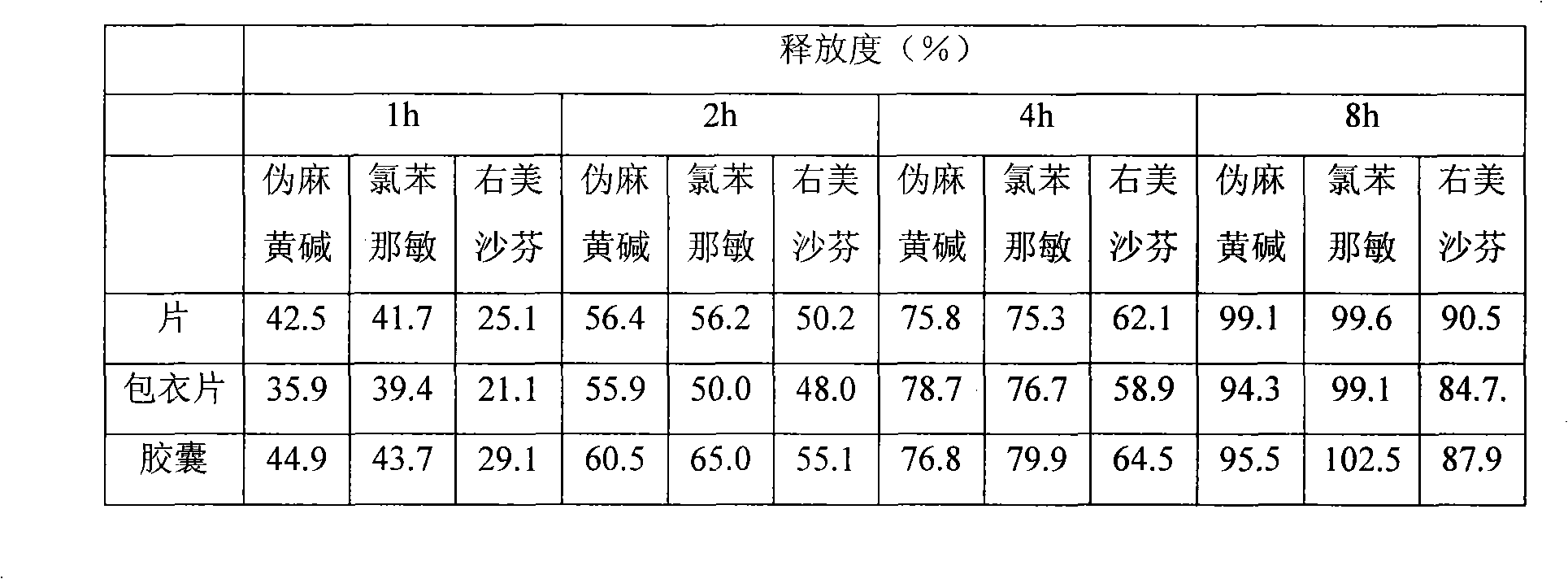
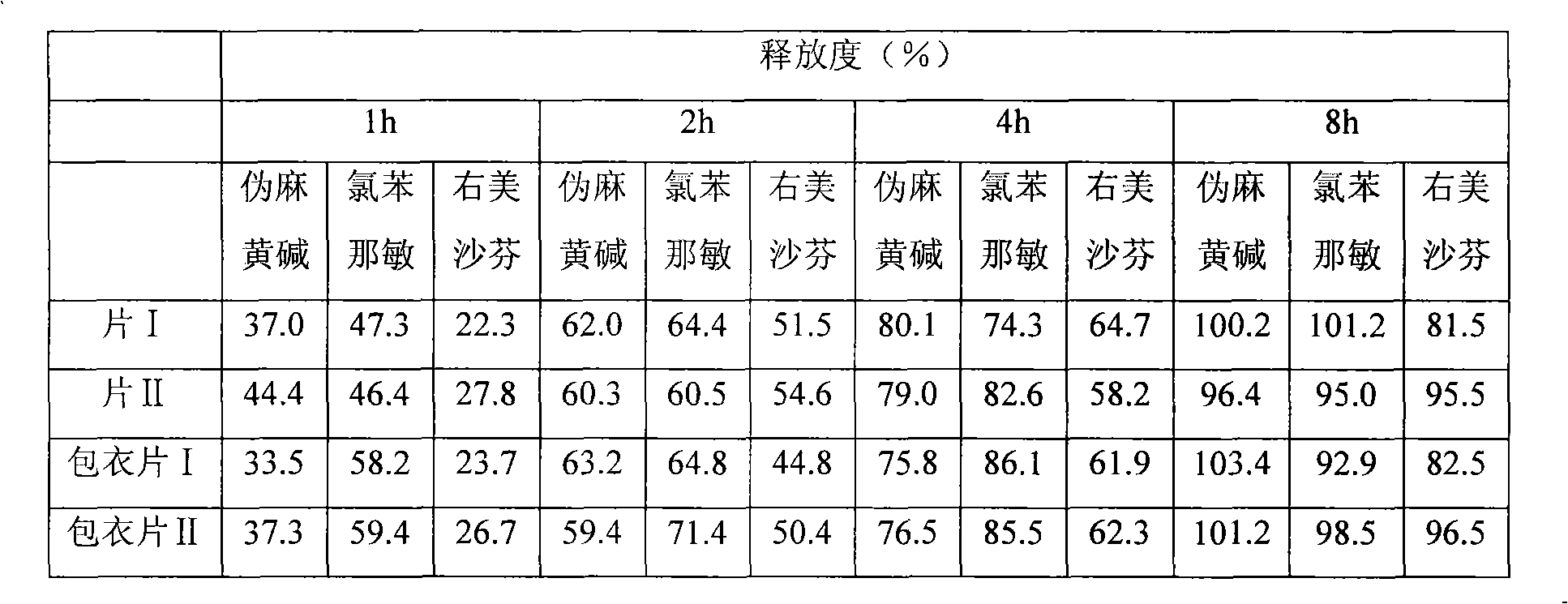
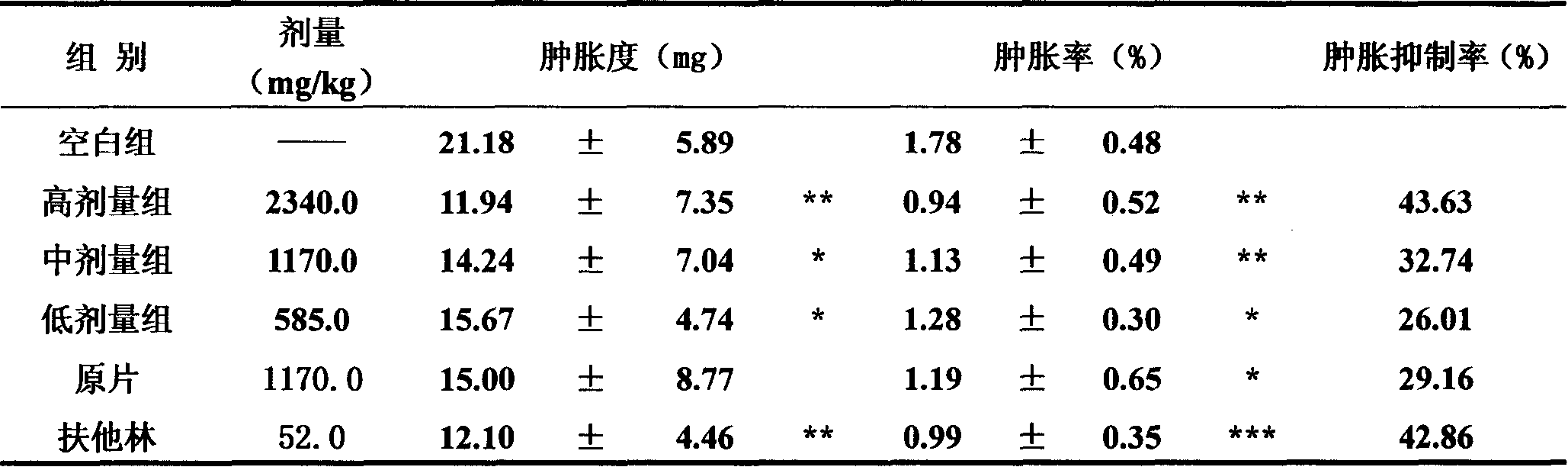
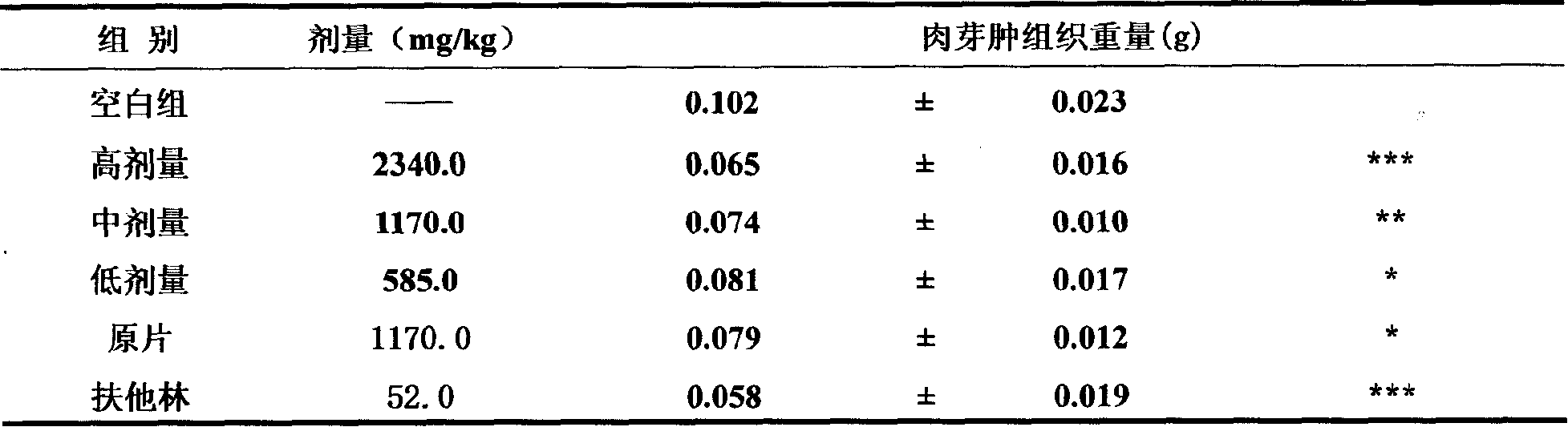
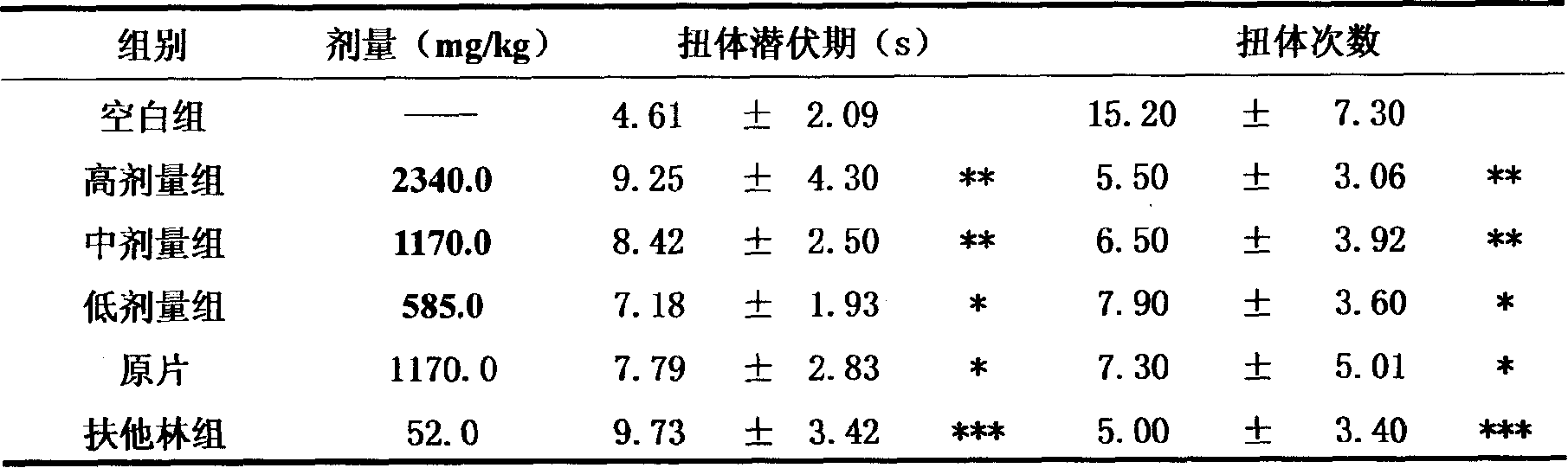
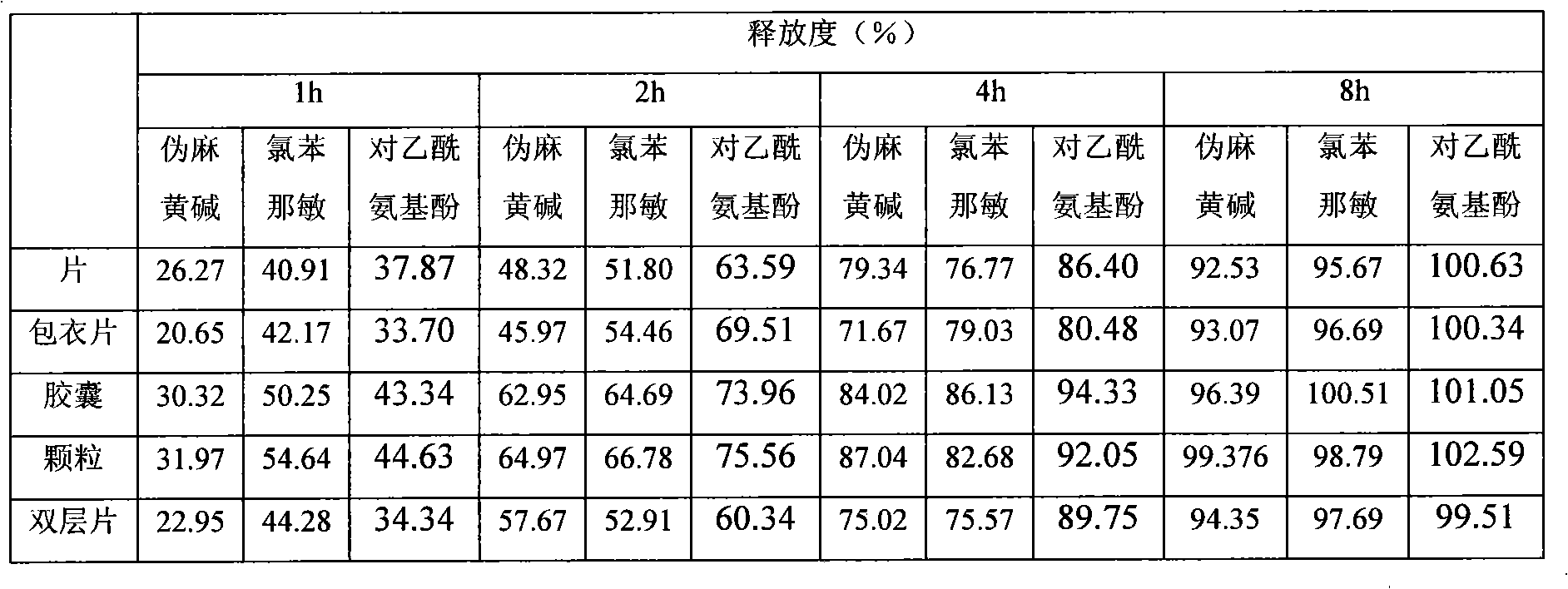
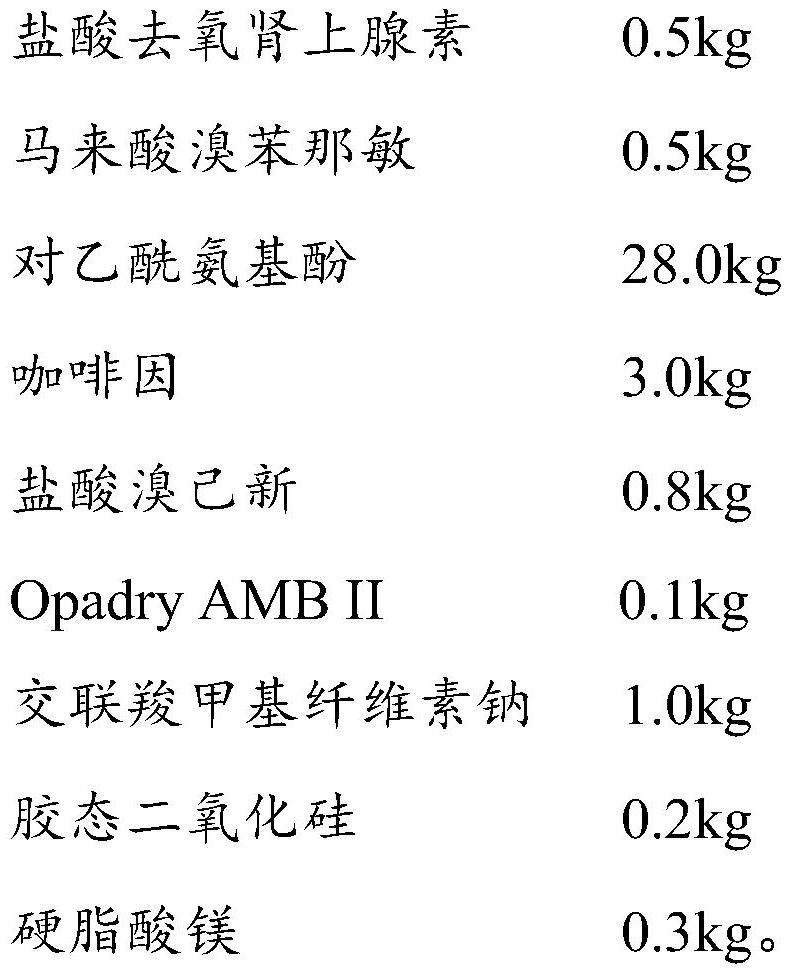
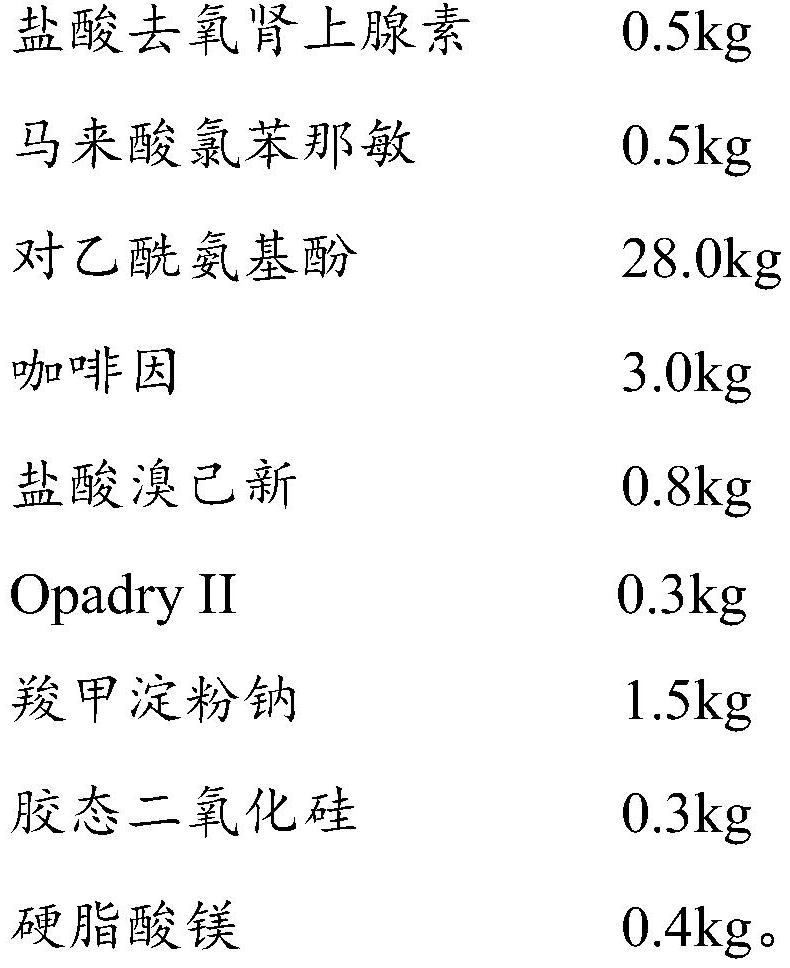
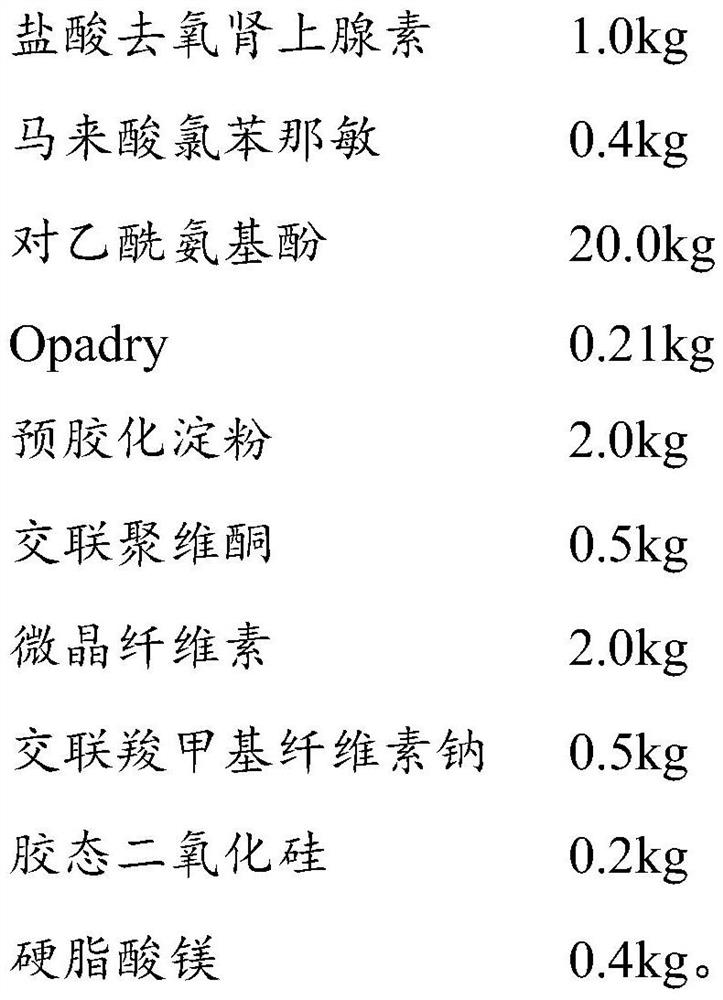
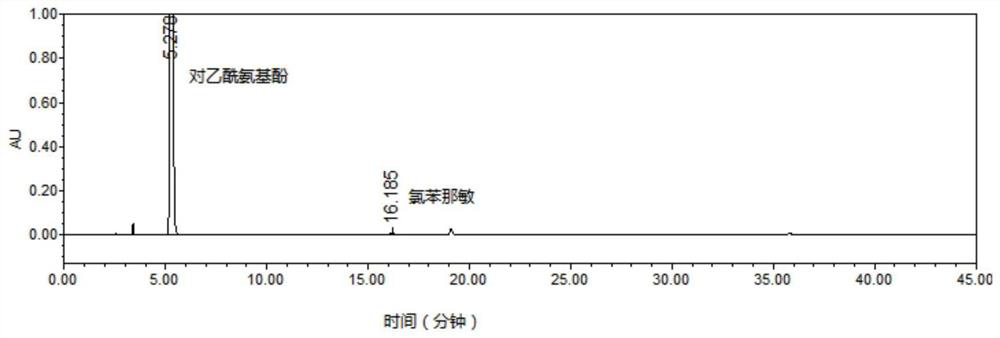
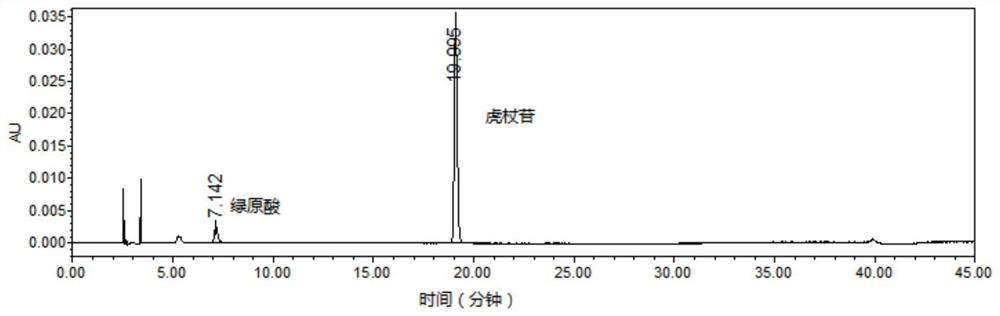
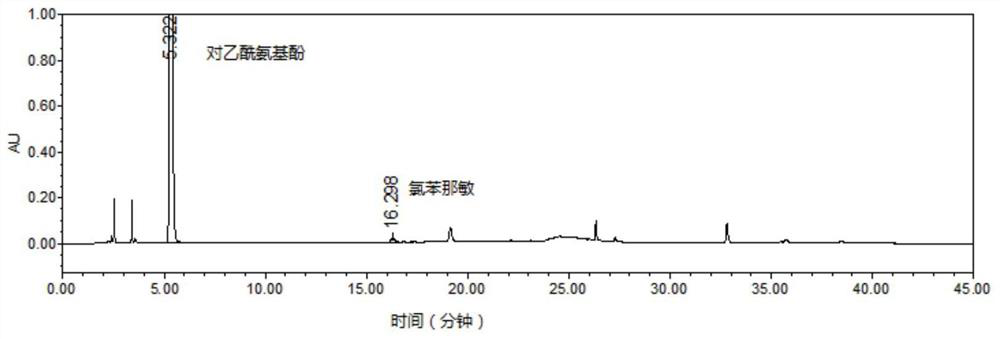
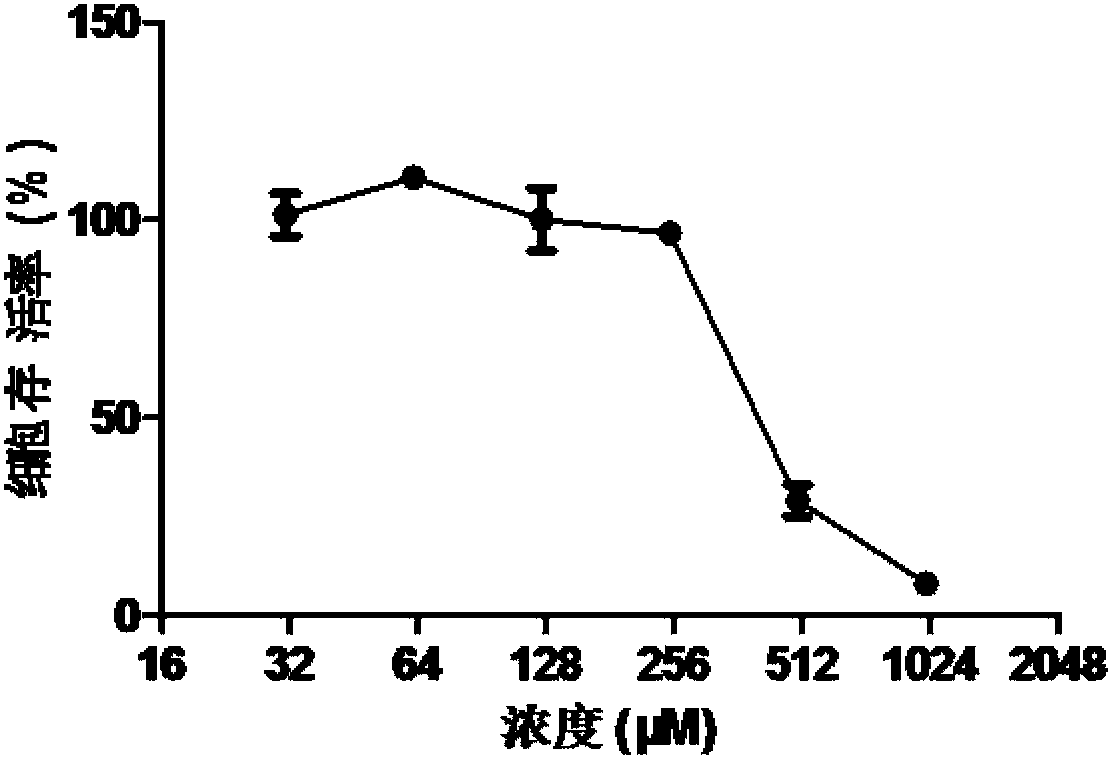
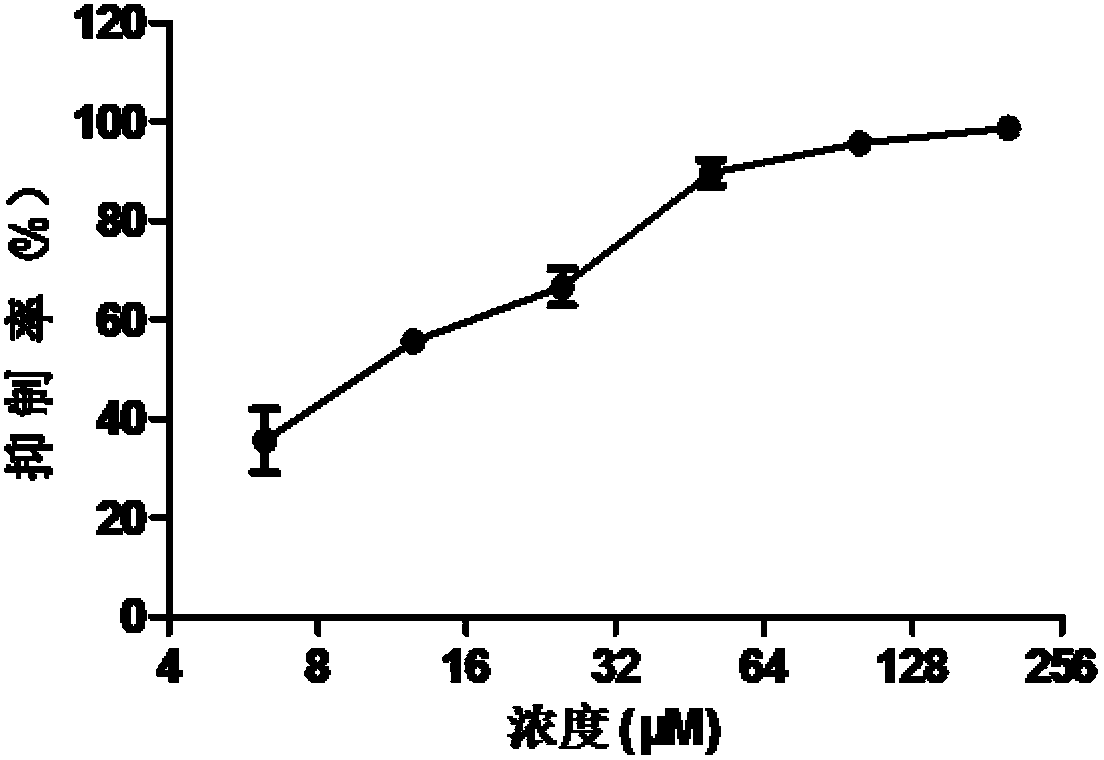
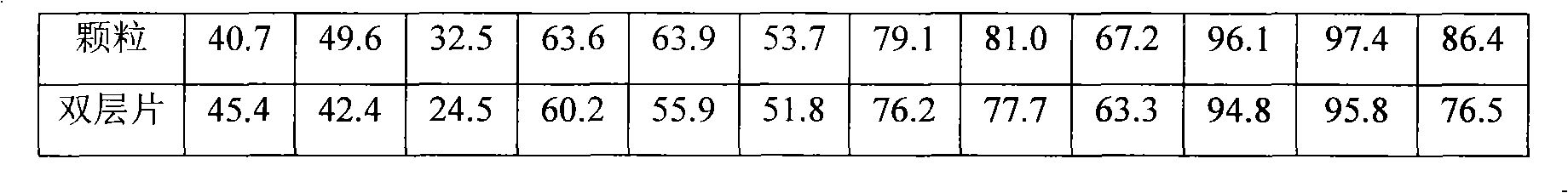Patents
Literature
30 results about "Chlorphenamine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
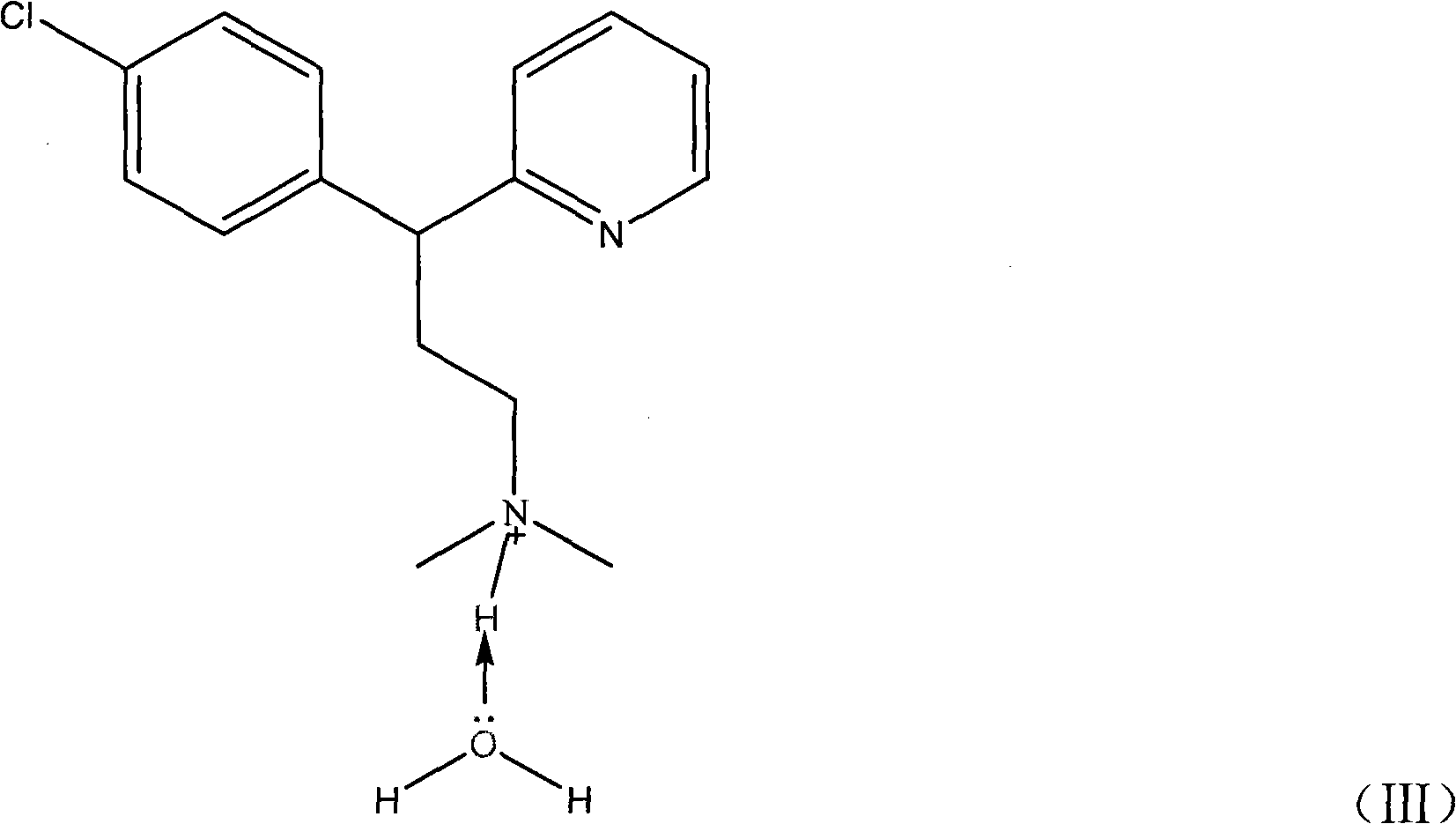
Chlorpheniramine is an antihistamine used to relieve symptoms of allergy, hay fever, and the common cold.
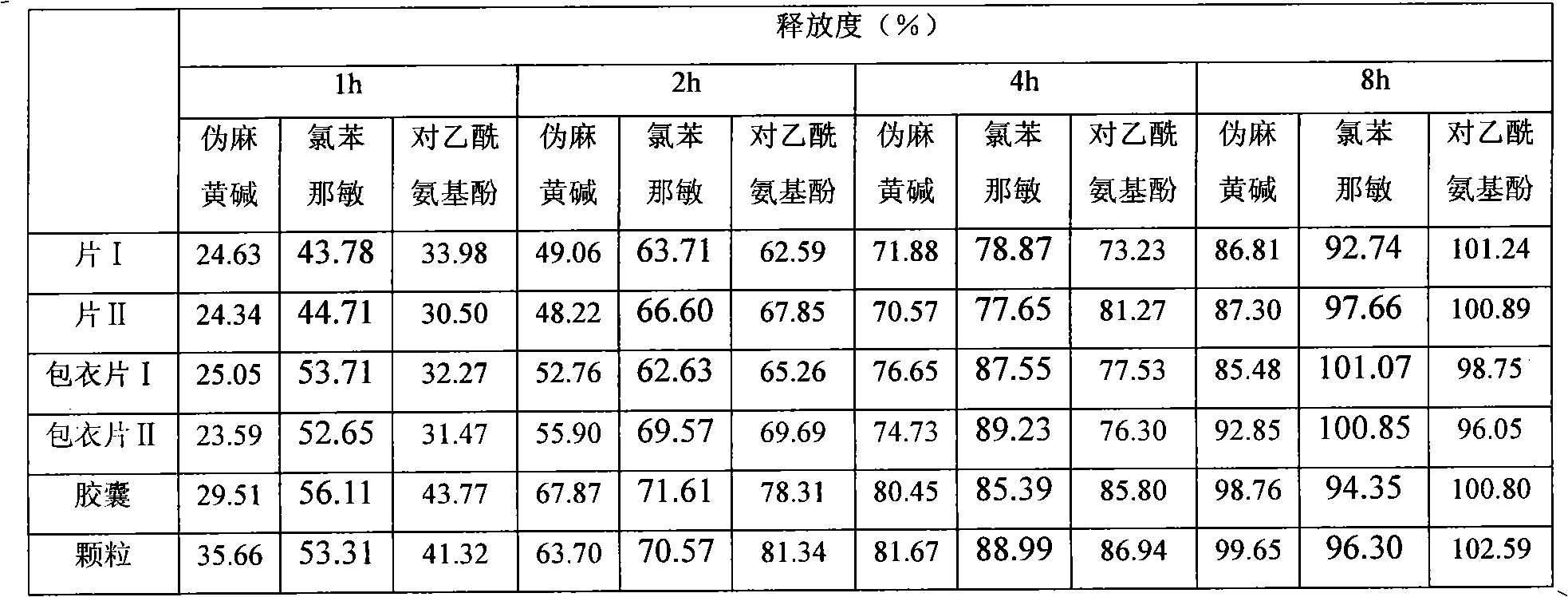
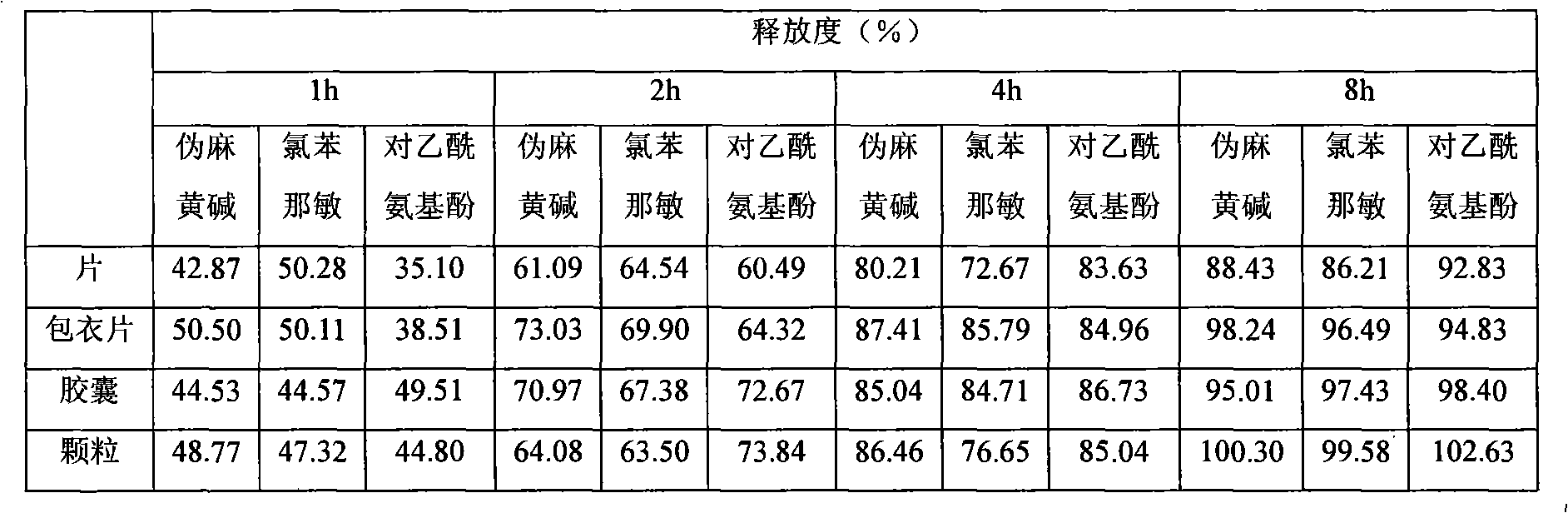
Oral liquid sustained-release preparation containing codeine and chlorphenamine and preparation method thereof
ActiveCN101474148AControl release behaviorNo grittinessOrganic active ingredientsSolution deliveryAdditive ingredientIon exchange
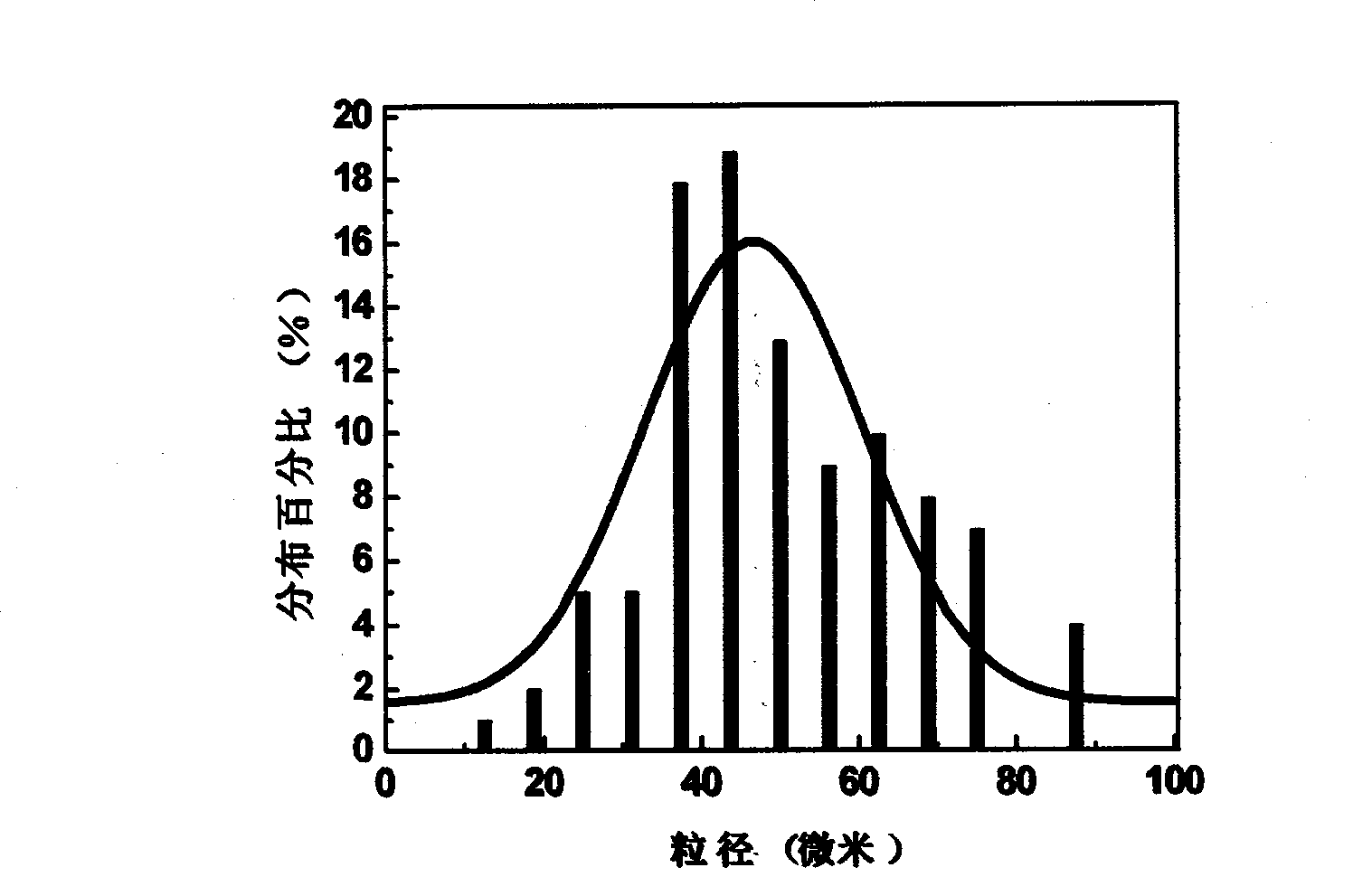
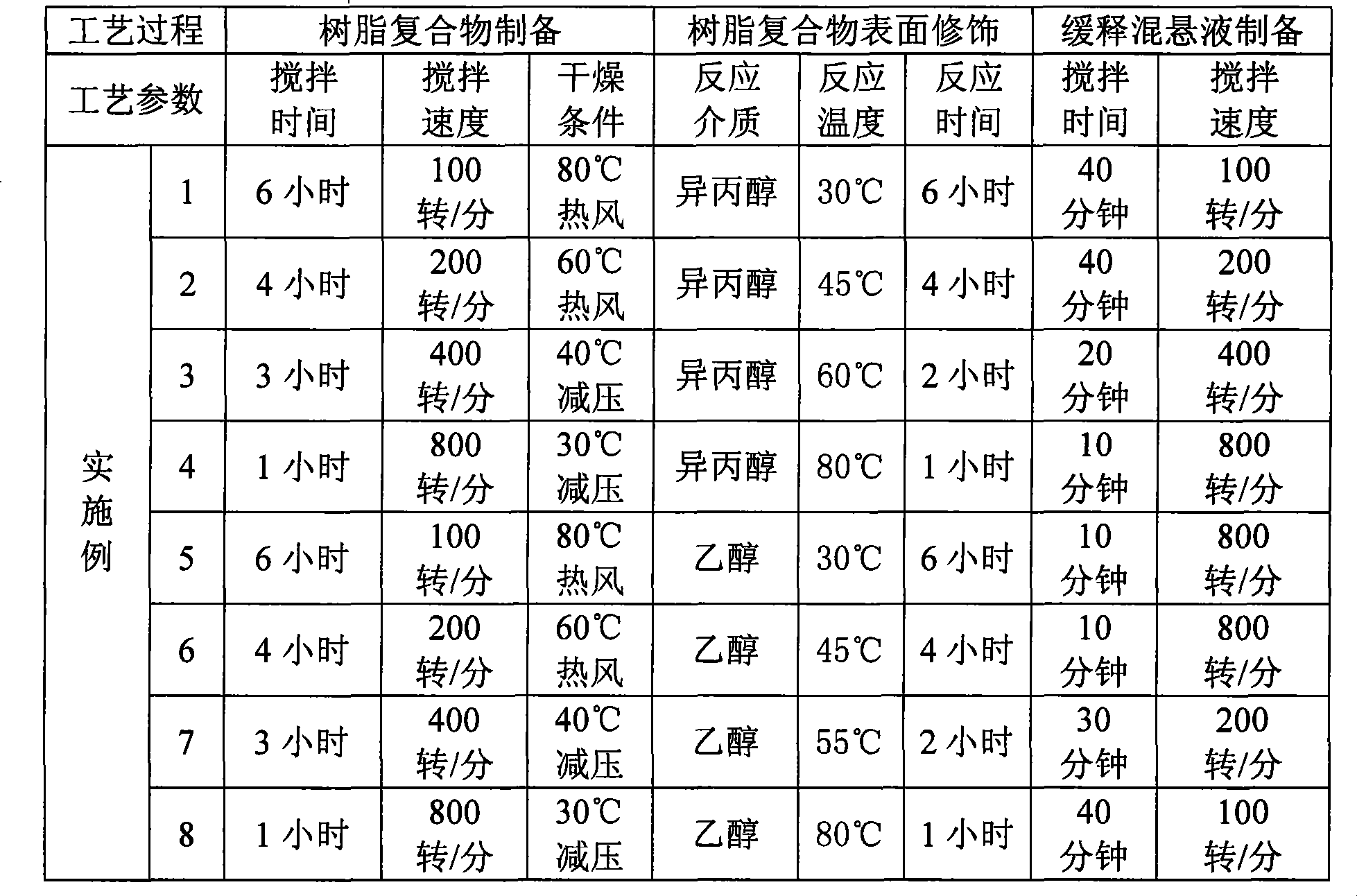
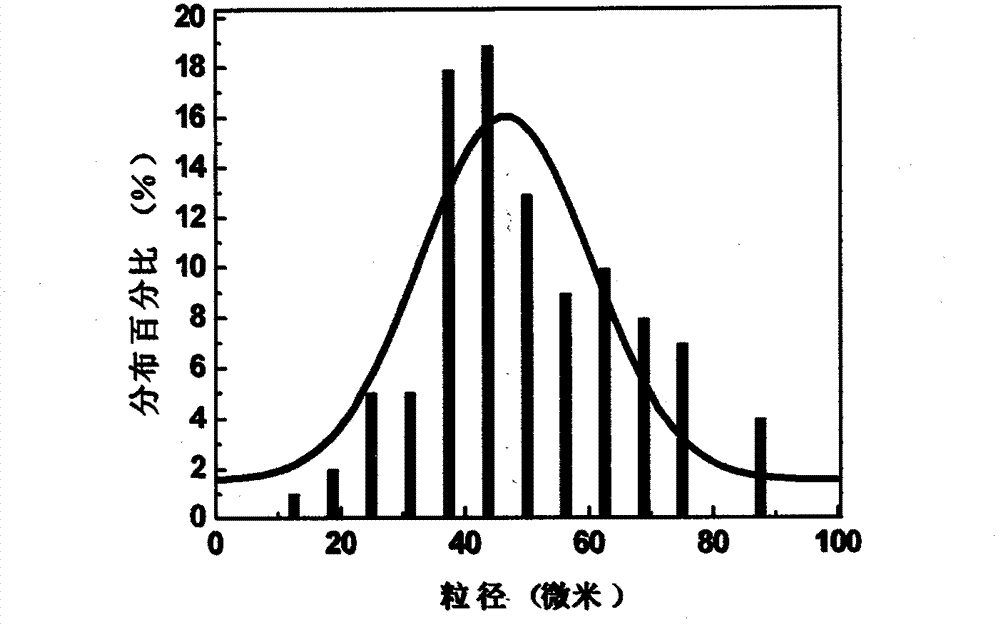
The invention discloses an oral liquid sustained-release preparation containing codein and chlorphenamine and a preparation method thereof, aiming at solving the technical problem of realizing sustained-release micro-capsules coating of subparticle with the particle size being less than 100 microns. The oral liquid sustained-release preparation comprises the ingredients based on the following proportion by weight: 0.1-1.0% of codein, 0.01-1.0% of chlorphenamine, 0.1-10.0% of ion exchange resin, 0.5-30.0% of suspending aid, 0.001-5.0% of sustained-release material, 0.1-5% of acidity regulators, 0.01-5% of heavy metal ion complexing agent and the rest of water. The preparation method comprises: compound codein, chlorphenamine resin particles and medium are stirred to be prepared. Compared with the prior art, the invention adopts a surface-modified method to combine the sustained-release material with ion exchange property radical and empty radical which is obtained after the medicine is absorbed by the ion exchange resin, a layer of compact sustained-release film is formed at the outer layer of medicine resin to control the releasing action of the medicine, thus realizing the sustained-release micro-capsule coating of subparticle with the particle size being less than 100 microns.
Owner:SINOPHARM ZHIJUN (SHENZHEN) PHARMA CO LTD
Compound formula dextro methaphen oral disintegration tablet and its preparation method
InactiveCN1830442AShort disintegration timeGreat tasteOrganic active ingredientsPill deliveryMANNITOL/SORBITOLPseudoephedrine
An oral disintegrating tablet of dextromethorphan for treating the cold caused cough, nasal congestion and rhinorrhea is proportionally prepared from dextromethorphan, chlorphenamine, pseudoephedrine, beta-cyclodextrin or ion exchange resin, mannitol or lactose starch, and disintegrant through sieving, flavouring, mixing and die pressing.
Owner:SINOPHARM ZHIJUN (SHENZHEN) PHARMA CO LTD
Preparation method of paracetamol pseudoephedrine hydrochloride and cholrphenamine maleate oral disintegration tablet
InactiveCN1803136AGood content uniformity of active ingredientsImprove bioavailabilityOrganic active ingredientsAntiinfectivesChlorphenamine maleateAdjuvant
The invention provides a process for preparing paracetamol pseudoephedrine hydrochloride and chlorphenamine malease orally disintegrating tablets, mixing paracetamol with bulking agent for granulating, making solution from pseudoephedrine hydrochloride, chlorphenamine maleate and spray packaging material, coating onto obtained paracetamol particles, then dressing to obtain flavor masked particles, finally mixing the particles with other adjuvant and tabletting.
Owner:HONGGUAN BIO PHARMA CO LTD
Method for detecting chlorpheniramine maleate
InactiveCN101865857AStrong specificityReduce dosageMaterial analysis by observing effect on chemical indicatorPhosphoric acidColor reaction
The invention discloses a rapid detection method of chlorpheniramine maleate doped in drugs, Chinese patent medicines and health products, which comprises the following steps: (1) taking a solid sample, adding 3-10 times the weight of water, shaking and dissolving, and then taking 3mL of extract, adding 2 drops of 2% (w / v) of sodium hydroxide solution and uniformly shaking to be used as a solution to be detected; or taking 3mL of liquid sample, and then adding 2 drops of 2% (w / v) of sodium hydroxide solution and uniformly shaking to be used as a solution to be detected; (2) adding 3mL of ethyl acetate in the solution to be detected to extract, standing and layering; (3) taking 2mL of upper ethyl acetate extract, adding 2mL of phosphoric acid water solution of 3% of volume concentration to extract, standing and layering; and (4) taking 1mL of lower phosphoric acid stripping solution, and adding 1-2 drops of trinitrophenol saturated water solution drop by drop, if a yellow precipitate is generated, then judging that the sample contains chlorpheniramine maleate. The detection method of the invention has simple operation, strong specificity and rapid color reaction, and can be suitable for rapidly detecting whether the drugs, the Chinese patent medicines and the health products are doped with chlorpheniramine maleate or not.
Owner:广东省药品检验所
Drug for clearing away heat and toxic material, relieving cough, reducing phlegm and preventing asthma
InactiveCN101559180AEasy to takeImprove bioavailabilityRespiratory disorderAluminium/calcium/magnesium active ingredientsPulmonary vasculatureTracheitis
The invention discloses a drug for relieving cough and preventing asthma. The drug is made from the following materials: Chinese ephedra, amygdale, gypsum, dried tangerine peel, rice husk, tendril-leaved fritillary bulb, alum, liquorice, carbetapentane citrate, prednisone, clenbuterol and chlorphenamine. The drug treats both symptoms and causes by combination of traditional Chinese medicines and western medicines, and has efficacies of antibiosis and anti-inflammation, improvement of pulmonary circulation, enhancement of immunological function of body and the like. The drug has obvious treatment effect on bronchial asthma, tracheitis, emphysema, pulmonary heart disease and the like and has advantages of quick effect, good curative effect, high curative ratio up to 68%, high effectiveness ratio above 96%, high safety and reliability and the like without toxic side effect and adverse reaction. The drug has simple preparation method, low price, easy popularization and wide application prospect.
Owner:北京汉潮联创中药科技有限公司
Compound slow release preparation of pseudoephedrine, chlorphenamine and dextromethorphan
InactiveCN101596157AStable blood concentrationImprove bioavailabilityOrganic active ingredientsPharmaceutical delivery mechanismPseudoephedrineBULK ACTIVE INGREDIENT
The invention relates to a slow release preparation taking pseudoephedrine or physiologically acceptable salt thereof, chlorphenamine or physiologically acceptable salt thereof and dextromethorphan or physiologically acceptable salt thereof as active ingredients. The slow release preparation is characterized in that a releasing system comprises a tablet core and / or pill core capable of enabling a medicament to slowly release and a coating, wherein one part and / or all of the active ingredients exist in the tablet core and / or pill core, and the rest of the active ingredients exist in the coating.
Owner:COSCI MED TECH CO LTD
Wet scabies treating ointment and its compounding process
InactiveCN1706390AEfficient killingIncrease profitOrganic active ingredientsWhite petrolatumMedicine
The present invention is wet scabies treating ointment as one kind of externally applied medicine for treating various kinds of wet scabies and its production process. The ointment is re-compounded with six kinds of medicine, including compound sulfamethoxazole, furazolidone, chlorphenamine, etc and vaseline. The preparation process includes mixing the medicine components to form mixture powder, and grinding the mixture and vaseline to obtain the ointment. The ointment has simple production process, high medicine utilization, capacity of killing bacteria, fungi and viruses and other advantages and may be used to cure various kinds of wet scabies.
Owner:刘朝辉
Jinling common cold preparation and controlled releasing preparations and preparation method thereof
The invention relates to Jinling cold controlled release preparation and the preparation method thereof. The invention consists of the following components by weight: cornu bubali concentration powder, cornu antelopis, caulis lonicerae, chrysanthemi indici, asiatic moonseed rhizome, aspirin, chlorphenamine and vitamin C. The controlled release preparation in the invention consists of sustained release part and immediate release part; wherein, aspirin, chlorphenamine and vitamin C are used as immediate release part, with the functions of relieving fever and pain, which can treat the early cold symptoms, aiming to cure the disease rapidly and effectively; cornu bubali concentration powder, cornu antelopis, caulis lonicerae, chrysanthemi indici, asiatic moonseed rhizome are used as the sustained release part, which focuses on the aspects such as anti-inflammation, antibiosis, anti-viral infection and regulating immunity, aiming to treat common cold at whole range and recovery stage. The invention has the advantage of integrating rapidness of western medicine with long-lasting of the traditional Chinese medicine, which reaches the real integration of traditional Chinese medicine with western medicine, thus improving bioavailability of the drug.
Owner:孙蓉
Adhesive plaster for treating prolapse of lumber intervertebral disc and its producing process
InactiveCN1682961AEliminate edemaSimple processAnthropod material medical ingredientsAerosol deliveryWestern medicineClematis
The present invention relates to a kind of plaster for treating protrusion of lumbar intervertebral disc and its preparation process. The plaster is prepared with prepared with over thirty kinds of Chinese medicinal materials, including nux vomica seed, momordica seed, clematis root, Radix Aconiti Brachpodi, aconite root, etc. and Western medicine chlorphenamine, and through frying partial of its Chinese medicinal materials in oil, filtering, mixing and other steps. It is pasted on to the affected part to treat protrusion of lumbar intervertebral disc.
Owner:杨献志
Powder pharmaceutical formulations for treating infantile diarrhea
InactiveCN101548990ARegulate stomachAntidiarrheal fastOrganic active ingredientsBacteria material medical ingredientsDiseaseCure rate
The present invention provides a powder pharmaceutical formulations for treating infantile diarrhea which overcomes weak pocket of other medicament for treating the disease. The powder pharmaceutical formulation is prepared by raw materials as follows: biofermin 0.1-1 g, compound diphenoxylate 0.25-2.5 mg, acetylsalicylic acid 0.3-0.15 g, chlorphenamine 0.04-0.2 mg and hydrochlorothiazidum tablet 1-5 mg, the above medicine are grinded to dead small, then are measured and packed for obtaining the powder pharmaceutical formulation. The powder pharmaceutical formulation for treating infantile diarrhea is used about twenty years and is consulted by patients 10000 times, wherein, males 5500 times, females 4500 times, curative rate 98% and efficiency rate 100%.
Owner:杨国强
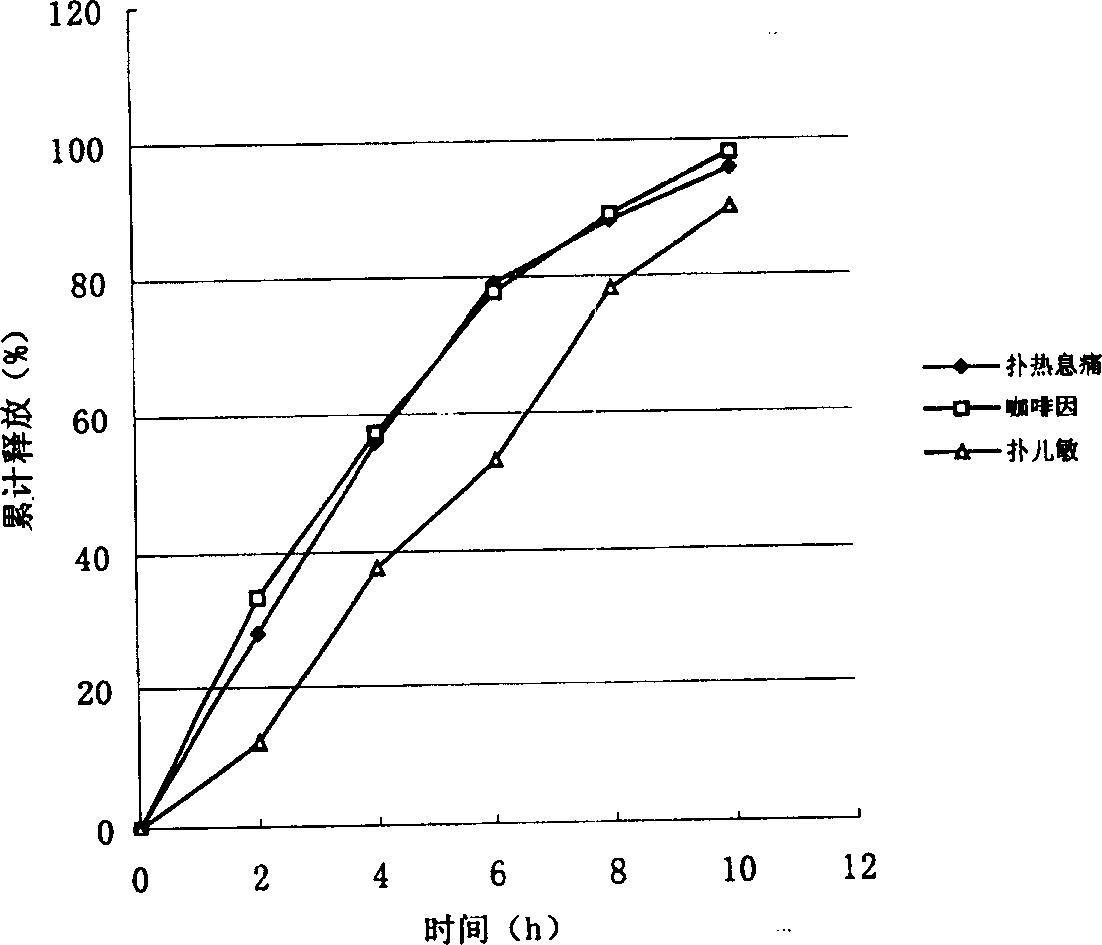
Paracetamol, pseudoephedrine and chlorphenamine compound sustained release preparation
InactiveCN101658521AStable blood concentrationImprove bioavailabilityOrganic active ingredientsAntiviralsActive componentPseudoephedrine
The invention relates to a sustained release preparation taking paracetamol and pseudoephedrine or physiologically acceptable salts thereof, and chlorphenamine or physiologically acceptable salts thereof as active components, and provides a compound preparation which can comprehensively overcome cold related symptoms and of which all the active components are slowly released.
Owner:COSCI MED TECH CO LTD
Dermatosis treating medicine and its prepn
InactiveCN1383873AGood curative effectUnknown materialsDermatological disorderAllergic dermatitisBenzoic acid
The dermatosis treating medicine is prepared with three kinds of Chinese medicinal materials; three kinds of Western medicine of dexamethasone, chlorphenamine and chloromycetin; pearl powder, benzoicacid, and vanishing cream. During its preparation, the three kinds of Chinese medicinal materials are washed, cut sun dried and ground to fine powder; the three kinds of Western medicine are ground into fine powder; and the two kinds of powder and the pearl powder are mixed, sieved and mixed with benzoic acid and vanishing cream to produce the medicine. The medicine is used for treating eczema, neurodermatitis, allergic dermatitis, psora, senile cutaneous pruritus, etc.
Owner:李书斋
Compound paracetamol and chlorphenamine maleate slow releasing tablet and its preparation
InactiveCN1241568CImprove complianceReduce the number of dosesPharmaceutical delivery mechanismAntiinfectivesAdjuvantGallstones
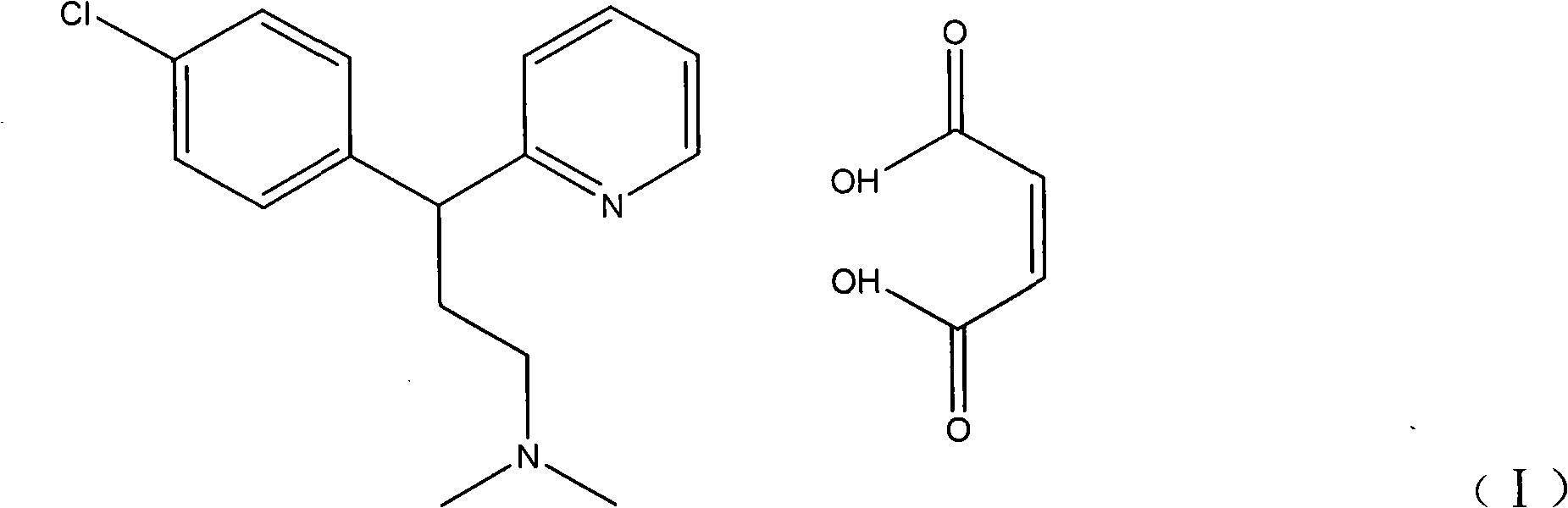
The invention relates to a compound paracetamol and chlorphenamine slow release tablet and method for preparation, which comprises paracetamol, chlorpheniramine maleate, caffeine, artificial ox gallstone and slow release matrix material, lubricating agent, core and damp-proof membrane consisting binding agent and flow adjuvant, wherein the proportion of the composition is, acetaminopher 30%-80%, chlorpheniramine maleate 0.5%-4%, caffeine 2%-10%, taurine 1%-10%.
Owner:TIANJIN PACIFIC PHARMA
Anti-cold nasal administration composition
InactiveCN1742747AAvoid destructionEasy to administerPowder deliveryOrganic active ingredientsCommon coldCurative effect
The present invention relates to a nasal medication composition for relieving common cold symptom and inhibiting common cold virus. Said composite is made up by using (by wt%) 0.1%-60% of pseudoephedrine hydrochloride, 0.2%-30% of chlorphenamine and 0.1%-75% of ribavirin.
Owner:YUNNAN INST OF MATERIA MEDICA
Medicine for treating perennial allergic rhinitis
InactiveCN1357329AInhibitory responsePrevent proliferationOrganic active ingredientsRespiratory disorderNasal cavityAllergic pharyngitis
The present invention is one kind of medicine for treating perennial allergic rhinitis. Oral antiallergic medicine chromoglycate sodium, chlorphenamine and Prednisolone acetate and externally used rhinitis treating medicine Fuma liquid are compounded in certain proportion to prepare the medicine for treating allergic rhinitis through local application. The medicine can resist allergic reaction, inhibit excitation of sympathetic nerve, reduce the reaction of nasal mucous membrane to histamine, reduce the pathological reaction of nasal mucous membrane to various irritative damage, inhibit proliferation and exudation of connective tissue and relieve various rhinitis symptoms. It has obvious treating effects and no negative reaction.
Owner:曾咏梅
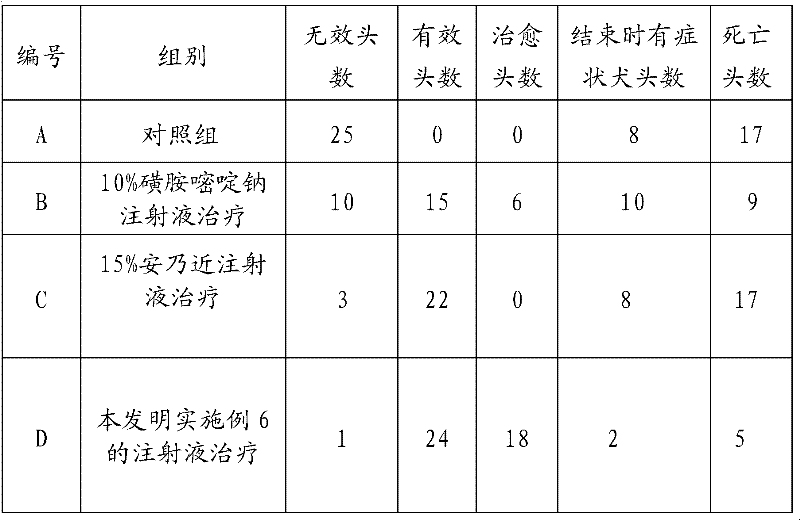
Injecta for treating bacterial encephalitis of dog and preparation method thereof
InactiveCN102327276AHas a broad-spectrum antibacterial effectImprove antibacterial propertiesAntibacterial agentsOrganic active ingredientsDiseaseSulfadiazine
The invention discloses injecta for treating bacterial encephalitis of a dog and a preparation method thereof, and aims at providing the injecta for treating bacterial encephalitis of the dog effectively and efficiently, with an effect for treating both principal and secondary aspect of the disease as well as the preparation method with a simple process and easiness in realization. Every 100L of injecta comprises 5-15kg of sulfadiazine sodium, 10-20kg of analgin, 1-3kg of trimethoprim, 0.1-0.2kg of chlorphenamine, 10kg of dimethylformamide, 10kg of 95 percent ethanol, 30kg of propanediol and the balance of water for injection. The sulfadiazine sodium adopted by the invention has a wide spectrum antimicrobial function and can be used for transmitting through a blood brain barrier and killing bacteria; the trimethoprim is a synergistic agent and can be used for enhancing the antimicrobial effect of the sulfadiazine sodium and changing the traditional bacteriostatic action into bactericidal action; the analgin has functions of defervescing and resisting inflammation and can be used for reducing the damage to an immunologic system and nerve of the dog caused by high fever; and the chlorphenamine has the functions of resisting hypersusceptibility and calming and can be used for reducing an anaphylactic reaction caused by toxin generated by bacteria.
Owner:TIANJIN SHENGJI GRP CO LTD
Phenylephrine hydrochloride and chlorphenamine maleate preparation and preparation method thereof
InactiveCN112472678AAvoid reactionImprove stabilityOrganic active ingredientsPharmaceutical non-active ingredientsChlorobenzenePharmaceutical medicine
The invention belongs to the technical field of pharmaceutical preparations, and particularly relates to a phenylephrine hydrochloride and chlorphenamine maleate preparation and a preparation method thereof. The phenylephrine hydrochloride and chlorphenamine maleate preparation comprises a mixture formed by a first coating and a second coating, wherein the first coating is composed of a first coreand a first coating film coating the surface of the first core, the second coating is composed of a second core and a second coating film coating the surface of the second core, the first core is phenylephrine or a pharmaceutically acceptable salt thereof, and the second core is chlorpheniramine maleate and / or bromopheniramine maleate. According to the phenylephrine hydrochloride and chlorphenamine maleate preparation disclosed by the invention, the first core and the second core are not in contact with each other, so that thorough isolation of phenylephrine from chlorpheniramine maleate and / or bromopheniramine maleate is realized, secondary amino groups on phenylephrine are prevented from reacting with maleate radicals, the obtained phenylephrine hydrochloride and chlorphenamine maleatepreparation has higher product stability, and the safety and effectiveness of clinical medication are improved.
Owner:BRIGHT FUTURE PHARMA LAB LTD (CN)
High performance liquid chromatography for determining the content of compound Polygonum cuspidatum and Aminmin tablets
ActiveCN108845062BAccurate measurementSensitive assayComponent separationO-Phosphoric AcidChlorobenzene
Owner:广西壮族自治区食品药品检验所
Application of chlorpheniramine maleate in preparation of medicaments for treating or preventing influenza virus
InactiveCN103239446BInhibition of adsorptionInhibit entryOrganic active ingredientsAntiviralsVirus strainInfluenza a
The invention discloses application of chlorpheniramine maleate in preparation of medicaments for treating or preventing influenza virus. The application comprises the following steps of: first, performing an antivirus experiment by selecting the chlorpheniramine maleate with completely non-toxic concentration, wherein the result shows that the low molecular weight compound has remarkable antiviral activity and has dose-dependent correlation; then, analyzing the anti-influenza virus action cycle of chlorpheniramine maleate, wherein the result shows that chlorpheniramine maleate mainly inhibits the early virus infection events of virus adsorption, entry and the like; and finally, detecting the antiviral activity of chlorpheniramine maleate on different types or subtypes of influenza virus, wherein the result shows that chlorpheniramine maleate can inhibit the replication of all detection virus strains and has a dose-dependent effect, and shows that the anti-influenza virus activity of chlorpheniramine maleate has certain broad spectrum. Therefore, chlorpheniramine maleate disclosed by the invention can be developed as a new anti-influenza virus medicament, and provides a new way and a new means for treating the influenza virus.
Owner:WUHAN INST OF VIROLOGY CHINESE ACADEMY OF SCI
Powder pharmaceutical formulations for treating infantile diarrhea
InactiveCN101548990BNormal stoolNormal dietOrganic active ingredientsBacteria material medical ingredientsDiseaseHydrochlorothiazide
The present invention provides a powder pharmaceutical formulations for treating infantile diarrhea which overcomes weak pocket of other medicament for treating the disease. The powder pharmaceutical formulation is prepared by raw materials as follows: biofermin 0.1-1 g, compound diphenoxylate 0.25-2.5 mg, acetylsalicylic acid 0.3-0.15 g, chlorphenamine 0.04-0.2 mg and hydrochlorothiazidum tablet1-5 mg, the above medicine are grinded to dead small, then are measured and packed for obtaining the powder pharmaceutical formulation. The powder pharmaceutical formulation for treating infantile diarrhea is used about twenty years and is consulted by patients 10000 times, wherein, males 5500 times, females 4500 times, curative rate 98% and efficiency rate 100%.
Owner:杨国强
Codein and chlorphenamine compound sustained release capsules
ActiveCN101537005BImprove bioavailabilityReduce the number of dosesOrganic active ingredientsAntipyreticMedicineAcrylic resin
The invention discloses codein and chlorphenamine compound sustained release capsules. The sustained release granules are made from the following raw materials by weight: 10-11.1 parts of codein, 2-2.2 parts of chlorphenamine, 1-2 parts of a lubricant, 18.9-86 parts of a sustained release matrix material and 0-12.4 parts of a filling agent. The sustained release matrix material is two or three ofhydroxypropyl emthylcellulose, acrylic resin and ethyl cellulose, wherein, the acrylic resin and the ethyl cellulose totally accounts for 10.9-31.5%. The capsules are obtained by the following steps:evenly mixing the codein, the chlorphenamine and the sustained release matrix material; adding a binder to prepare a soft material and sieving with a 6-24-mesh sieve; preparing damp granules, drying and granulating; and adding the lubricant, evenly mixing and then encapsulating to obtain the finished product.
Owner:SINOPHARM GRP ZHIJUN SHENZHEN PINGSHAN PHARMA CO LTD
Oral liquid sustained-release preparation containing codeine and chlorphenamine and preparation method thereof
ActiveCN101474148BControl release behaviorNo grittinessOrganic active ingredientsSolution deliveryIon exchangeIon-exchange resin
The invention discloses an oral liquid sustained-release preparation containing codein and chlorphenamine and a preparation method thereof, aiming at solving the technical problem of realizing sustained-release micro-capsules coating of subparticle with the particle size being less than 100 microns. The oral liquid sustained-release preparation comprises the ingredients based on the following proportion by weight: 0.1-1.0% of codein, 0.01-1.0% of chlorphenamine, 0.1-10.0% of ion exchange resin, 0.5-30.0% of suspending aid, 0.001-5.0% of sustained-release material, 0.1-5% of acidity regulators, 0.01-5% of heavy metal ion complexing agent and the rest of water. The preparation method comprises: compound codein, chlorphenamine resin particles and medium are stirred to be prepared. Compared with the prior art, the invention adopts a surface-modified method to combine the sustained-release material with ion exchange property radical and empty radical which is obtained after the medicine isabsorbed by the ion exchange resin, a layer of compact sustained-release film is formed at the outer layer of medicine resin to control the releasing action of the medicine, thus realizing the sustained-release micro-capsule coating of subparticle with the particle size being less than 100 microns.
Owner:SINOPHARM ZHIJUN (SHENZHEN) PHARMA CO LTD
Anisodamine ointment for treating frostbite and sclerema neonatorum and method for preparing same
InactiveCN1306933CEasy to makeLow cost of treatmentAerosol deliveryOintment deliverySclerema neonatorumMedicine
The present invention relates to medicine with organic effective component, and aims at one kind of ointment compounded with anisodamine and capable of curing frostbite and sclerema neonatorum in relatively short time. The ointment is compounded with anisodamine in 15-20 wt% and chlorphenamine 5-15 wt% except medical vaseline. The supplementary material includes metronidazole and / or prednisone and proper amount of vitamin E. The preparation process includes heating vaseline, mixing with other components, cooling and packing in box. The present invention has simple preparation process, low cost, convenient use and obvious treating effect.
Owner:康佳兰
Injection for treating livestock bacterial dysentery and preparing method thereof
InactiveCN104173357ARelieves the body's stress responseReduce stress responseAntibacterial agentsDigestive systemTreatment effectDissolution
The invention relates to an injection for treating livestock bacterial dysentery and a preparing method thereof. The injection is prepared by (1) adding 100-400 kg / L of sodium salicylate into a part of water for injection, adding 10-150 kg / L of mequindox, and stirring for 10-20 min until the liquid is clear; (2) weighing a part of water for injection, adding 5-110 kg / L of apramycin sulfate, 1-20 kg / L of atropine sulfate and 1-20 kg / L of chlorphenamine in order, and stirring for dissolution; and (3) combining the solution prepared in the step (1) and the solution prepared in the step (2), adding the water for injection to a labeled volume, stirring uniformly, and maintaining for 15-20 min, then after the medicine solution is clear, the compound apramycin sulfate injection is obtained. The compound preparation comprising the apramycin sulfate and the mequindox has obvious treating effects for swine bacterial dysentery and can effectively relieve body stress responses of the livestock with diseases. Experiments prove that: combination of the apramycin sulfate, the mequindox and the atropine sulfate can obviously enhance curative effects; the injection is widely used in clinical; and the injection can be widely used for treatment of bacterial dysentery of swine, cattle, sheep, and other livestock, and can increase the survival rate and the body weight of swine, cattle and sheep.
Owner:TIANJIN JIHE TECH
Preparation method of paracetamol pseudoephedrine hydrochloride and cholrphenamine maleate oral disintegration tablet
InactiveCN100358523CGood content uniformity of active ingredientsImprove bioavailabilityOrganic active ingredientsAntiinfectivesChlorphenamine maleateAdjuvant
The invention provides a process for preparing paracetamol pseudoephedrine hydrochloride and chlorphenamine malease orally disintegrating tablets, mixing paracetamol with bulking agent for granulating, making solution from pseudoephedrine hydrochloride, chlorphenamine maleate and spray packaging material, coating onto obtained paracetamol particles, then dressing to obtain flavor masked particles, finally mixing the particles with other adjuvant and tabletting.
Owner:HONGGUAN BIO PHARMA CO LTD
Compound slow-release preparation of benorilate, pseudoephedrine and chlorphenamine
ActiveCN101757001AStable blood concentrationImprove bioavailabilityOrganic active ingredientsAntiviralsPseudoephedrineBULK ACTIVE INGREDIENT
Owner:北京科信聚润医药科技有限公司
Adhesive plaster for treating prolapse of lumber intervertebral disc and its producing process
InactiveCN100353991CEliminate edemaSimple processAnthropod material medical ingredientsAerosol deliveryCyathula officinalisMomordica
The present invention relates to a kind of plaster for treating protrusion of lumbar intervertebral disc and its preparation process. The plaster is prepared with prepared with over thirty kinds of Chinese medicinal materials, including nux vomica seed, momordica seed, clematis root, Radix Aconiti Brachpodi, aconite root, etc. and Western medicine chlorphenamine, and through frying partial of its Chinese medicinal materials in oil, filtering, mixing and other steps. It is pasted on to the affected part to treat protrusion of lumbar intervertebral disc.
Owner:杨献志
Compound slow-release preparation of benorilate, pseudoephedrine and chlorphenamine
ActiveCN101757001BStable blood concentrationImprove bioavailabilityOrganic active ingredientsAntiviralsHuman bodyPseudoephedrine
The invention provides a compound preparation which can comprehensively overcome the symptom relative to cold and can slowly release all the active ingredients. The slow-release preparation of the invention not only can overcome the symptom relative to cold but also can perform the synergistic function of compound medicine. The three active ingredients of the slow-release preparation of the invention can be released and absorbed synchronously so as to acquire expected releasing action in human body. The preparation of the invention can be taken for two times per day, namely, can be taken for one time in morning and evening instead of taking four times per day in the past. The preparation of the invention is characterized by less taking times, slow release in body, stable blood concentration, small fluctuation, high bioavailability and high safety.
Owner:北京科信聚润医药科技有限公司
Chinese and western medicine composition for treating tuberculous pleuritis and preparation method and application thereof
InactiveCN106420737ANo financial burdenGood treatment effectAntibacterial agentsAnhydride/acid/halide active ingredientsWestern medicineSide effect
The invention discloses a Chinese and western medicine composition for treating tuberculous pleuritis and a preparation method and application thereof; the Chinese and western medicine composition is prepared from, by weight, 1-3 parts of artemisinin, 3-7 parts of chlorphenamine, 15-23 parts of taurine, and 11-19 parts of amoxicillin; the preparation method comprises mixing and grinding chlorphenamine and amoxicillin, adding deionized water, and stirring at 66 DEG C for 48-50 min to obtain mixture A; mixing taurine and the mixture A, stirring at 60 DEG C for 23-25 min, adding artemisinin, ultrasonically treating at 43 DEG C for 33 min, stirring at 55 DEG C until dryness, and granulating. The Chinese and western medicine composition has significant effect in treating tuberculous pleuritis, has high cure rate, short treatment time and high acting speed, is free of toxic and side effects and allergic reaction, has high cure rate and low recurrence rate, is low in manufacture cost and low in usage and is an ideal drug.
Owner:郑州莉迪亚医药科技有限公司
Pharmaceutical composition for treating motion sickness
InactiveCN110772524AQuickly relieve dizzinessQuick relief of nauseaOrganic active ingredientsDigestive systemVitamin b6Chlorobenzene
The invention relates to the technical field of medicines, in particular to a pharmaceutical composition for treating motion sickness. The pharmaceutical composition comprises chlorphenamine meleate,glucose, vitamin B6 and a medicinal carrier, wherein the total weight ratio of the components including the chlorphenamine meleate, the glucose and the vitamin B6 is (1-6):(50-200):(1-10). The pharmaceutical composition has the beneficial effects as follows: the pharmaceutical composition can effectively eliminate coordinated movement disorders and can prevent carsickness, seasickness and airsickness when taken 15-30 min before taking of a vehicle; the pharmaceutical composition can quickly relieve symptoms such as dizziness, nausea and vomiting when taken during attack and recover health, besides, the efficacy lasts for 6-8 h or longer, and the pharmaceutical composition has better effects and maintains longer effective time than existing medicines.
Owner:方科伟
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com