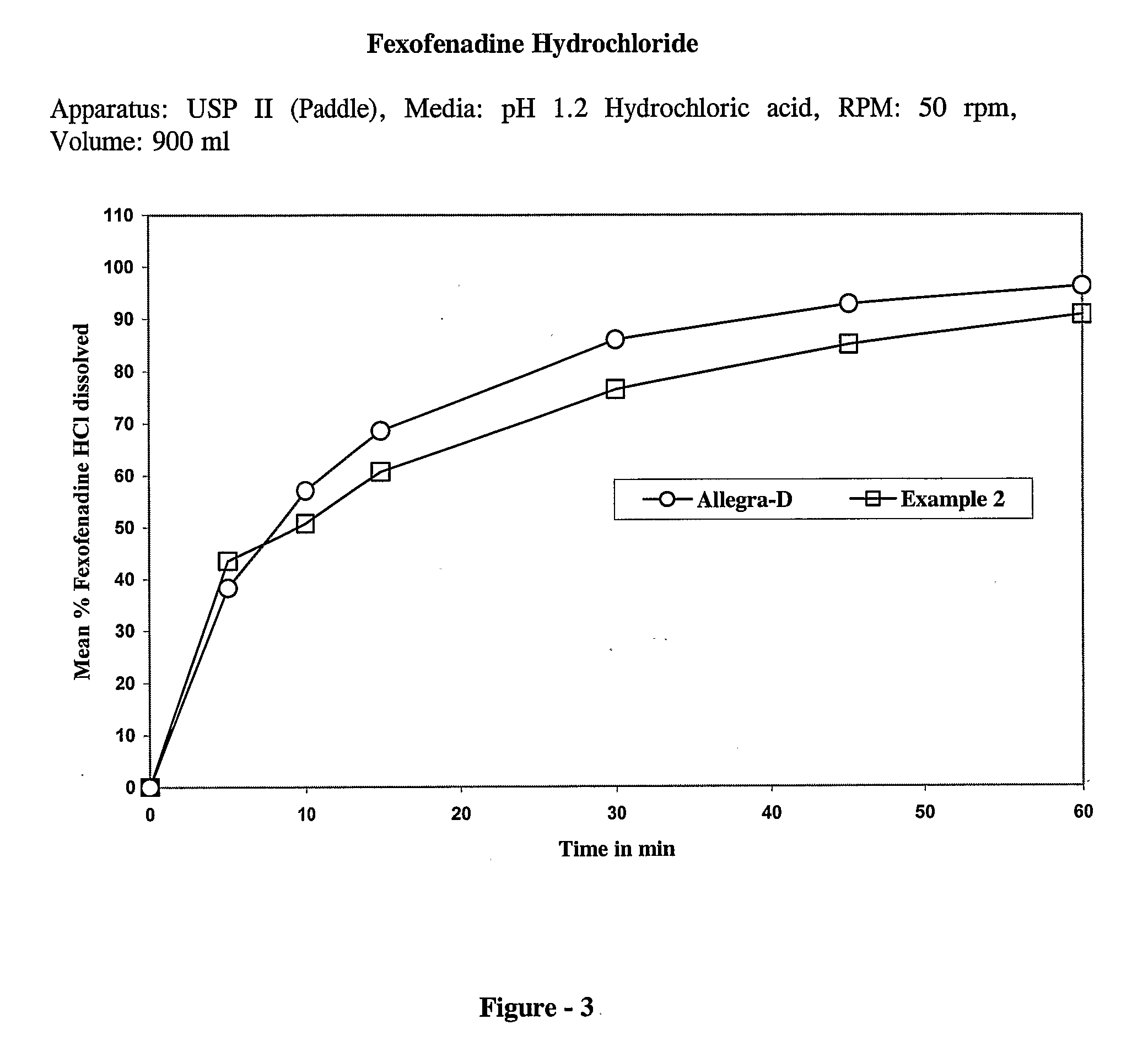
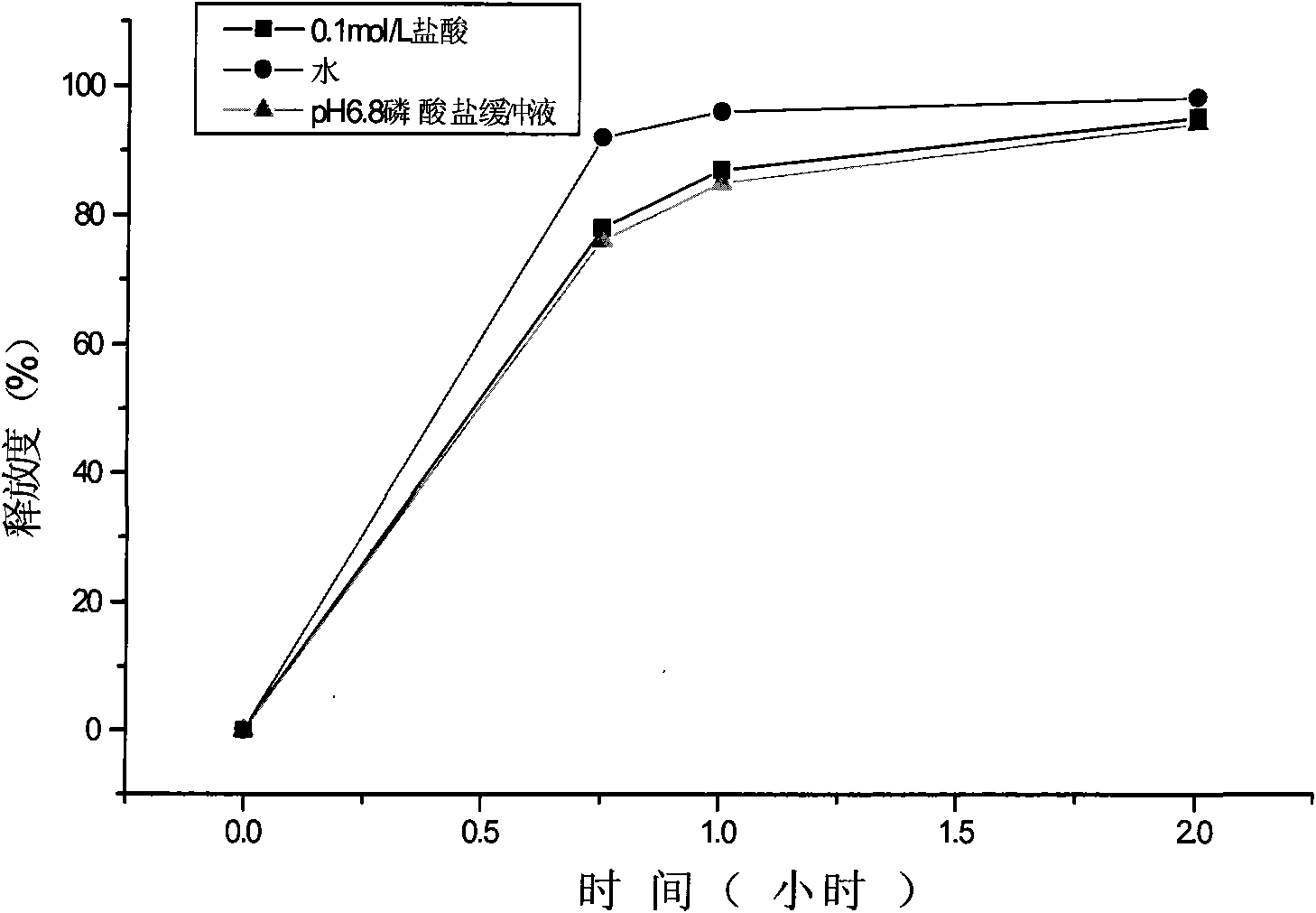
The present invention discloses a bilayer tablet composition comprising controlled or modified release decongestants such as pseudoephedrine or its salts or derivatives thereof with suitable pharmaceutical excipients with immediate release second layer comprising piperidinoalkanol compounds such as fexofenadine or its salts and derivatives thereof manufactured using just one functional excipient. The composition is useful particularly in the prophylaxis and treatment of allergic rhinitis.
Owner:MUKHERJI GOUR +2
Pharmaceutical composition, preparation method and application thereof
InactiveCN101919853AQuick effectExtended half-lifePharmaceutical delivery mechanismAntiinfectivesChronic urticariaBlood concentration
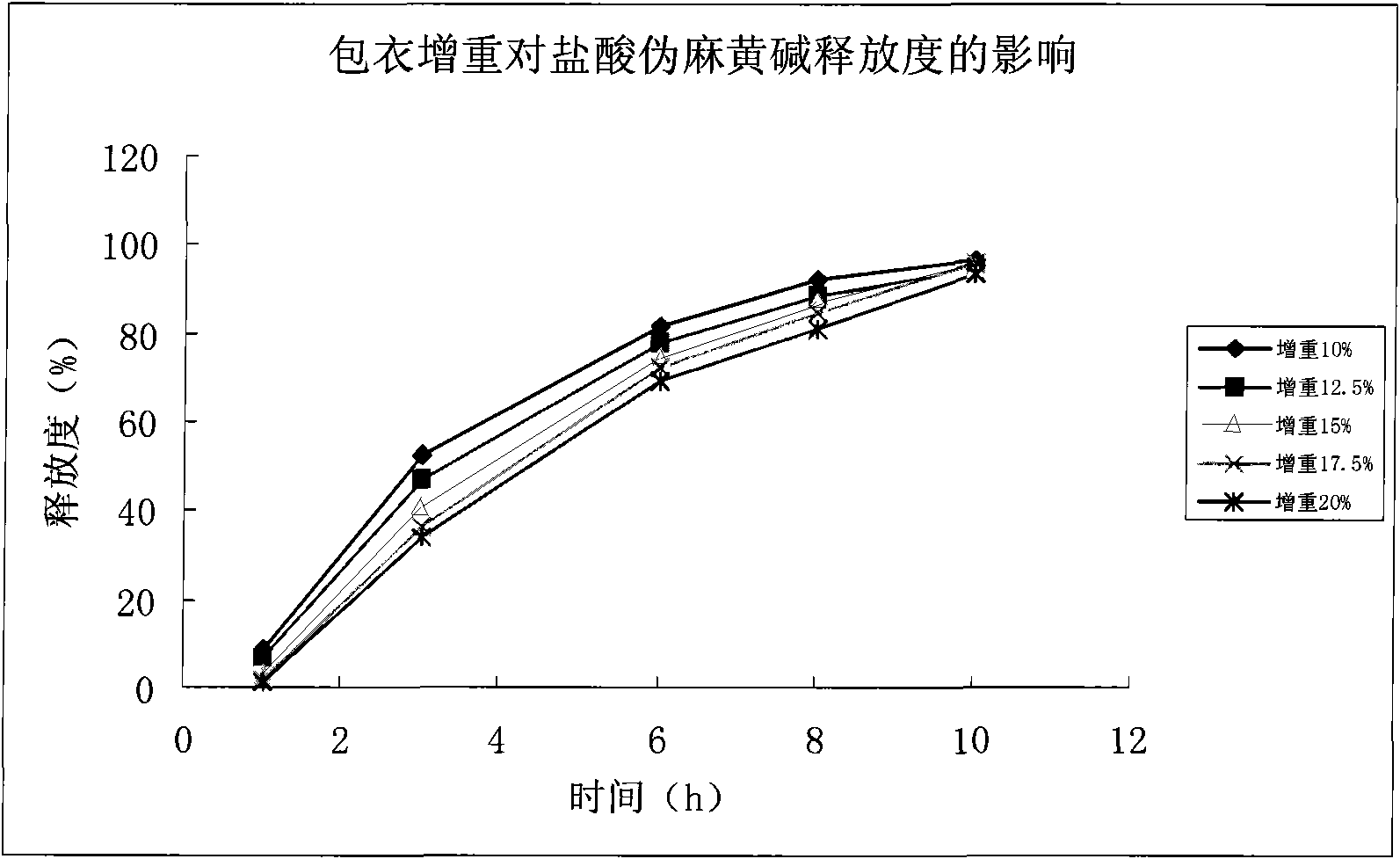
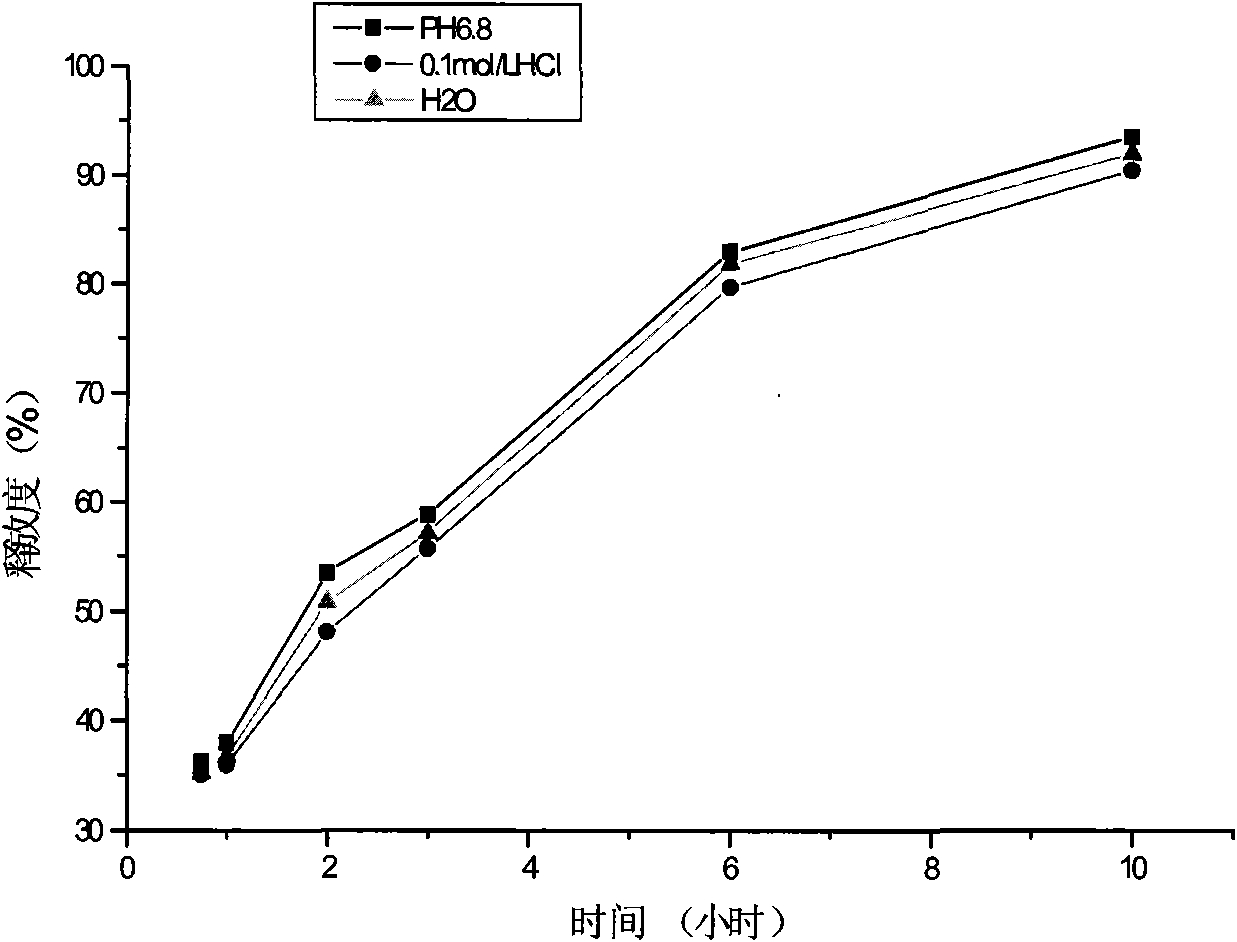
The invention relates to a pharmaceutical composition, a preparation method and application thereof. The invention provides a sustained release capsule which contains fexofenadine or pharmaceutically acceptable salt thereof and pseudo-ephedrine or pharmaceutically acceptable salt thereof, wherein the pseudo-ephedrine or the pharmaceutically acceptable salt thereof is a sustained release part, and the fexofenadine or the pharmaceutically acceptable salt thereof is an ordinary release part. The invention also provides the preparation method of the sustained release capsule and the application of the capsule in preparing drugs for treating allergic rhinitis, chronic urticaria and influenza. Compared with tablets, the sustained release capsule is easy to be absorbed, and has the advantages of rapid action, smaller individual difference of blood concentration, less irritation on gastrointestinal tracts, less possibility of drug collapse, good safety and larger dose flexibility.
Owner:SINOPHARM GUANGDONG GLOBAL PHARMA CO LTD +1
Methods and compositions to inhibit dependence on opioids
ActiveUS10675261B2Nervous disorderPharmaceutical delivery mechanismFexofenadineNon steroid anti inflammatory drug
The present invention provides a method of inhibiting dependence to an opioid by a human subject in need thereof. The method comprises administering an effective amount of a pharmaceutical composition to the subject during opioid therapy. The pharmaceutical composition comprises a) a non-steroidal anti-inflammatory drug (NSAID); and b) a co-agent selected from the group consisting of: fexofenadine, ketotifen, desloratadine, cetirizine, salts thereof and combinations thereof.
Owner:SEN JAM PHARMA LLC
Process for the preparation of keto compounds
InactiveUS8236961B2Obtained is highMercury content in wastes is remarkably reducedOrganic chemistryCompound aFexofenadine
A process for the preparation of 4-[1-oxo-4-[4-(hydroxydiphenylmethyl)-1-piperidinyl]butyl]-α,α-dimethylbenzeneacetic acid, useful as an intermediate for the preparation of fexofenadine, is provided.
Owner:DIPHARMA FRANCIS
Methods and compositions to inhibit tolerance to opioids
ActiveUS11129803B2Nervous disorderAnhydride/acid/halide active ingredientsFexofenadineNon steroid anti inflammatory drug
The present invention provides a method of inhibiting tolerance to an opioid by a human subject in need thereof. The method comprises administering an effective amount of a pharmaceutical composition to the subject during opioid therapy. The pharmaceutical composition comprises a) a non-steroidal anti-inflammatory drug (NSAID); and b) a co-agent selected from the group consisting of: fexofenadine, ketotifen, desloratadine, cetirizine, salts thereof and combinations thereof.
Owner:SEN JAM PHARMA LLC
Process for the preparation of fexofenadine
The invention relates to highly pure fexofenadine and a process for preparing highly pure fexofenadine. The invention also relates to pharmaceutical compositions that include the highly pure fexofenadine and use of said compositions for treating a patient for allergic reactions.
Owner:RANBAXY LAB LTD
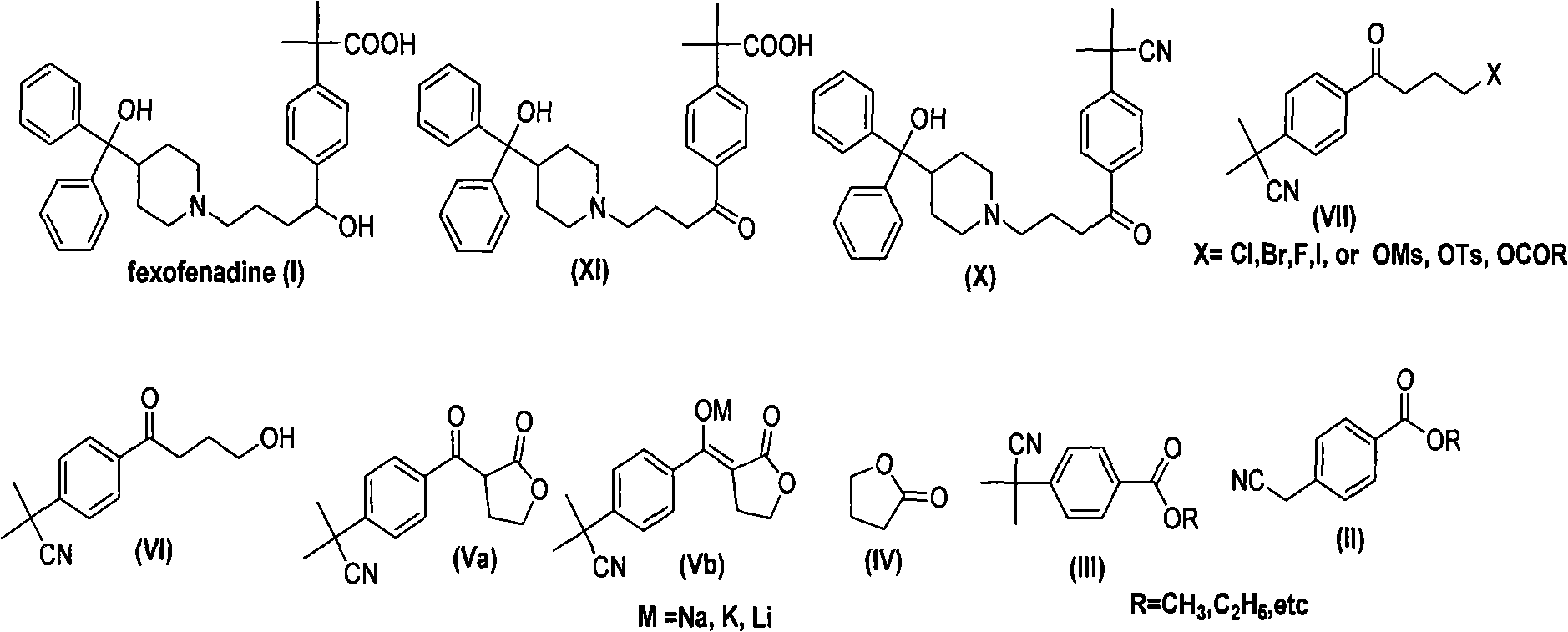
Synthesizing route and preparation method of high-purity fexofenadine and intermediate thereof
ActiveCN102070512AReduce total usageRaw materials are easy to getCarboxylic acid nitrile preparationOrganic compound preparationFexofenadinePara position
The invention relates to a preparation method of high-purity fexofenadine (chemical name (+ / -)-4-[1-hydroxyl-4-[4-(hydroxyldiphenylmethyl)-1-piperidyl]butyl]-alpha, alpha-dimethylphenylacetic acid) and an intermediate thereof and a novel synthesizing route. The method comprises the following steps of: with p-cyanomethylbenzoate (II) as a raw material, and with a new compound (Va) or salt (Vb) thereof as a key intermediate, hydrolyzing, esterifying or halogenating to obtain a compound (VII); and condensing, hydrolyzing and reducing with a piperdinol compound (VIII) to obtain a high-purity fexofenadine type compound (I) without para-position substitution. The invention has the advantages of simple reaction, convenience for postprocessing, higher yield and high purity of generated products and is a more ideal preparation method of fexofenadine.
Owner:ZHEJIANG HUAHAI PHARMACEUTICAL CO LTD +1
External preparation of Fexofenadine
ActiveCN1957941AAntagonism of sensitizationIncreased free drug concentrationAerosol deliveryOintment deliveryFexofenadineWhole body
An exterior-applied Feisuofeinading for preventing and treating the anaphylaxia and inflammation without untoward effect is disclosed.
Owner:LUNAN PHARMA GROUP CORPORATION
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
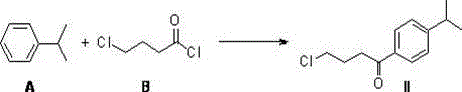
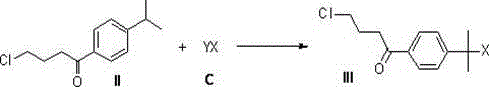
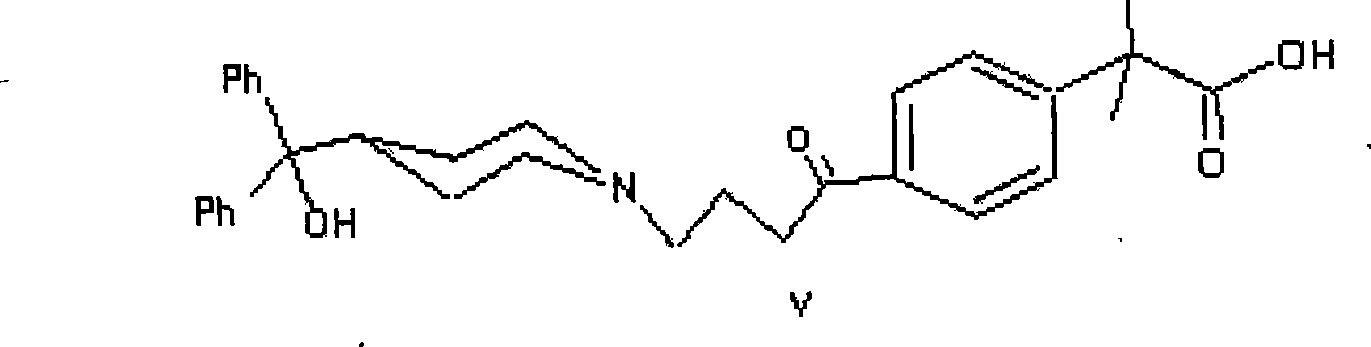
![Process for the preparation of 4-[1-hydroxy -4-[4-(hydroxydiphenylmethly)-1-piperidinyl]-butyl]-alpha, alpha-dimethylbenzeneacetic acid Process for the preparation of 4-[1-hydroxy -4-[4-(hydroxydiphenylmethly)-1-piperidinyl]-butyl]-alpha, alpha-dimethylbenzeneacetic acid](https://images-eureka.patsnap.com/patent_img/2b1ccb8d-4ca8-4330-9167-01776e0a37e2/US06815549-20041109-C00001.png)
![Process for the preparation of 4-[1-hydroxy -4-[4-(hydroxydiphenylmethly)-1-piperidinyl]-butyl]-alpha, alpha-dimethylbenzeneacetic acid Process for the preparation of 4-[1-hydroxy -4-[4-(hydroxydiphenylmethly)-1-piperidinyl]-butyl]-alpha, alpha-dimethylbenzeneacetic acid](https://images-eureka.patsnap.com/patent_img/2b1ccb8d-4ca8-4330-9167-01776e0a37e2/US06815549-20041109-C00002.png)
![Process for the preparation of 4-[1-hydroxy -4-[4-(hydroxydiphenylmethly)-1-piperidinyl]-butyl]-alpha, alpha-dimethylbenzeneacetic acid Process for the preparation of 4-[1-hydroxy -4-[4-(hydroxydiphenylmethly)-1-piperidinyl]-butyl]-alpha, alpha-dimethylbenzeneacetic acid](https://images-eureka.patsnap.com/patent_img/2b1ccb8d-4ca8-4330-9167-01776e0a37e2/US06815549-20041109-C00003.png)
![Process for the preparation of 4-[1-hydroxy-4-[4-(hydroxydiphenylmethly)-1-piperidinyl]-butyl]-alpha,alpha-dimethylbenzeneacetic acid Process for the preparation of 4-[1-hydroxy-4-[4-(hydroxydiphenylmethly)-1-piperidinyl]-butyl]-alpha,alpha-dimethylbenzeneacetic acid](https://images-eureka.patsnap.com/patent_img/05ec8953-52fc-4580-83af-226a598feb47/US20020198233A1-20021226-C00001.png)
![Process for the preparation of 4-[1-hydroxy-4-[4-(hydroxydiphenylmethly)-1-piperidinyl]-butyl]-alpha,alpha-dimethylbenzeneacetic acid Process for the preparation of 4-[1-hydroxy-4-[4-(hydroxydiphenylmethly)-1-piperidinyl]-butyl]-alpha,alpha-dimethylbenzeneacetic acid](https://images-eureka.patsnap.com/patent_img/05ec8953-52fc-4580-83af-226a598feb47/US20020198233A1-20021226-C00002.png)
![Process for the preparation of 4-[1-hydroxy-4-[4-(hydroxydiphenylmethly)-1-piperidinyl]-butyl]-alpha,alpha-dimethylbenzeneacetic acid Process for the preparation of 4-[1-hydroxy-4-[4-(hydroxydiphenylmethly)-1-piperidinyl]-butyl]-alpha,alpha-dimethylbenzeneacetic acid](https://images-eureka.patsnap.com/patent_img/05ec8953-52fc-4580-83af-226a598feb47/US20020198233A1-20021226-C00003.png)