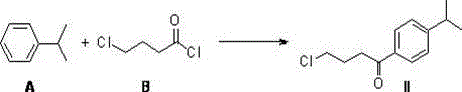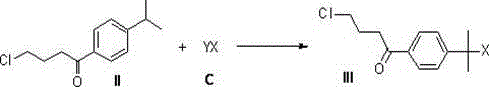Novel synthetic method of high-purity fexofenadine and intermediate
A fexofenadine, high-purity technology, applied in the field of chemical pharmacy, can solve the problems of difficulty in obtaining high-purity fexofenadine, affecting the quality of finished products, and high cost of raw materials, achieving mild conditions, convenient operation, and simple operation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
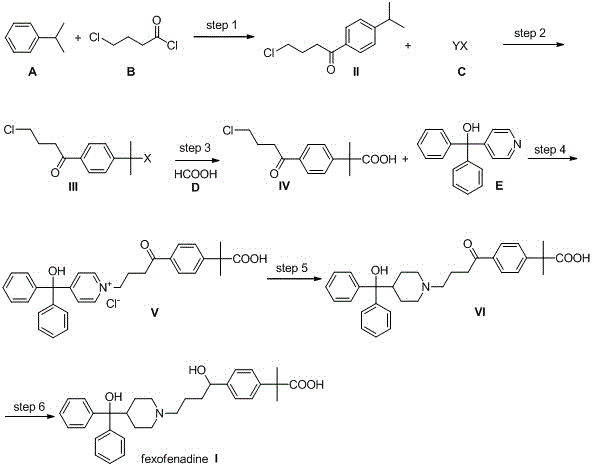
[0056] (1) Add 166.7 g (1.25 mol) of aluminum trichloride and 450 ml of dichloromethane to a 2-liter three-necked flask equipped with a mechanical stirrer and under the protection of nitrogen. After cooling to 0-5°C, add 140 ml dropwise (1.25mol) 4-Chlorobutyryl chloride (B), add dropwise within 30 minutes. Then 143g (1.19mol) cumene (A) was added dropwise within 40 minutes, keeping the internal temperature at 5-10°C. After continuing the reaction for 30 minutes, the reaction solution was carefully poured into 1 kg of ice water, slowly returned to room temperature under stirring, and the phases were separated, and the aqueous phase was extracted with 400 ml of dichloromethane. Combine the organic phases and wash once with 500 ml saturated sodium bicarbonate. Revolve the dichloromethane below 30℃ to obtain 280 g of yellow oil, crystallize it with a mixed solvent of isopropanol and water at -10℃, filter, wash the filter cake with isopropanol and water, and dry under vacuum to ob...
Embodiment 2
[0063] The 4-(4-chlorobutyryl)-α,α-dimethylbenzyl bromide (III) prepared in Example 1 above was used as the raw material. Add 300 ml of concentrated sulfuric acid, 60 g (0.2 mol) of raw material (III) into a 1L three-necked flask equipped with a mechanical stirrer and nitrogen protection, and then slowly add 55 g of anhydrous formic acid (D) dropwise at 20°C. Add time for about 3 hours and continue to react at room temperature for 2 hours. The reaction solution was slowly poured into 3L ice water, adjusted to pH 2.5 with 20% sodium hydroxide, extracted twice with 500 ml of toluene, combined the organic phases and washed once with 300 ml of saturated brine, dried with anhydrous sodium sulfate, filtered, and the filtrate was rotary evaporated Most of the toluene was distilled off, leaving about 300 ml of toluene solution, cooled and crystallized, filtered, the filter cake was washed with a small amount of cold toluene, and dried under vacuum to obtain 45 g of compound (IV) with a...
Embodiment 3
[0065] The 4-(4-chlorobutyryl)-α,α-dimethylbenzyl bromide (III) prepared in Example 1 above was used as the raw material. Add 300 ml of concentrated sulfuric acid into a 1L three-necked flask equipped with a mechanical stirrer and under the protection of nitrogen, and then slowly and dropwise add 55 g of anhydrous formic acid (D) and 60 g (0.2 mol) of raw material (III) at 20°C. 200 ml of carbon tetrachloride solution, dripped for about 3 hours, and continued to react at room temperature for 2 hours. The reaction solution was slowly poured into 3L ice water, adjusted to pH 2.5 with 20% sodium hydroxide, extracted twice with 500 ml of toluene, combined the organic phases and washed once with 300 ml of saturated brine, dried with anhydrous sodium sulfate, filtered, and the filtrate was rotary evaporated Distill out most of the toluene and carbon tetrachloride, leaving about 300 ml of mixed solution, cooling and crystallization, filtration, the filter cake is washed with a small a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com