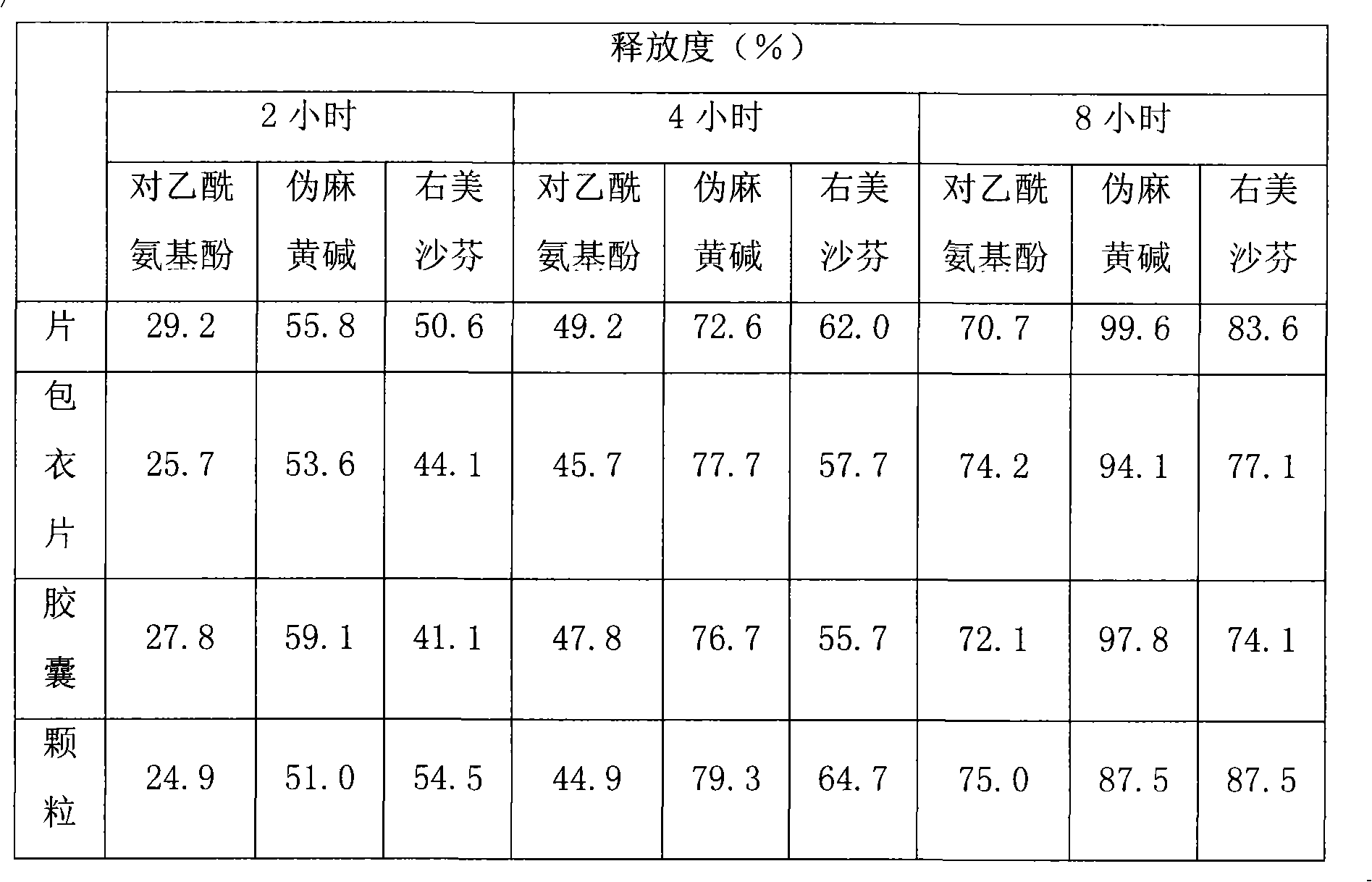
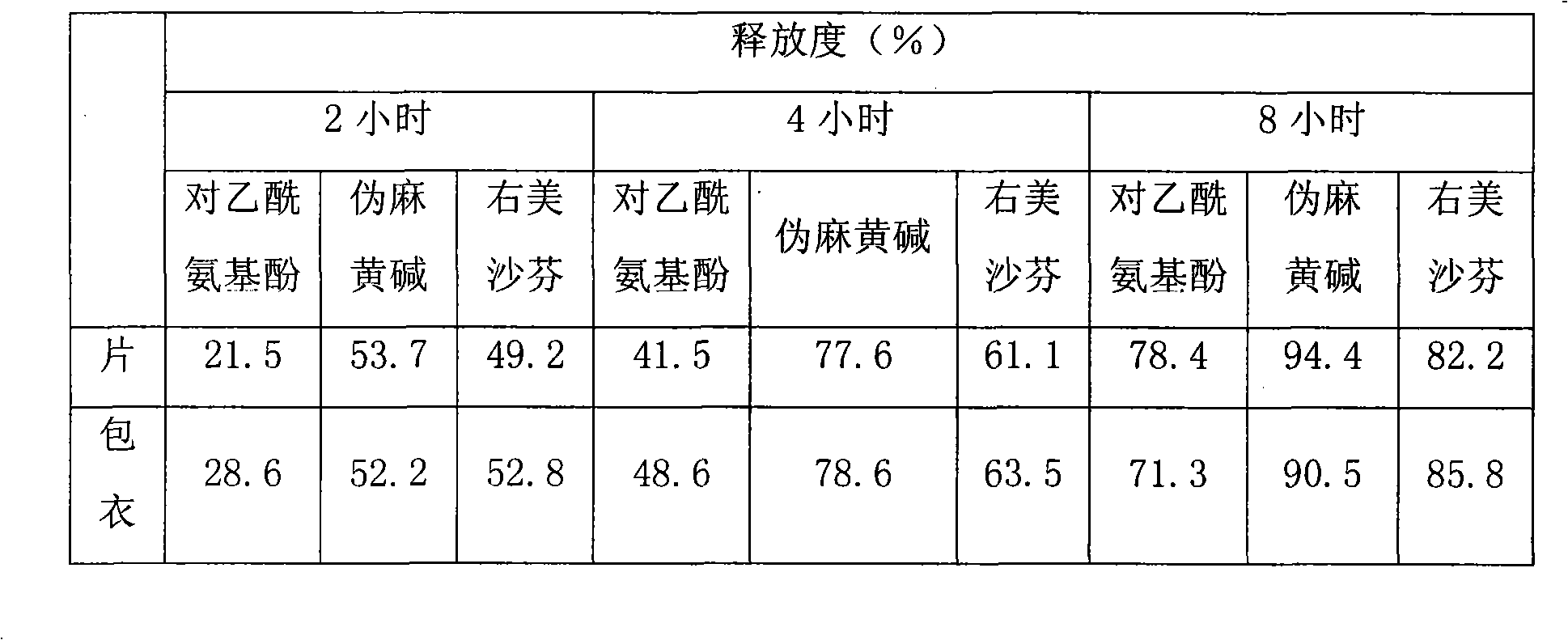
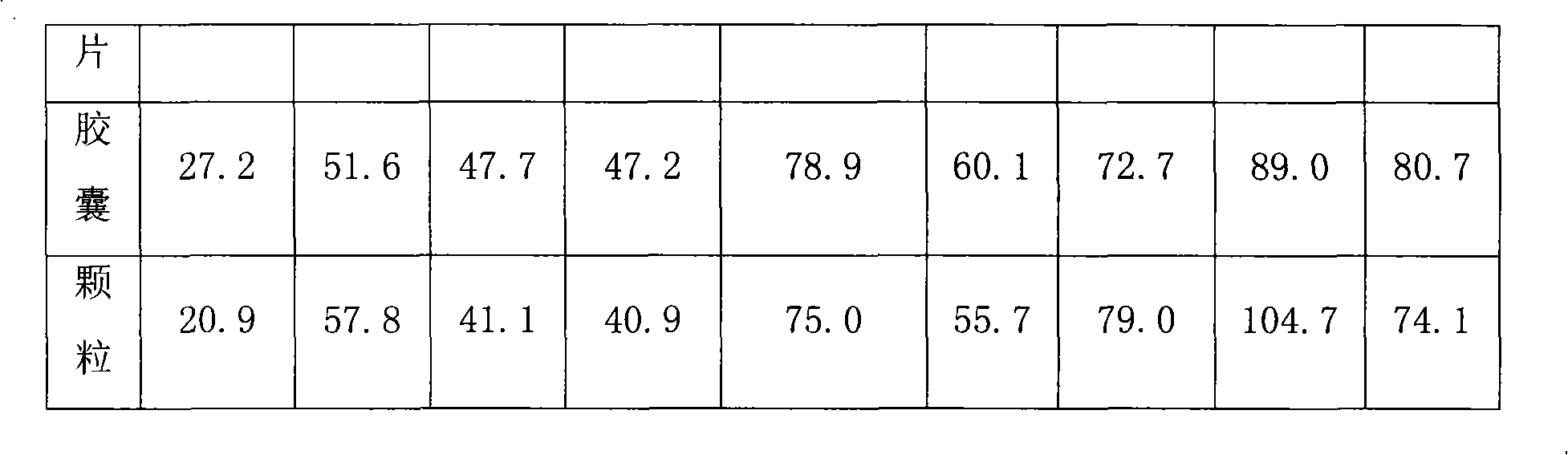
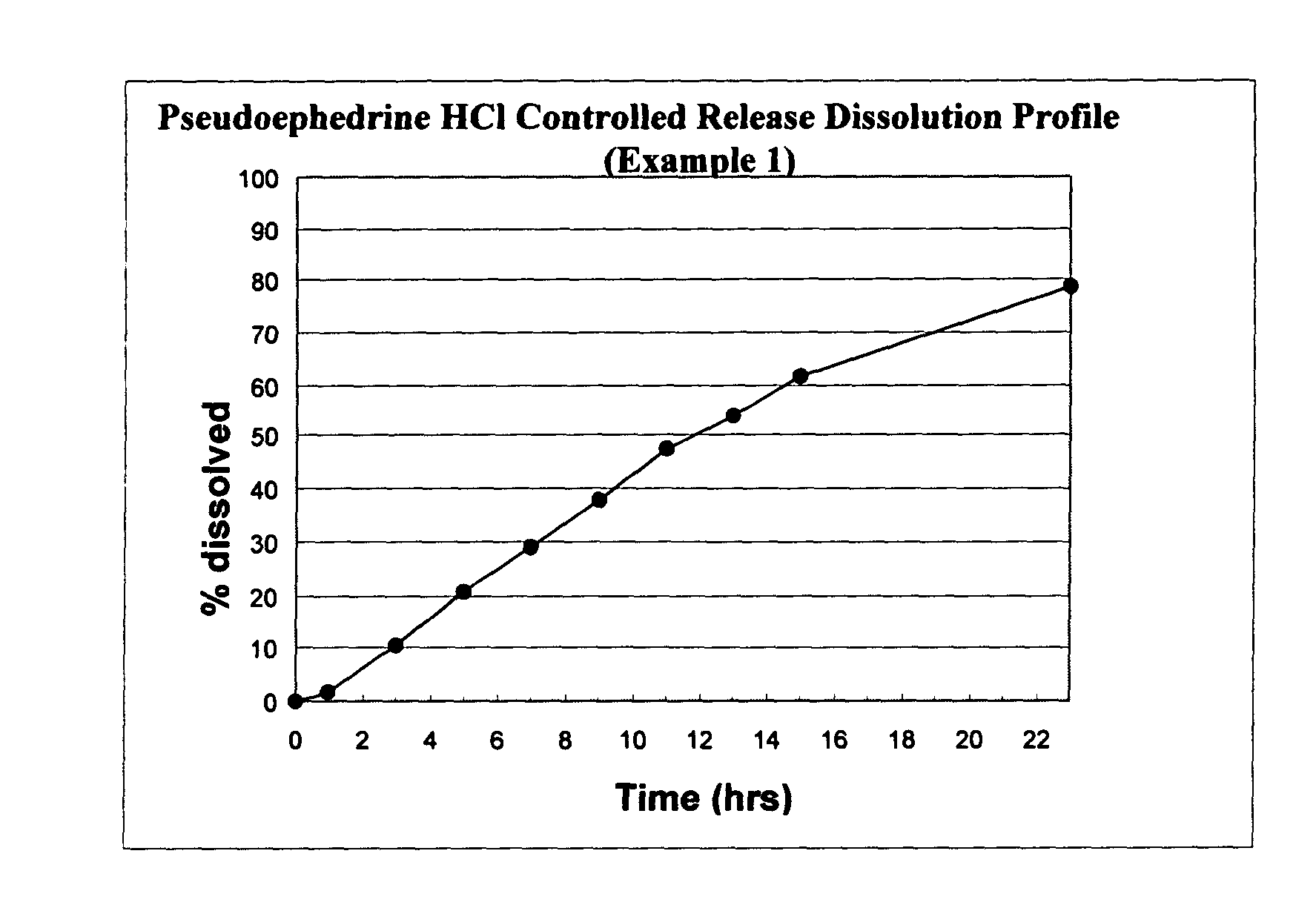
Patents
Literature
103 results about "Pseudoephedrine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Pseudoephedrine is used for the temporary relief of stuffy nose and sinus pain/pressure caused by infection (such as the common cold, flu) or other breathing illnesses (such as hay fever, allergies, bronchitis).
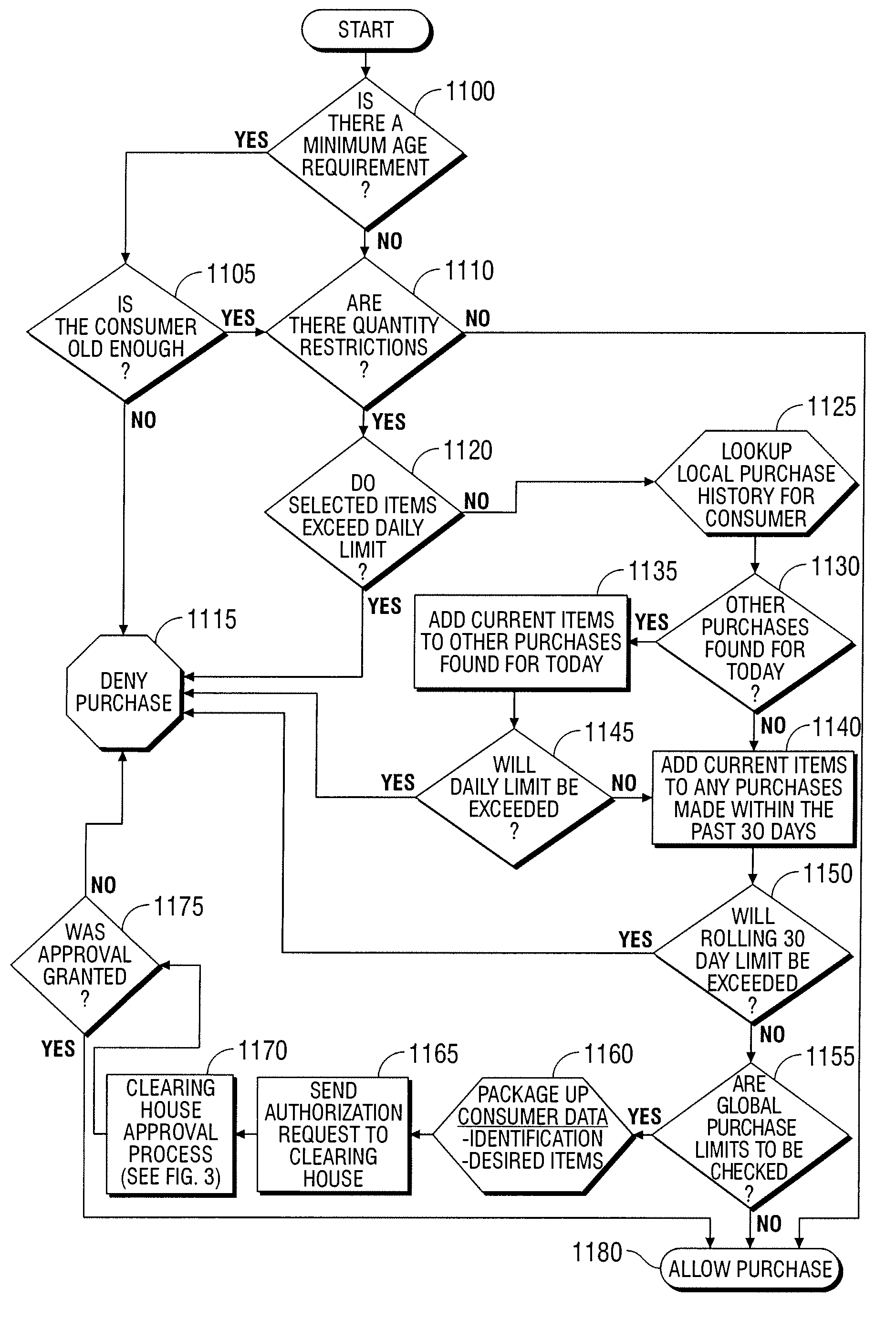
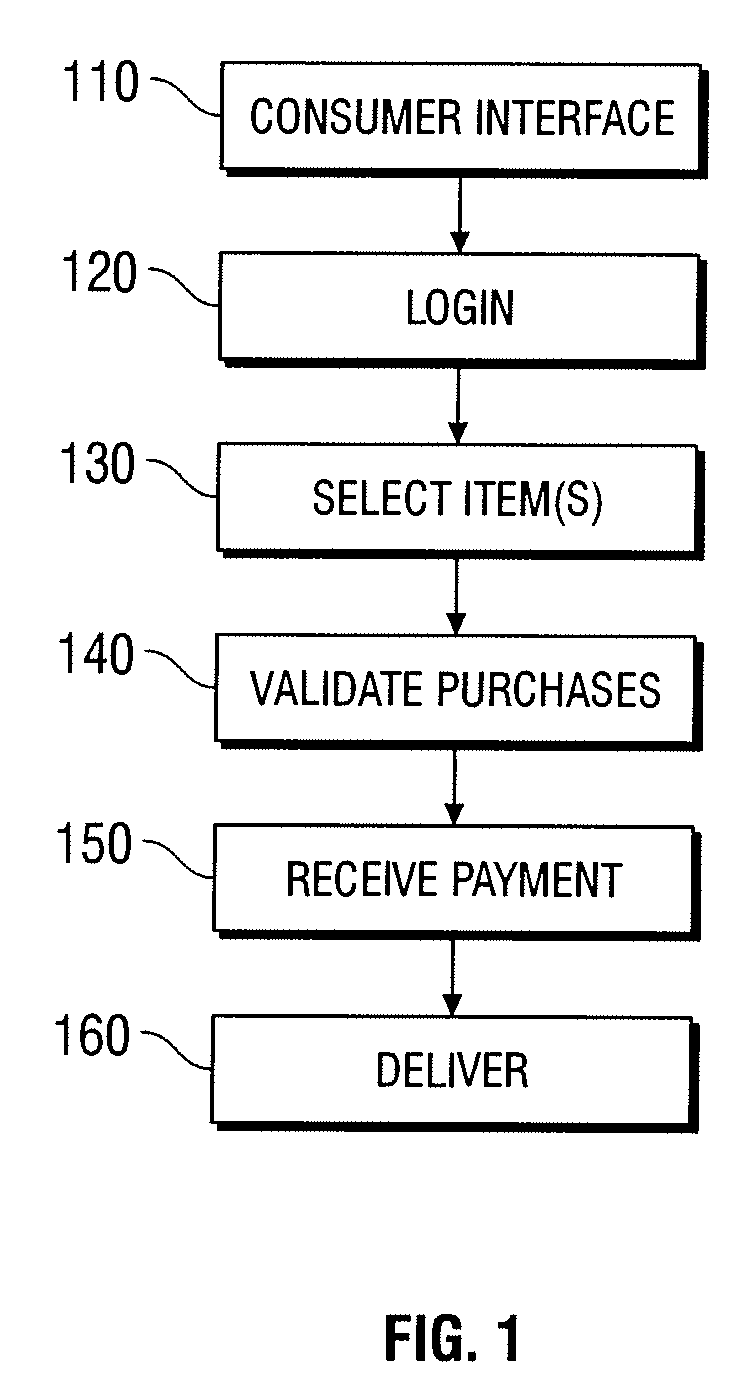
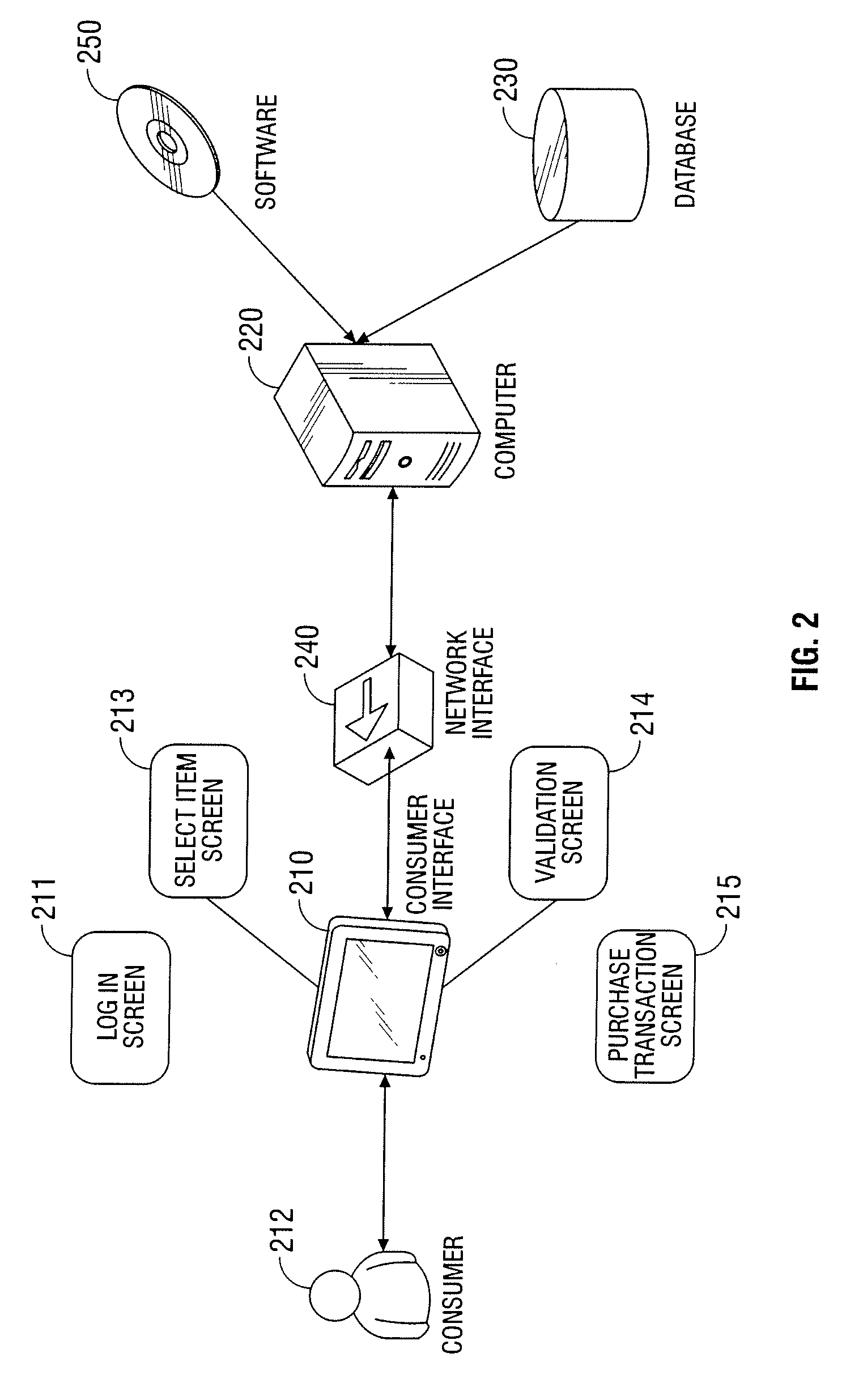
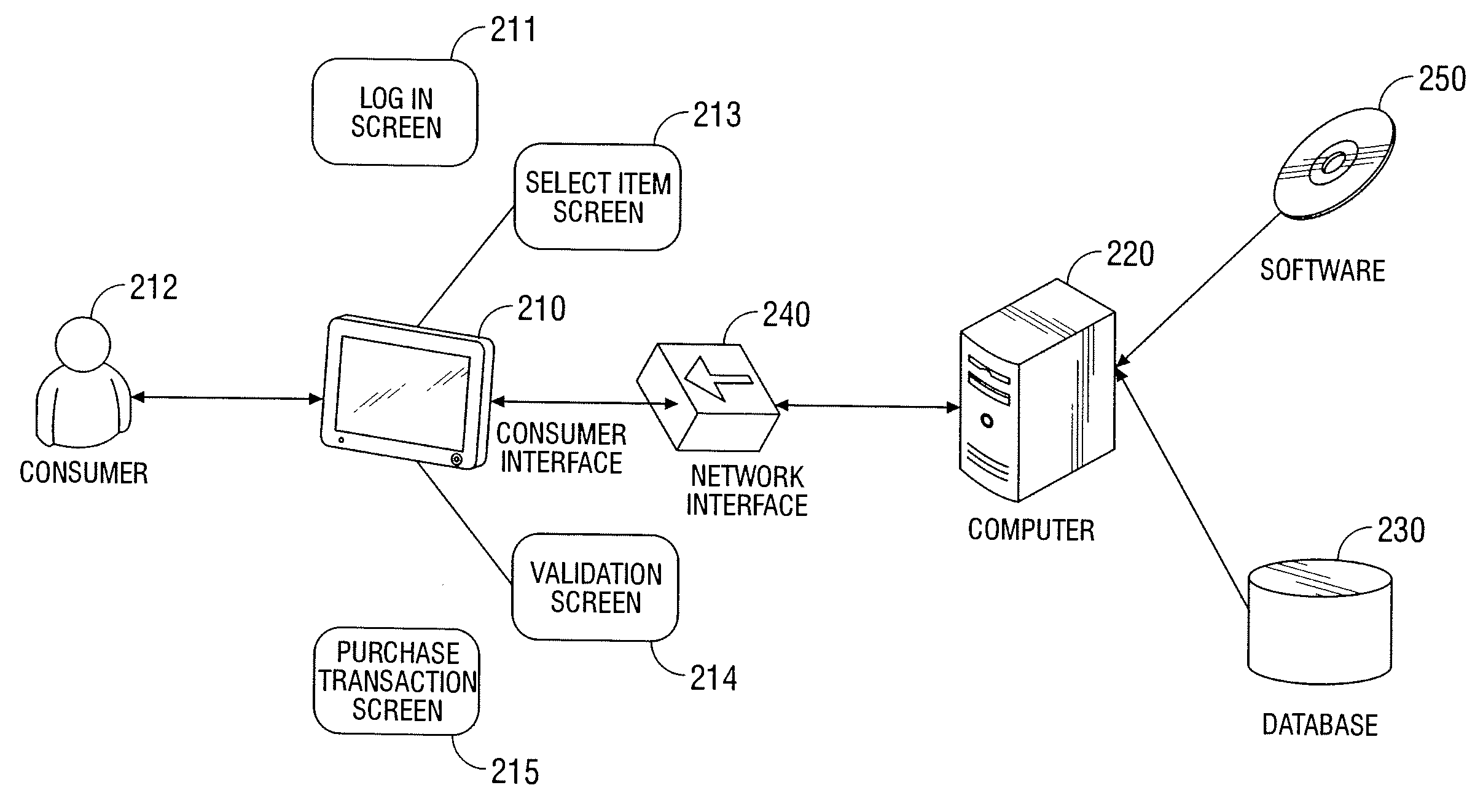
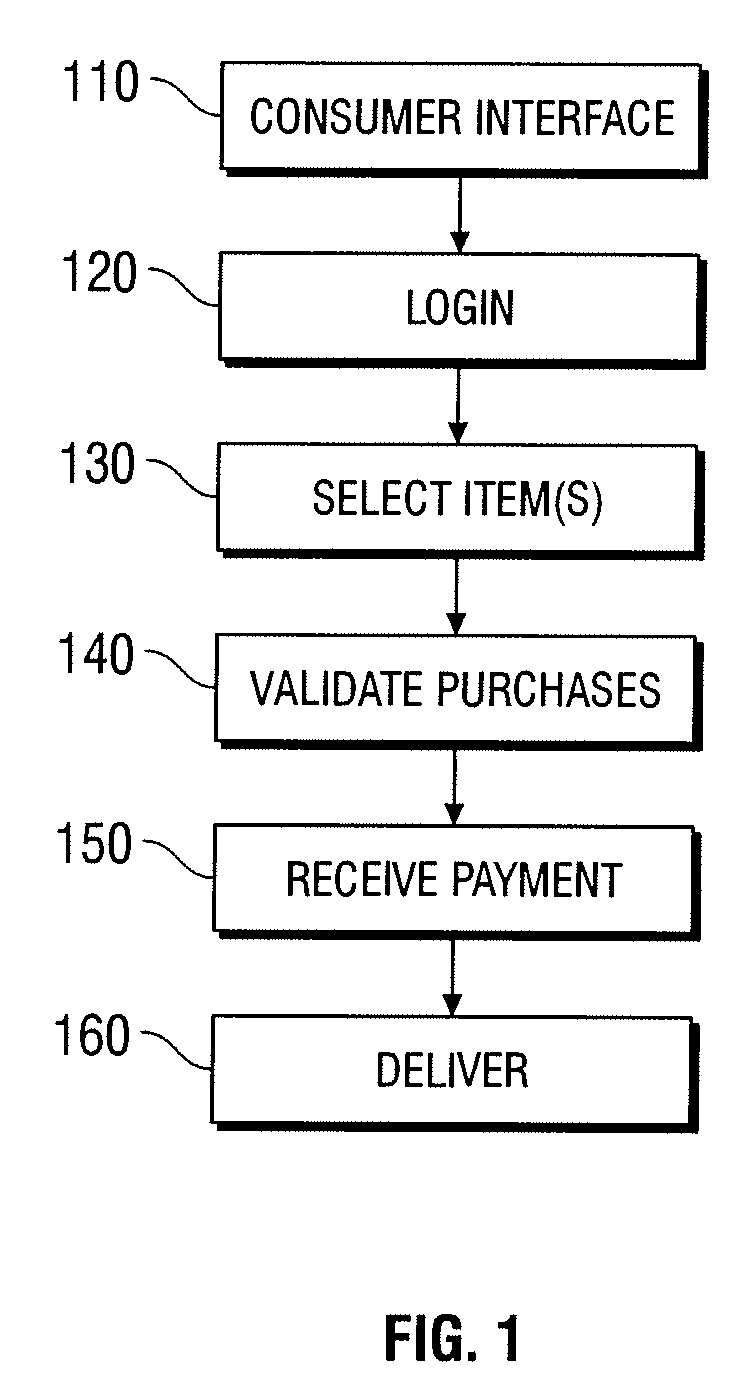
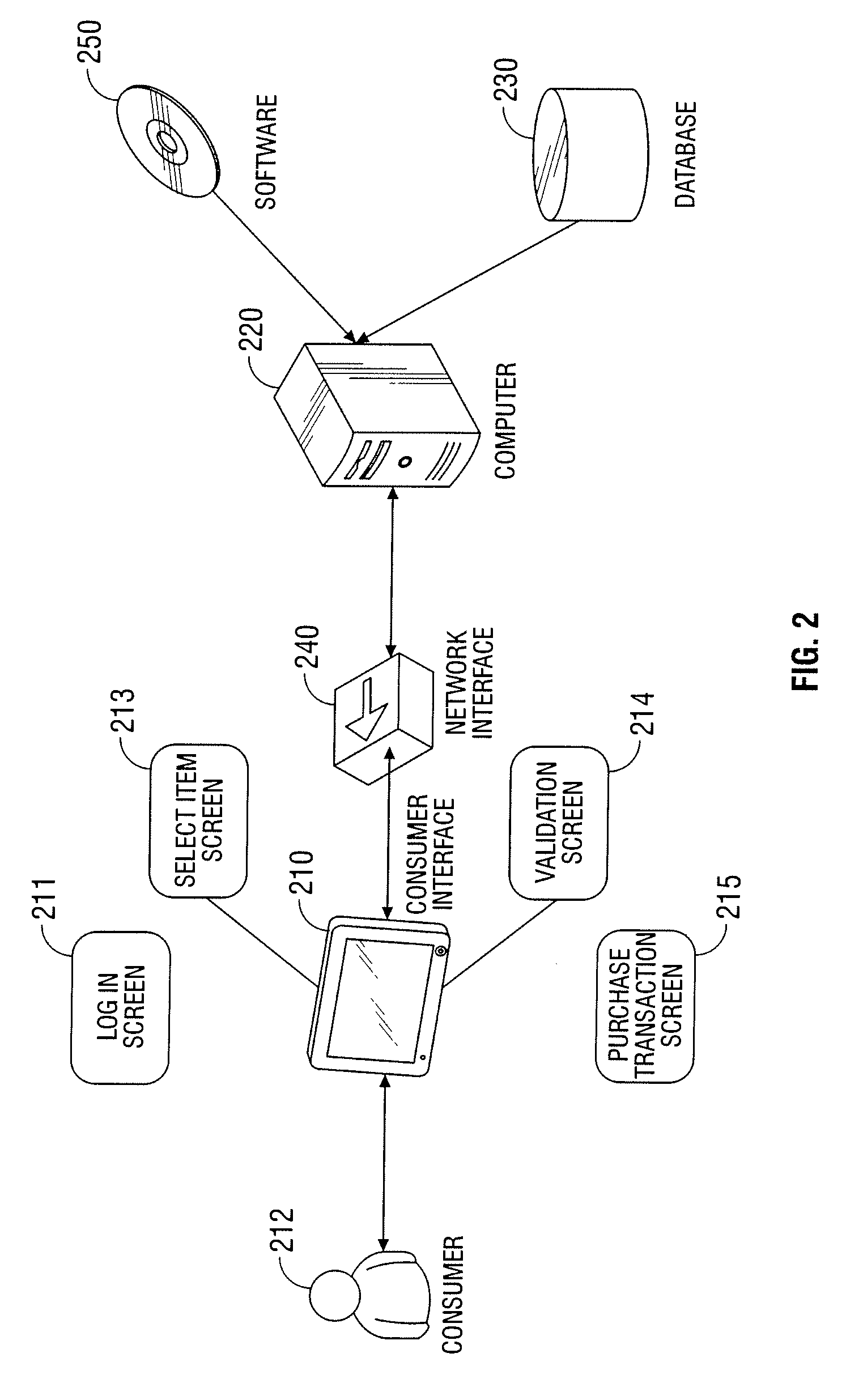
Automated vending of products containing controlled substances
ActiveUS7783379B2Efficient and widespread enforcementReduced resourceCoin-freed apparatus detailsCommercePseudoephedrineControl substances
The present invention provides for devices and methods for vending regulated products, particularly controlled substances, including those containing pseudoephedrine. The present invention allows for the identification of consumers through reliable log-in-procedures, allows the consumer to select items, validates whether the purchase request complies with regulations, to facilitate the delivery of the requested product to a consumer. Other embodiments include a vending machine that is placed into a retail environment in which software enforces validation of the purchasers' identities, limits the amount of pseudoephedrine for each purchaser within the regulations of local, state and federal agencies.This invention reduces the resources which must be expended in retail locations to comply with regulatory agencies, to implement effective counter measures against illegal purchases of regulated and controlled substances, and to ensure the effective limitation of these substances within reasonable limits required for normal consumption.
Owner:ASTERES
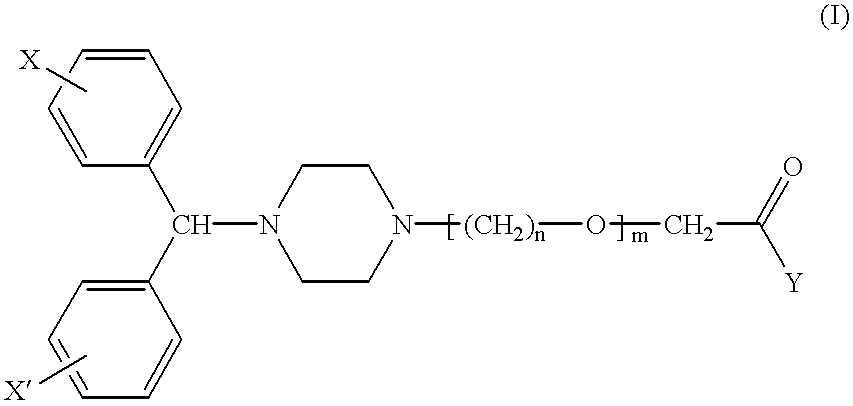
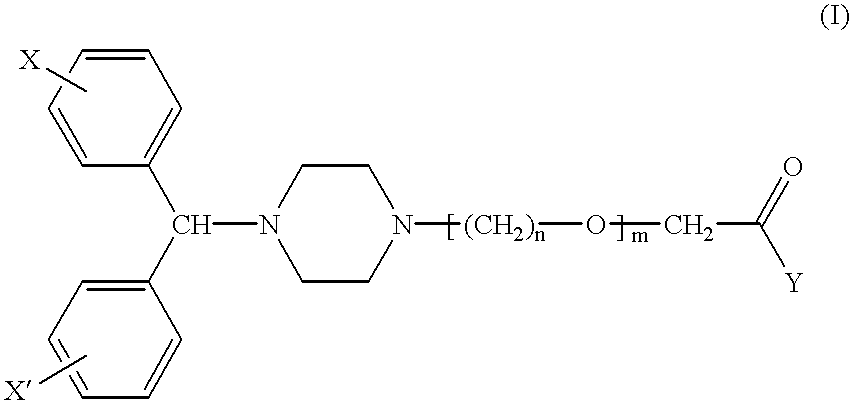
Combination dosage form comprising cetirizine and pseudoephedrine
A dosage form containing cetirizine as an immediate release component and pseudoephedrine or a pharmaceutically acceptable salt thereof as a controlled release component. A portion of the pseudoephedrine can also be incorporated as an immediate release component. The dosage form is free of alcohols having a molecular weight lower than 100 and reactive derivatives thereof.
Owner:JOHNSON & JOHNSON CONSUMER COPANIES
Automated Vending of Products Containing Controlled Substances
ActiveUS20080269947A1Efficient and widespread enforcementReduced resourceAcutation objectsCoin-freed apparatus detailsPseudoephedrineControl substances
The present invention provides for devices and methods for vending regulated products, particularly controlled substances, including those containing pseudoephedrine. The present invention allows for the identification of consumers through reliable log-in-procedures, allows the consumer to select items, validates whether the purchase request complies with regulations, to facilitate the delivery of the requested product to a consumer. Other embodiments include a vending machine that is placed into a retail environment in which software enforces validation of the purchasers' identities, limits the amount of pseudoephedrine for each purchaser within the regulations of local, state and federal agencies.This invention reduces the resources which must be expended in retail locations to comply with regulatory agencies, to implement effective counter measures against illegal purchases of regulated and controlled substances, and to ensure the effective limitation of these substances within reasonable limits required for normal consumption.
Owner:ASTERES
Solid Pharmaceutical Dosage Form
InactiveUS20110028456A1High drug loadingEasy to manufacturePowder deliveryBiocideValsartanTrenbolone
A pharmaceutical composition comprising a solid unit dosage form comprising: one or more of pharmaceutically active ingredients selected from valacyclovir, olanzapine, voriconazole, topotecan, artesunate, amodiaquine, guggulosterone, ramipril, telmisartan, tibolone, atorvastatin, simvastatin, amlodipine, ezetimibe, fenofibrate, tacrolimus, valgancyclovir, valsartan, clopidrogel, estradiol, trenbolone, efavirenz, metformin, pseudoephedrine, verapamil, felodipine, valproic acid / sodium valproate, mesalamine, hydrochlorothiazide, levosulpiride, nelfinavir, cefixime and cefpodoxime proxetil in combination with a water insoluble polymer and / or a water soluble polymer. Methods for making the pharmaceutical composition are also disclosed.
Owner:CIPLA LTD
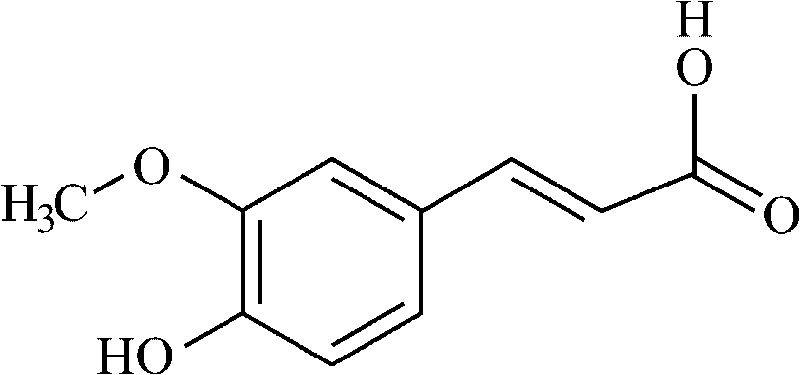
Action of ferulic acid on enhancing drug effect of some medicaments and purpose thereof
InactiveCN101721400AOrganic active ingredientsPharmaceutical non-active ingredientsCatharanthineChemical reaction
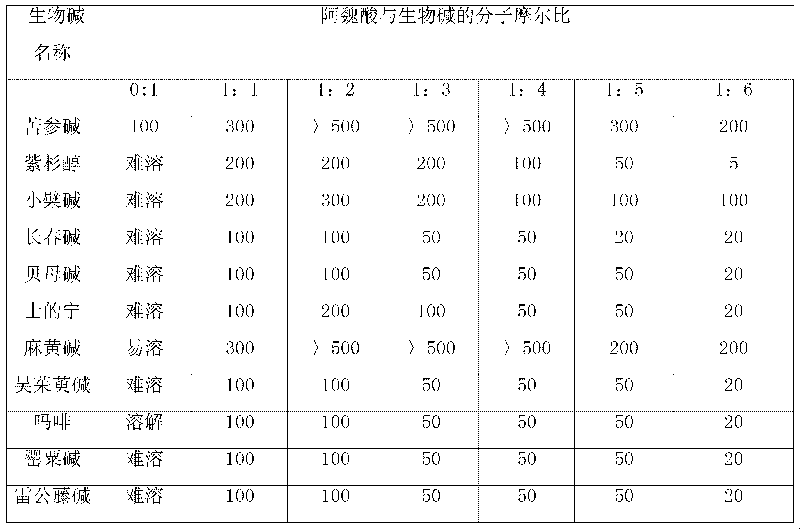
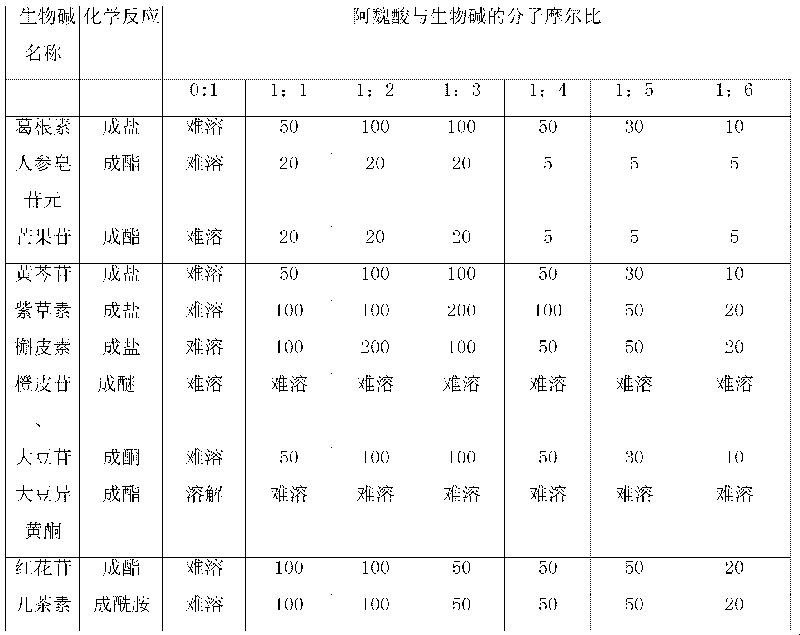
The invention relates to an action of ferulic acid on enhancing the drug effect of some medicaments and a purpose thereof, wherein the medicaments mainly comprise alkaloid medicaments including matrine, kushenin, sophocarpine, hyoscyamine, narceine, hanfangchin A, jamaicin, morphine, codeine, evodiamine, strychnine, catharanthine, vincristine, taxol, verticine, peimine, peiminine, ephedrine, pseudoephedrine, wilfordine, triptolide, tripdiolide and the like, and flavonoid medicaments including puerarin, ginsenoside, ginsengenin, mangiferin, scutelloside, alkannin, meletin, rutin, hesperidin, daidzin, soybean isoflavone, daidzein, carthamin, catechin and the like. The ferulic acid and the medicaments can form a compound or a medicament compound, or the ferulic acid and the medicaments can generate a chemical reaction (including salification, esterification, amidation, ketonization, etherification and the like), and / or the ferulic acid and the medicaments can generate a synergistic effect and an additive effect.
Owner:QINGDAO QIYUAN BIO TECH CO LTD
Orally disintegrating tablets comprising diphenhydramine
The compositions of the present invention comprise a therapeutically effective amount of particles consisting of diphenhydramine or pharmaceutically acceptable salts thereof, optionally in combination with another drug such as pseudoephedrine, or phenylephrine and hydrocodone, in combination with rapidly-dispersing microgranules comprising a disintegrant and a sugar alcohol and / or a saccharide. These compositions are useful in treating the symptoms of one or more diseases or conditions in which diphenhydramine (alone or in combination with one or two other drugs) is a therapeutically effective, e.g. allergic rhinitis, sinusitis, upper respiratory tract infections, motion sickness, Parkinson's disease, insomnia, the common cold, and nighttime pain management, particularly for subjects or patients with dysphagia, and people ‘on the move’.
Owner:ADARE PHARM INC
Prevention of Illicit Manufacutre of Methamphetamine from Pseudoephedrine Using Food Flavor Excipients
The invention relates generally to ephedrine or pseudoephedrine compositions containing biocompatible organoleptic (food flavoring) excipients that would prevent the illicit manufacture of methamphetamine from ephedrine or pseudoephedrine.
Owner:SATARA PHARMA
Pharmaceutical compositions for the treatment of rhinitis
InactiveUS6489329B2Increasing possible adverse effects of the treatmentImproved efficiency over each pharmaceutical substanceBiocideOrganic active ingredientsAcetic acidPseudoephedrine
Owner:UCB PHARMA SA
Tamper resistant lipid-based oral dosage form for sympathomimetic amines
ActiveUS8420700B1Not always easyNot effectiveBiocidePharmaceutical non-active ingredientsPseudoephedrineSympathomimetic Amines
A tamper resistant drug delivery system made of at least one lipid, at least one gelling agent and at least one sympathomimetic amine, such as for example pseudoephedrine, wherein the system gels in the presence of water or a solution containing water and ethanol, wherein the sympathomimetic amine releases into the digestive system when ingested, and wherein the weight ratio of gelling agent to lipid is less than 1:1.4.
Owner:BAUSCH JAMES M +3
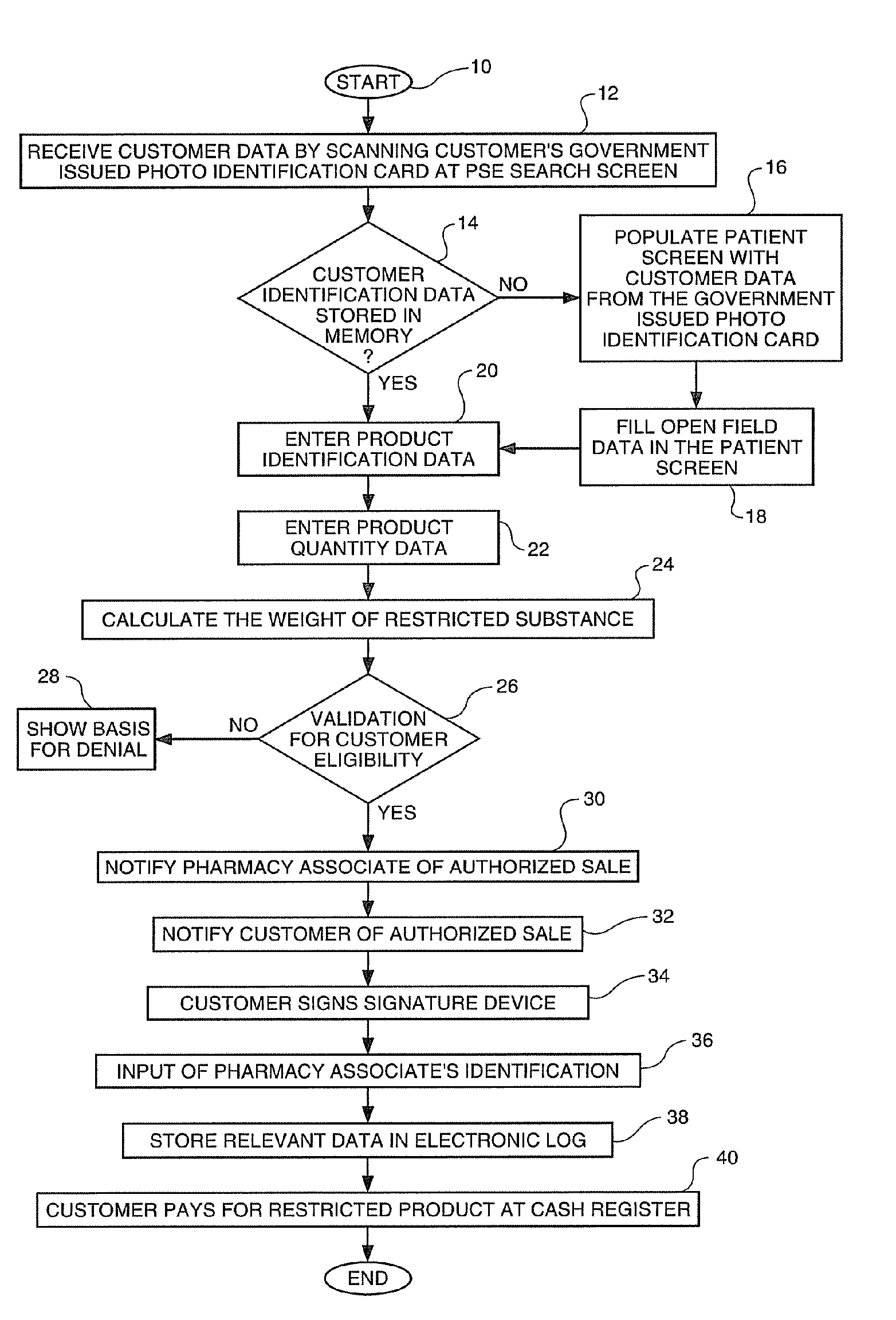
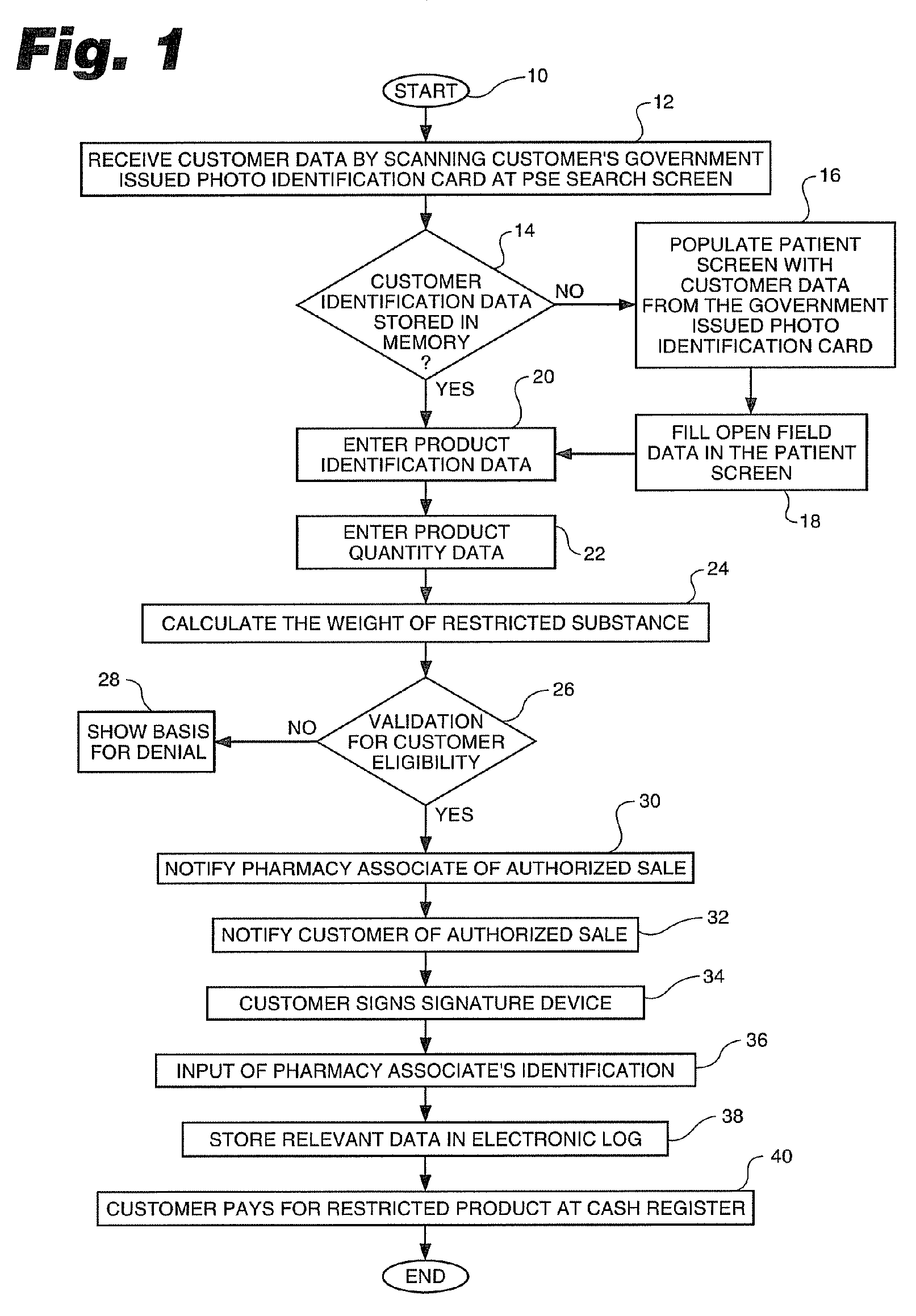
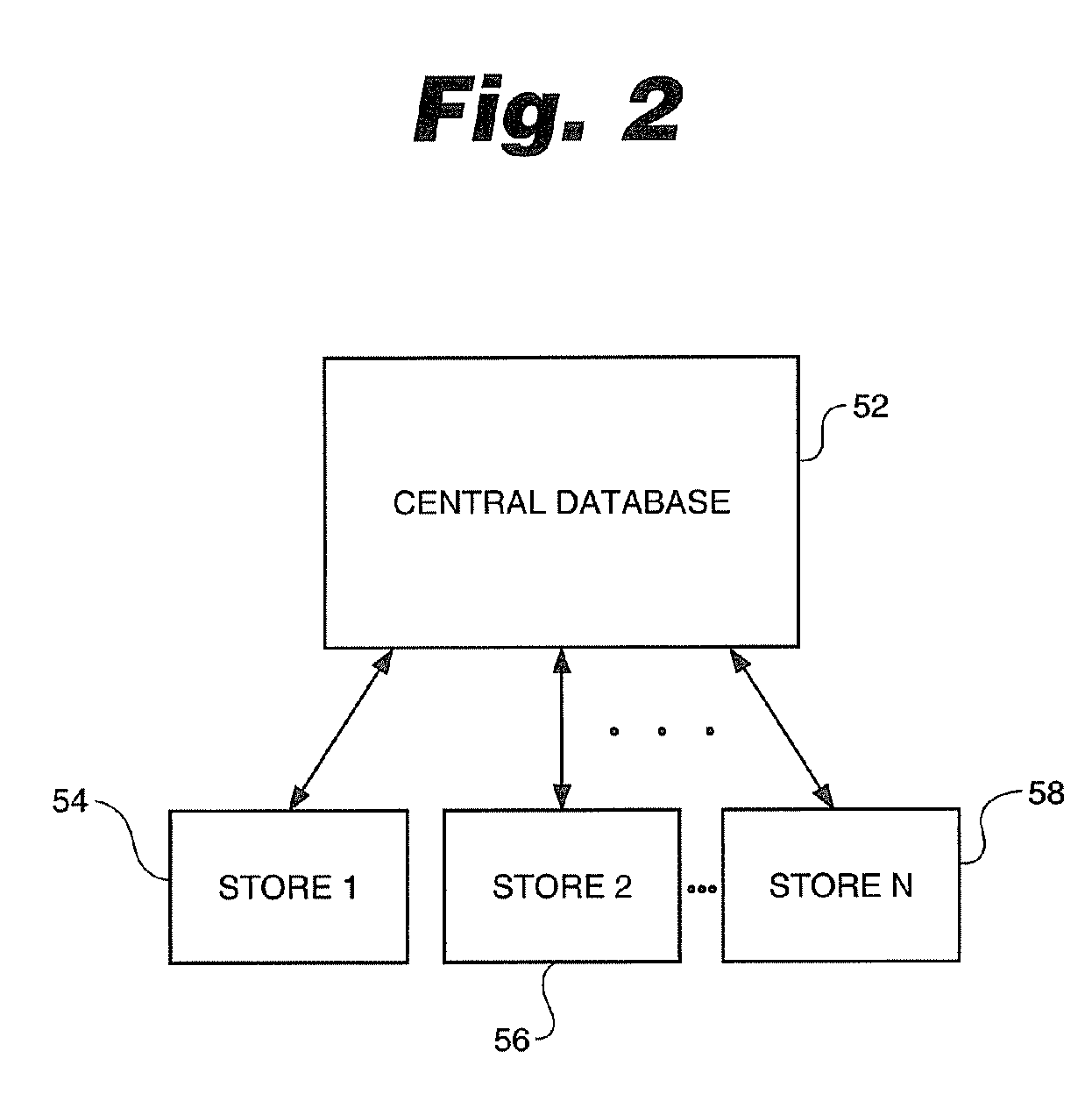
Process for control of restricted product sales in accordance with legal restrictions and expedited creation of a customer log
InactiveUS20070124170A1SpeedProcessing speedOffice automationIndividual entry/exit registersPharmacyCommon cold
In an effort to control the production of illegal drugs such as methamphetamine, new statutes have placed restrictions on the sale of common cold remedies that contain pseudoephedrine and other precursors used in the production of these illegal drugs. Many common products, such as Sudafed® cold medicine, have been removed from store shelves and are now behind the counter at most pharmacies. These legal restrictions vary from state to state, but most restrict the amount of product that can be purchased by quantity and time. These legal restrictions also require creation of a customer log that often includes the customer's name, address, government-issued photo identification number and / or signature. Clearance of these restricted product sales and creation of the customer log are time consuming tasks that result in long lines during the winter season. The present invention is an automated process to speed up the authorization process and creation of the customer logs. The process can be applied to a single store or multiple stores. The invention also includes a clearance terminal especially adapted for this process.
Owner:WALMART APOLLO LLC
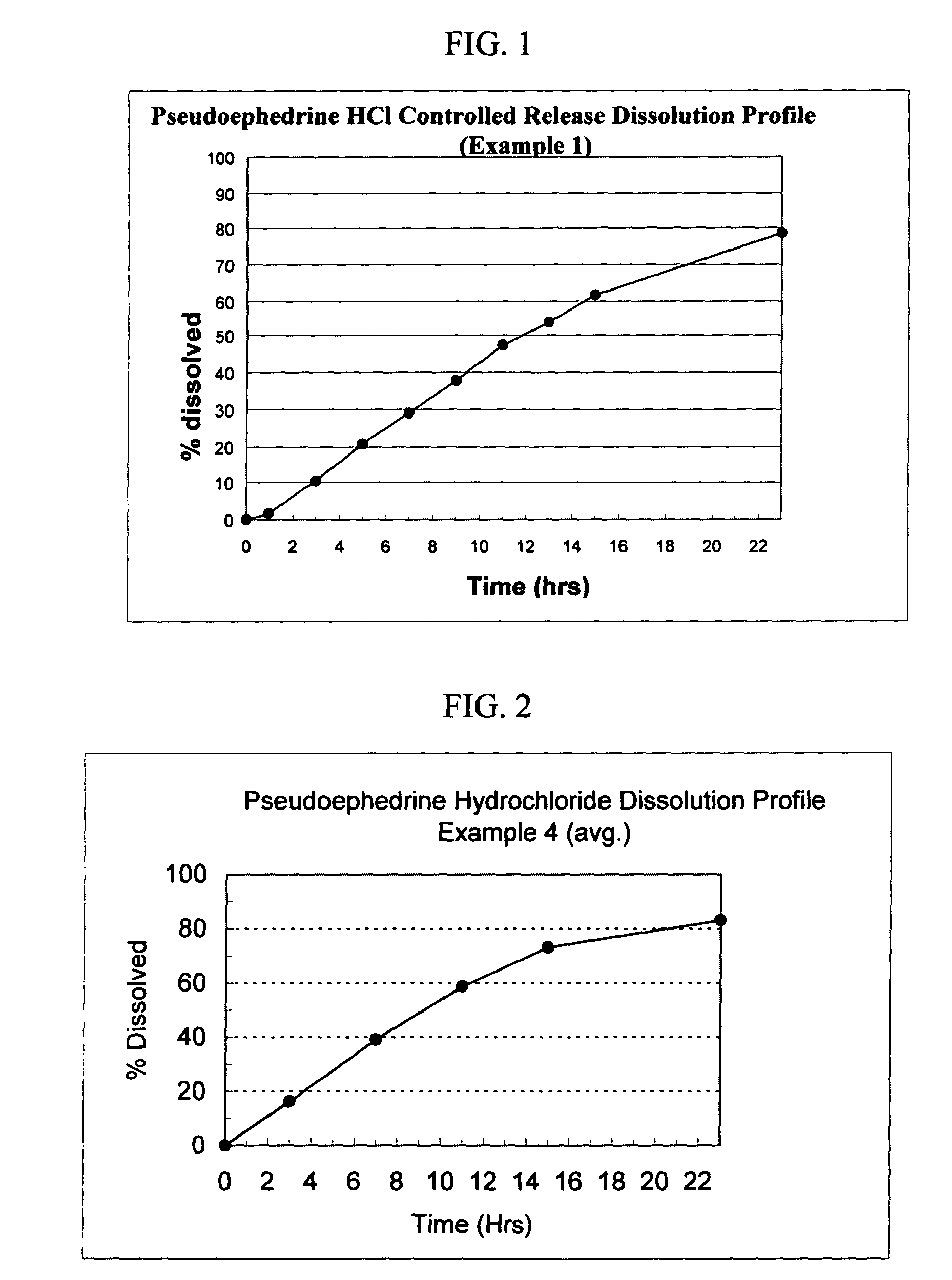
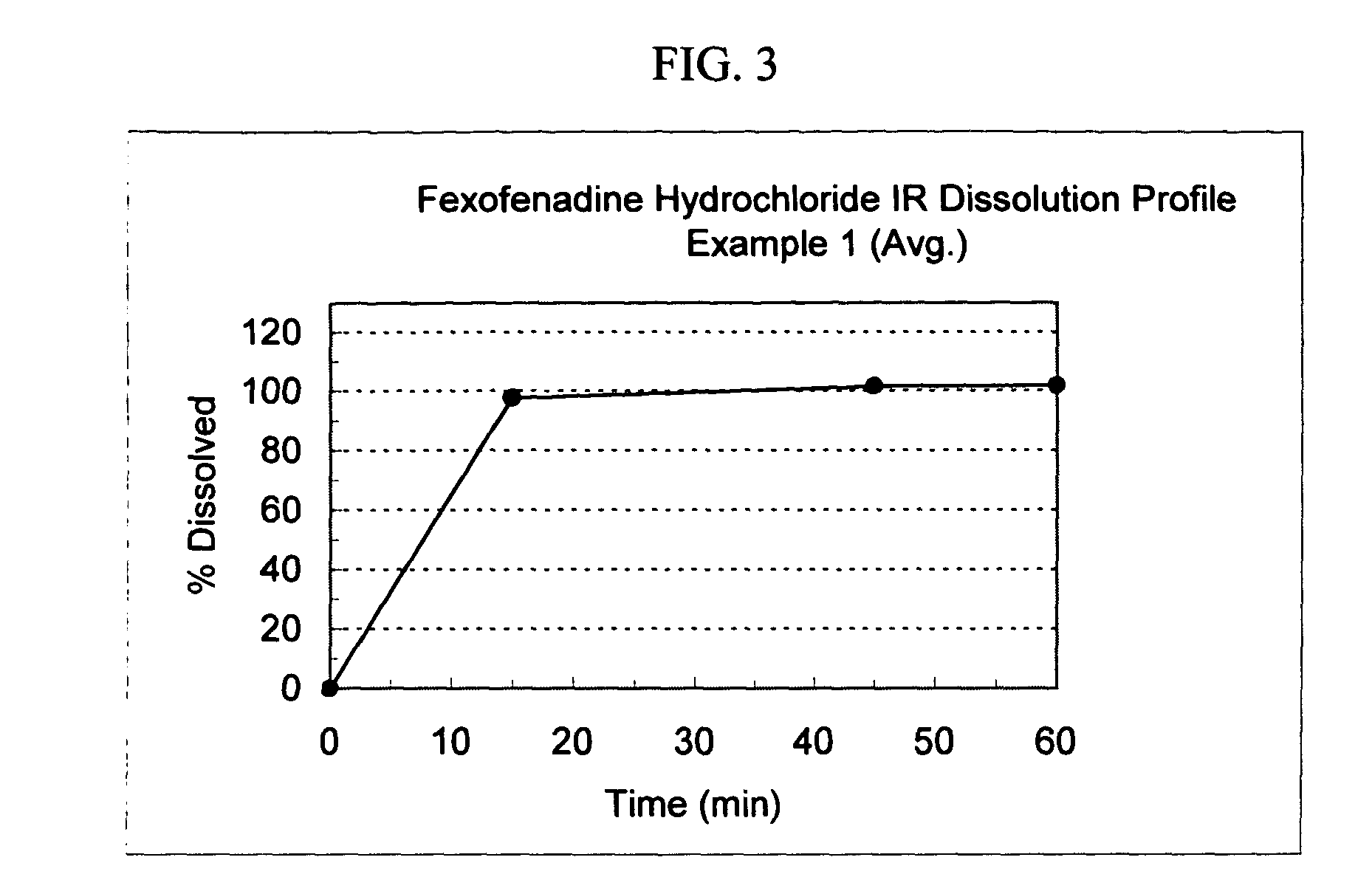
Pseudoephedrine pharmaceutical formulations
InactiveUS20100260842A1Simple and cost-effective drug delivery technologyPromoting nasal drainageBiocideOrganic active ingredientsControlled releasePseudoephedrine
Controlled-release pharmaceutical formulations comprising pseudoephedrine or any of its pharmaceutically acceptable salts, processes for preparing the pharmaceutical formulations, and methods of using the formulations.
Owner:DR REDDYS LAB LTD +1
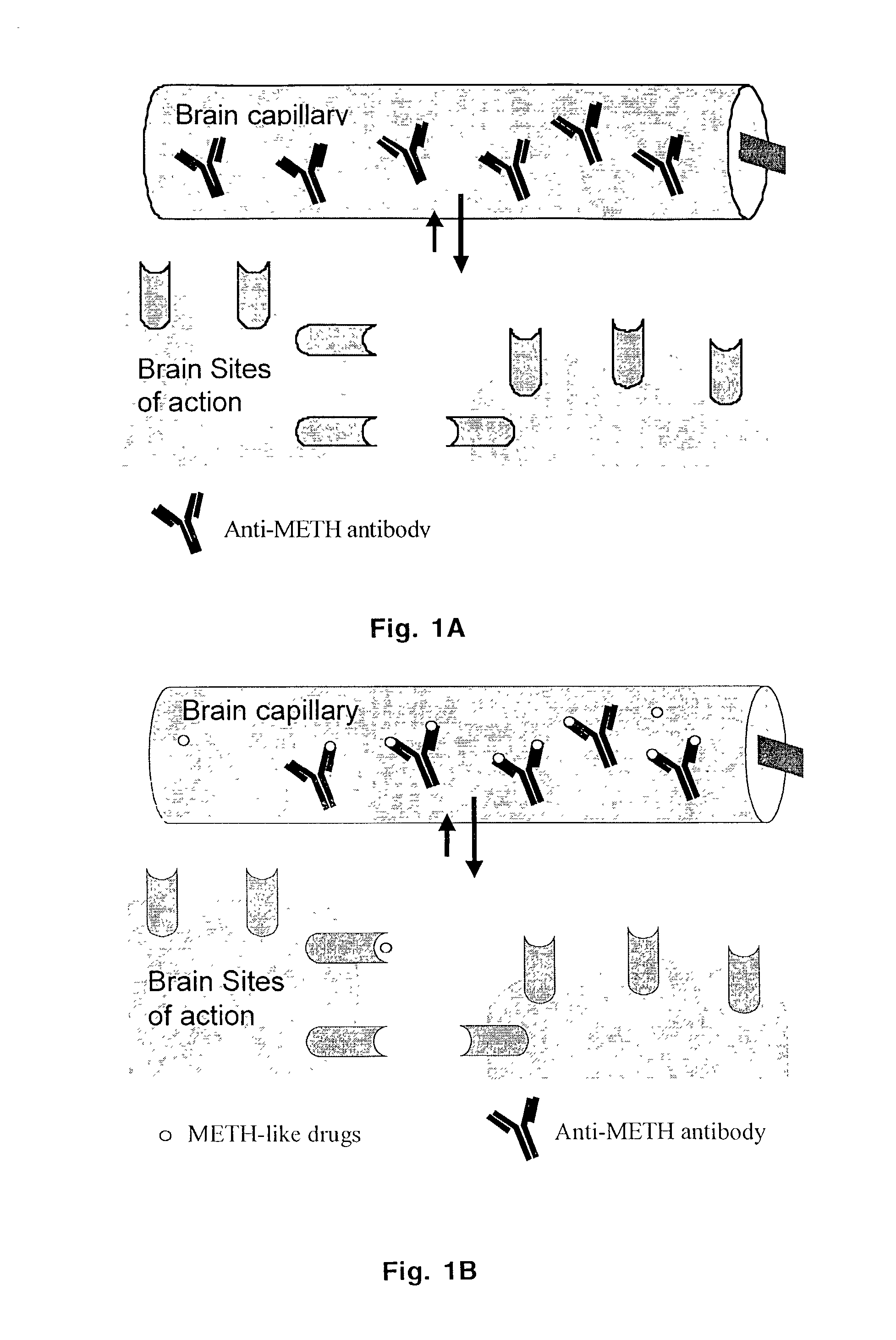
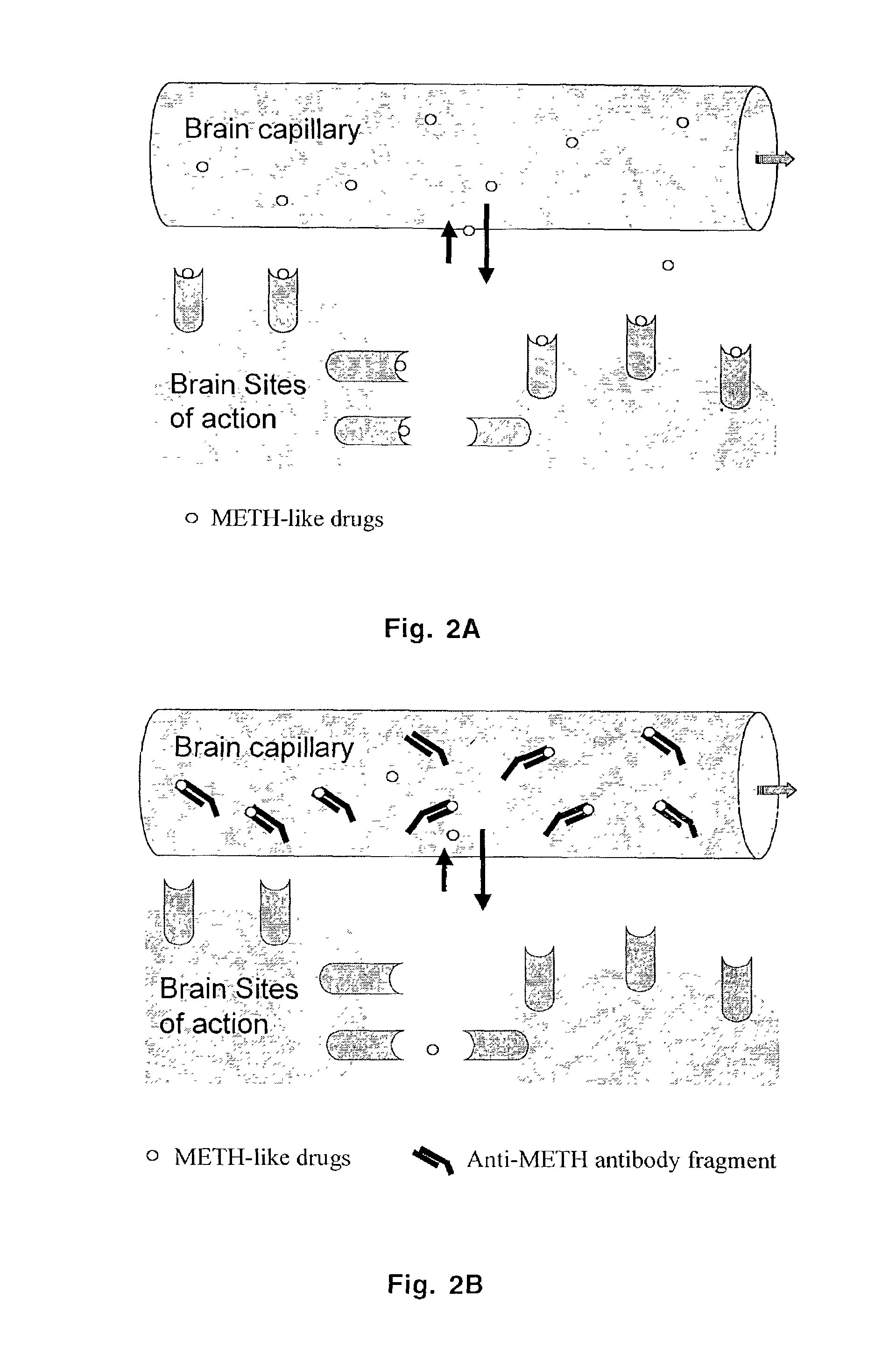
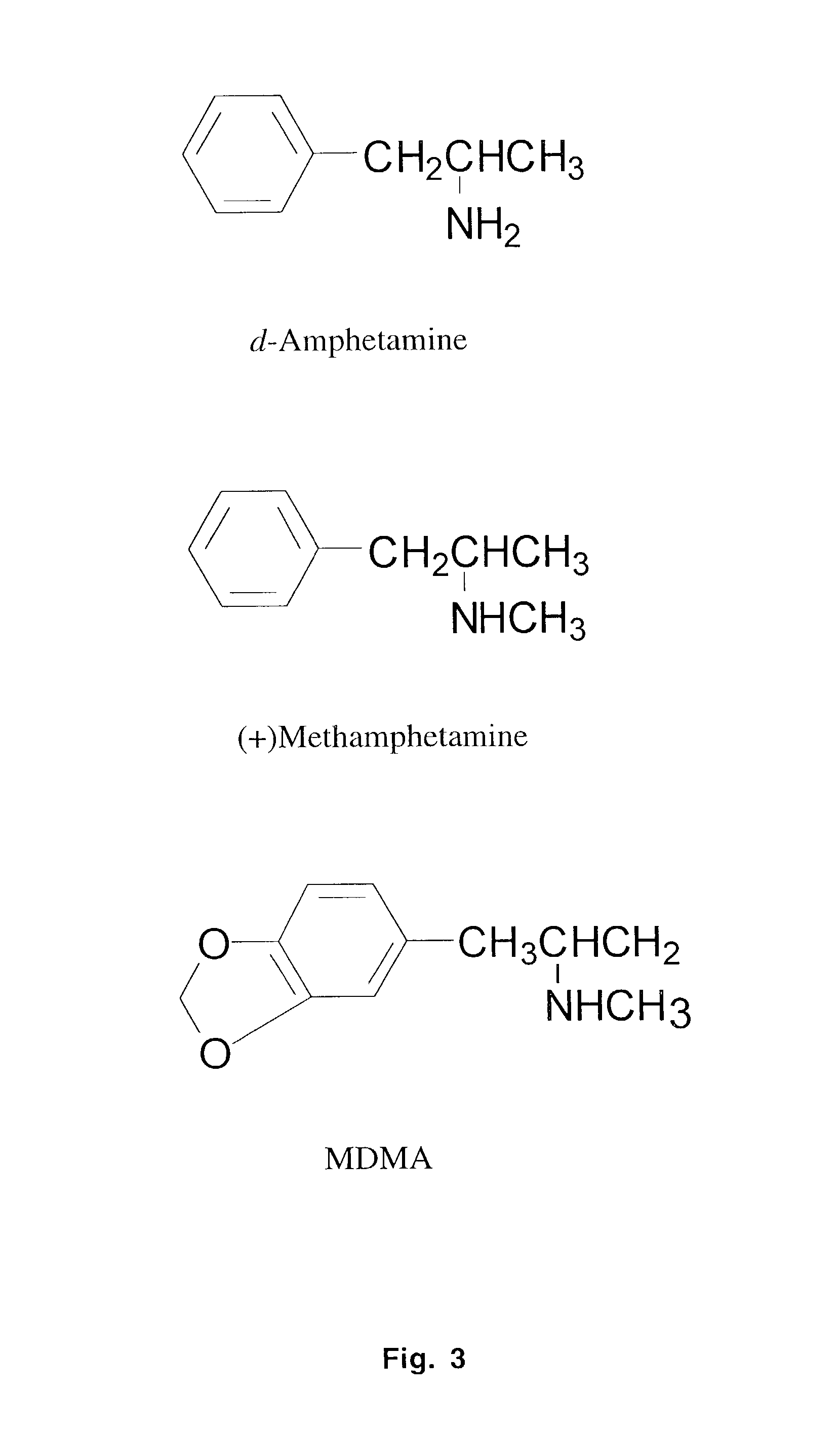
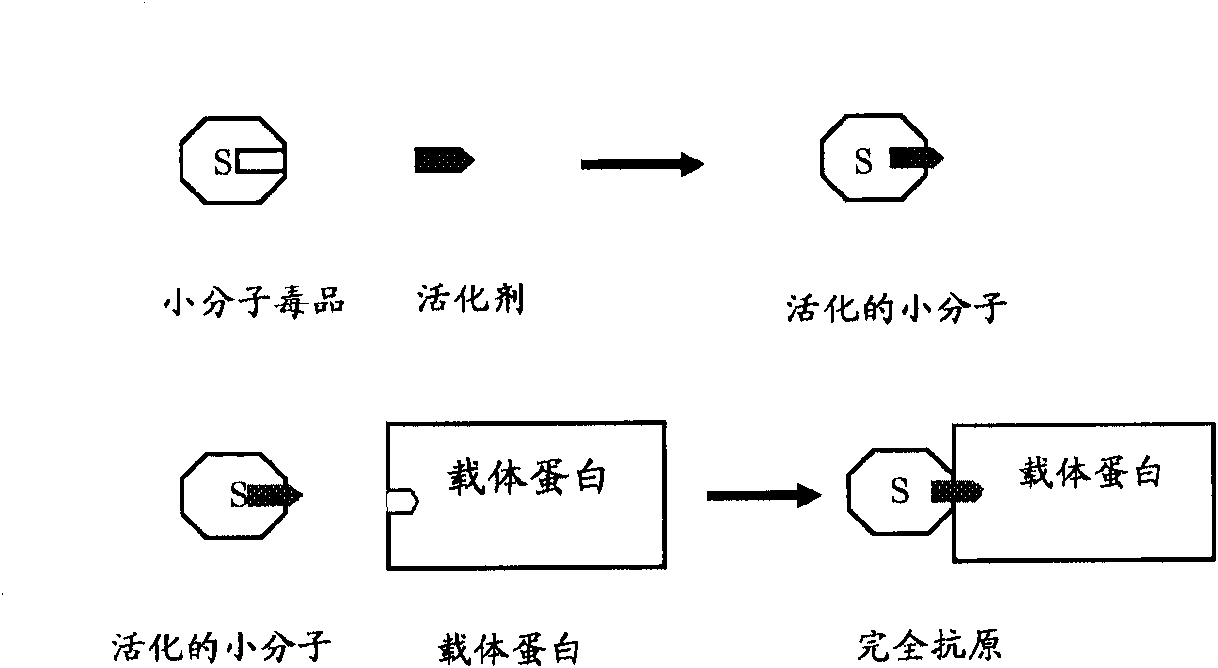
Monoclonal antibody antagonists for treating medical problems associated with d-amphetamine-like drugs
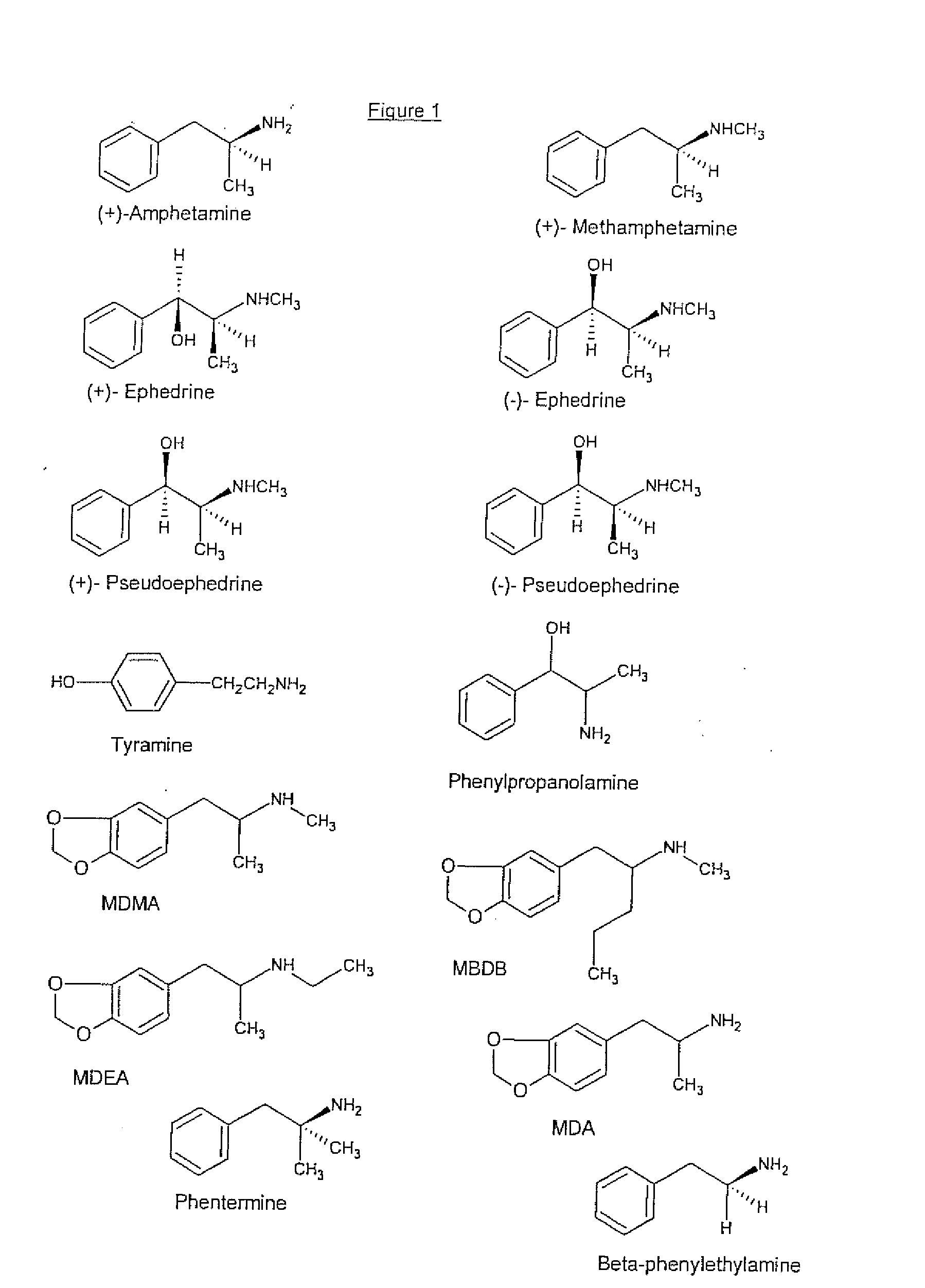
The present invention provides synthetic immunochemical haptens for the generation of antibodies that are designed to recognize the common molecular features of d-methamphetamine-like abused stimulants with insignificant cross-reactivity to endogenous substrates (e.g. dopamine) or over-the-counter medications (e.g. 1-methamphetamine, pseudoephedrine, phenylpropanolamine and ephedrine). These monoclonal antibodies and their antigen binding fragments are useful in treatment plans for recovering addicts, in emergency room settings for rapidly reversing a drug overdose, in protection of fetuses from drug-abusing pregnant mothers or in a psychiatric setting to reduce the exacerbation of psychotic disorders caused by stimulant drugs.
Owner:ARKANSAS FOR MEDICAL SCI THE UNIV OF +1
Automated vending of products containing controlled substances
ActiveUS20110047043A1Efficient and widespread enforcementReduced resourceCoin-freed apparatus detailsData acquisition and loggingPseudoephedrineControl substances
The present invention provides for devices and methods for vending regulated products, particularly controlled substances, including those containing pseudoephedrine. The present invention allows for the identification of consumers through reliable log-in-procedures, allows the consumer to select items, validates whether the purchase request complies with regulations, to facilitate the delivery of the requested product to a consumer. Other embodiments include a vending machine that is placed into a retail environment in which software enforces validation of the purchasers' identities, limits the amount of pseudoephedrine for each purchaser within the regulations of local, state and federal agencies.This invention reduces the resources which must be expended in retail locations to comply with regulatory agencies, to implement effective counter measures against illegal purchases of regulated and controlled substances, and to ensure the effective limitation of these substances within reasonable limits required for normal consumption.
Owner:ASTERES
Arbidol containing compound preparation
InactiveCN1572298AProlonged cough incubation periodLess frequent coughingOrganic active ingredientsAntiviralsAdjuvantNose
The present invention relates to a compound preparation containing antiviral drug arbidol, which is especially adapted to respiratory tract infection disease caused by virus. During the respiratory tract infection period, the patient has the discomfort symptoms of fever and nose stuff, therefore, the anti influenza compound preparation on the invention also includes one, two or three of the ibuprofen, alcaine pseudoephedrine, and dextromethorphan hydrobromide on the basis of including alcaine arbidol, and is then added with take orally preparation medicinal adjuvant to make take orally solid dosage forms. The medical effect experiment shows that the cooperation of the active components in the compound preparation is well, and the preparation is convenient in used by patient.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD +1
Gingival retraction cord with wetting agent
A retraction cord for retracting gingival tissue is pre-impregnated with a chemical agent for chemically retracting gingival tissue and / or a hemostatic agent for arresting bleeding. A wetting agent is further applied to the cord to ensure product efficacy and shelf stability. The retracting agent may be an astringent water soluble organic salt such as aluminum potassium sulfate, aluminum sulfate, aluminum chloride, or ferric sulfate-or an inorganic salt of organic vascoconstrictors such as epinephrine, pseudoephedrine, or VISINE. The wetting agent is preferably a non-ionic surface active surfactant such as SILWET L-7607 at a concentration of 0.05% by volume.
Owner:DUX INDS
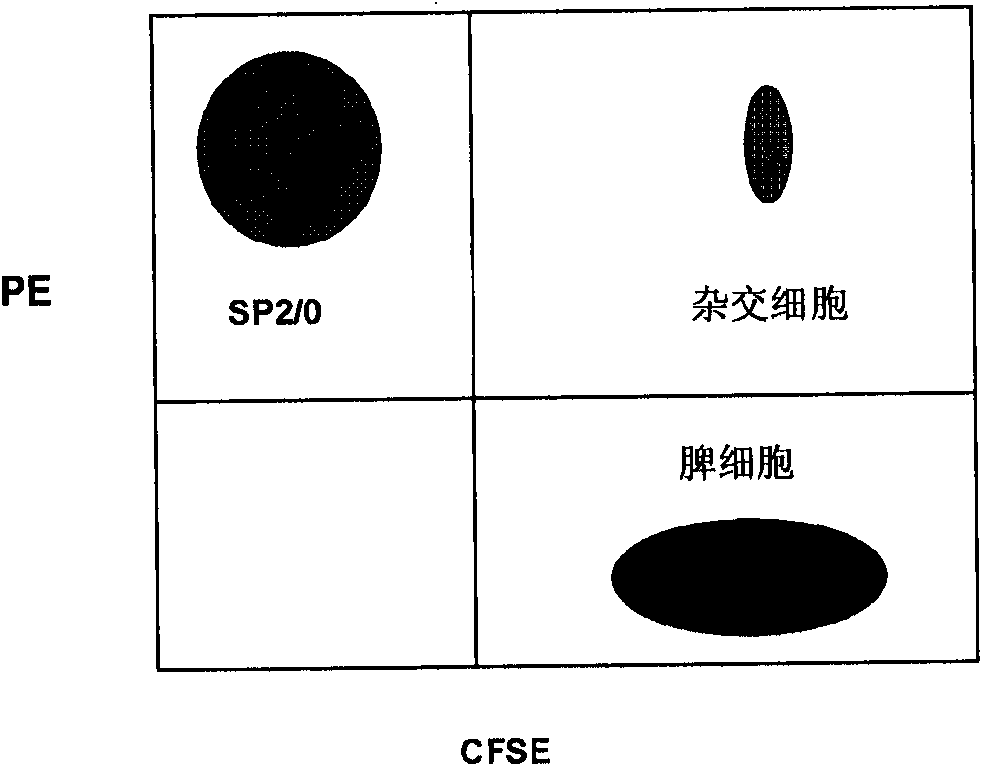
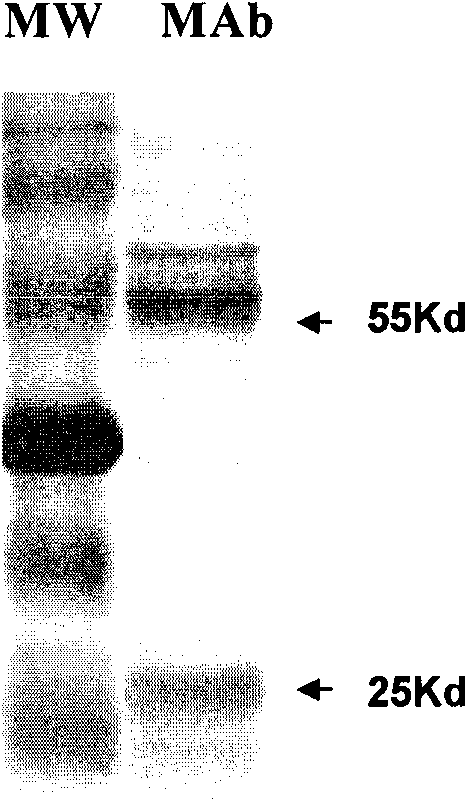
Methamphetamine monoclonal antibody kit having no cross-reactions with ephedrine and pseudoephedrine
ActiveCN101597233AImproving immunogenicityPreserved immunoreactivityOvalbuminSerum albuminPseudoephedrineMonoclonal antibody
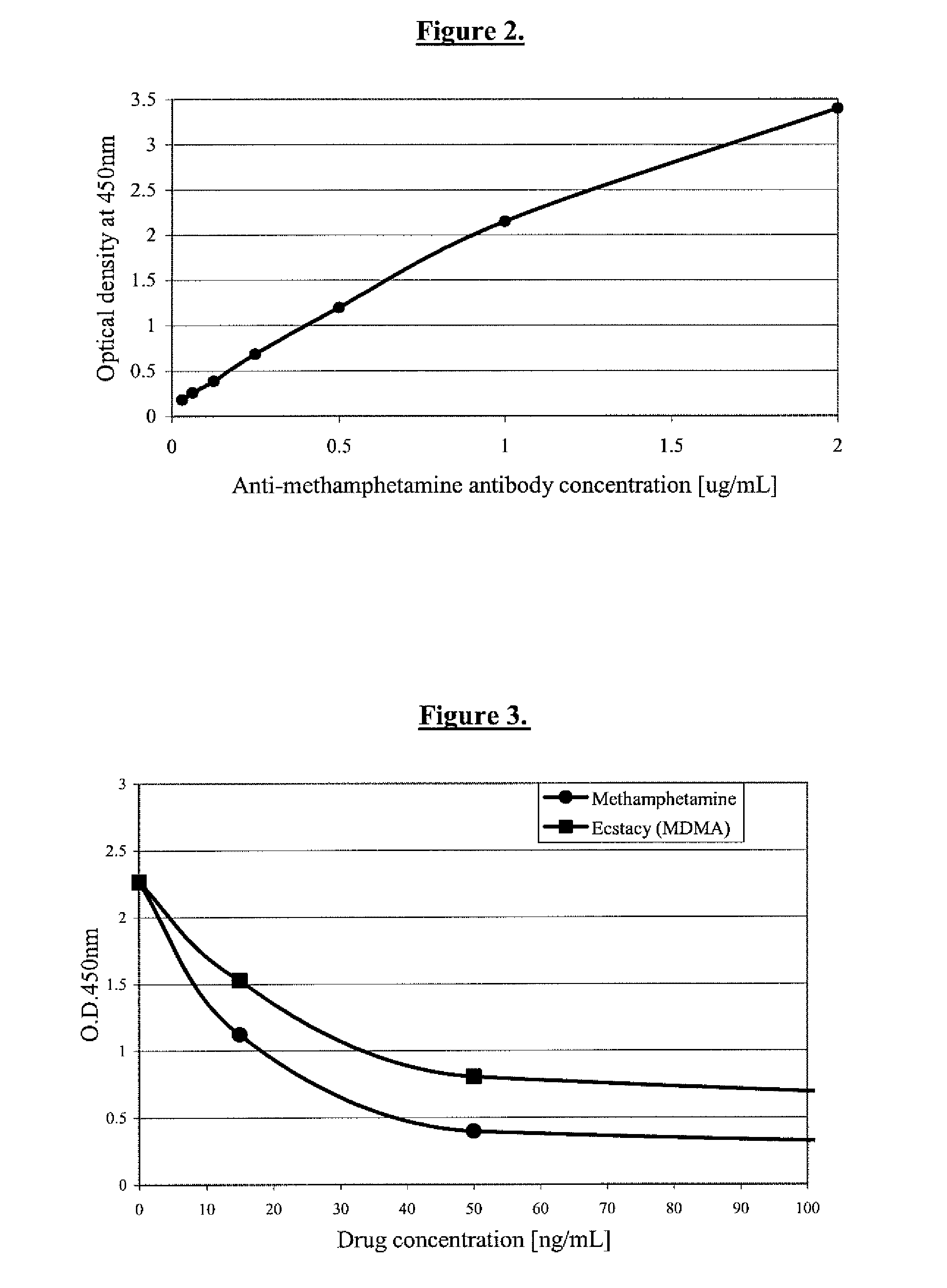
The invention discloses a hapten and a complete antigen which are used for methamphetamine detection and antibody preparation. The invention also discloses an anti-methamphetamine monoclonal antibody prepared from the complete antigen, and a colloidal gold marked methamphetamine monoclonal antibody immunoassay plate, which are used for detecting the methamphetamine in pharmaceutical products and human specimens of urine samples and the like. Compared with chromatographic processes of HPLC and the like, the immunoassay plate is simple and portable and is easy to carry, can perform field detection, and needs no expensive devices. By using the immunoassay plate to detect the methamphetamine, the entire test can be finished in 3 to 5 minutes, the detection sensitivity can reach 300 ng, and the immunoassay plate has no cross-reactions with 60 common medicaments and poisons, in particular ephedrine and pseudoephedrine.
Owner:SHANGHAI CRIMINAL SCI TECH RES INST +1
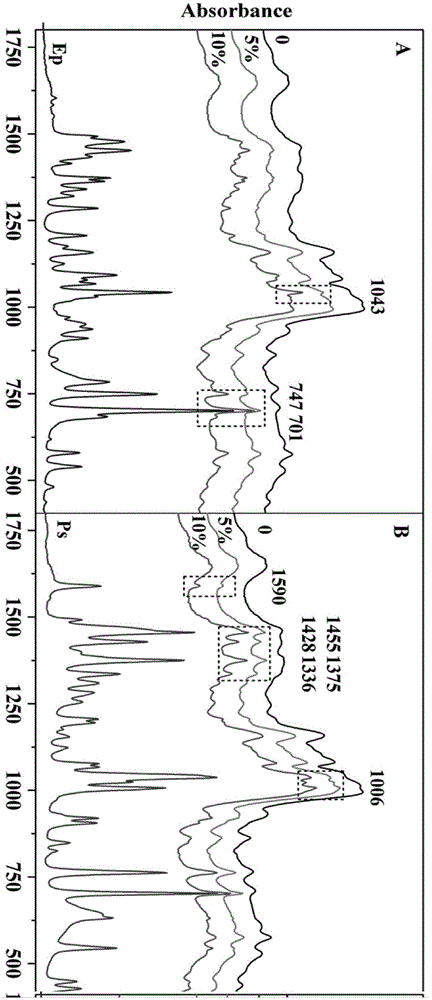
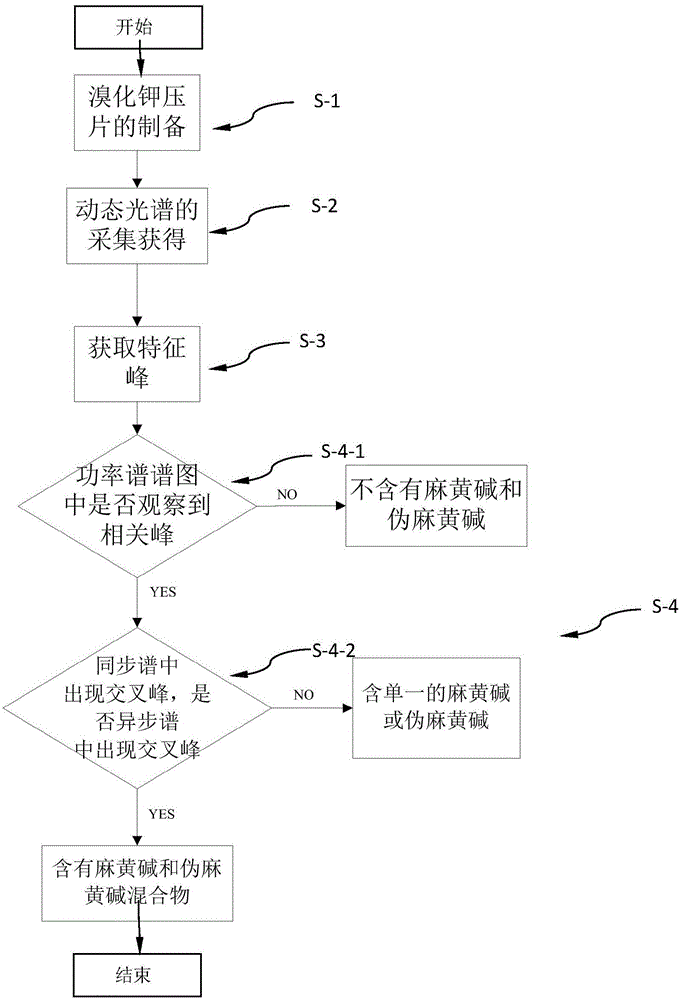
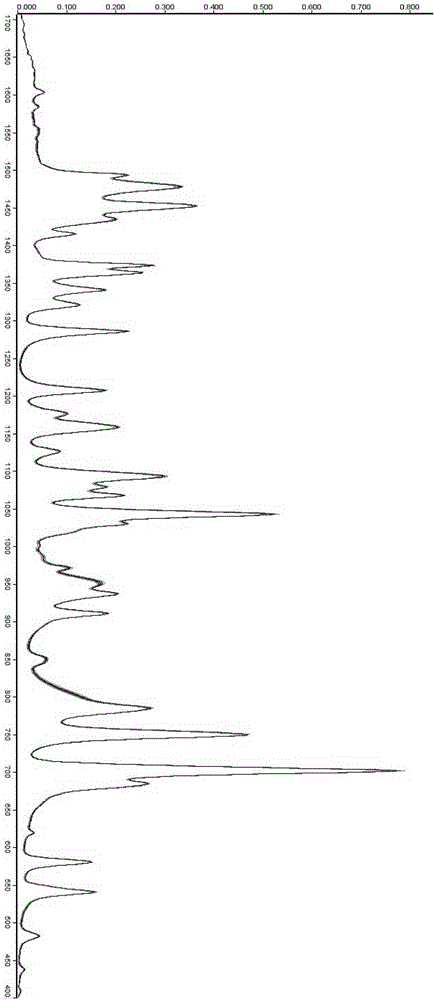
Method for identifying whether ephedrine and/or pseudo ephedrine are/is added to weight-reducing type traditional Chinese medicine or health care products
InactiveCN105158194AHigh-resolutionLower requirementMaterial analysis by optical meansTest sampleMedicine
A method for identifying whether ephedrine and / or pseudo ephedrine are / is added to weight-reducing type traditional Chinese medicine or health care products rapidly identifies whether illicit ephedrine and / or pseudo ephedrine are / is added to weight-reducing type traditional Chinese medicine preparations or health care food through a two-dimensional correlation infrared spectroscopy method and includes the following steps of preparing potassium bromide tablets, collecting a dynamic spectrum, obtaining characteristic peaks, conducting comparison and judgment to determine whether a to-be-tested sample contains ephedrine and / or pseudo ephedrine, wherein two-dimensional correlation spectra of the obtained to-be-tested sample, an ephedrine standard sample and a pseudo ephedrine standard sample are compared and judged, and characteristic peaks, correlation peaks and cross peaks are sequentially contrasted for a two-dimensional correlation power spectrum, a two-dimensional correlation synchronous spectrum and a two-dimensional correlation asynchronous spectrum.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Therapeutic formulations for the treatment of cold and flu-like symptoms
A pharmaceutical formulation of therapeutically effective amounts of acetaminophen, ibuprofen, and a sympathomimetic drug, such as pseudoephedrine (or its prodrug), or phenylephrine used in the treatment of cold and flu-like symptoms. Such symptoms may include fever, pain, nasal congestion, sinus congestion, runny nose, sore throat, myalgia, ear pressure and fullness, and headache. The formulation further includes various excipients used in the formulation process.
Owner:KINGSWAY PHARMA
Compound formula dextro methaphen oral disintegration tablet and its preparation method
InactiveCN1830442AShort disintegration timeGreat tasteOrganic active ingredientsPill deliveryMANNITOL/SORBITOLPseudoephedrine
An oral disintegrating tablet of dextromethorphan for treating the cold caused cough, nasal congestion and rhinorrhea is proportionally prepared from dextromethorphan, chlorphenamine, pseudoephedrine, beta-cyclodextrin or ion exchange resin, mannitol or lactose starch, and disintegrant through sieving, flavouring, mixing and die pressing.
Owner:SINOPHARM ZHIJUN (SHENZHEN) PHARMA CO LTD
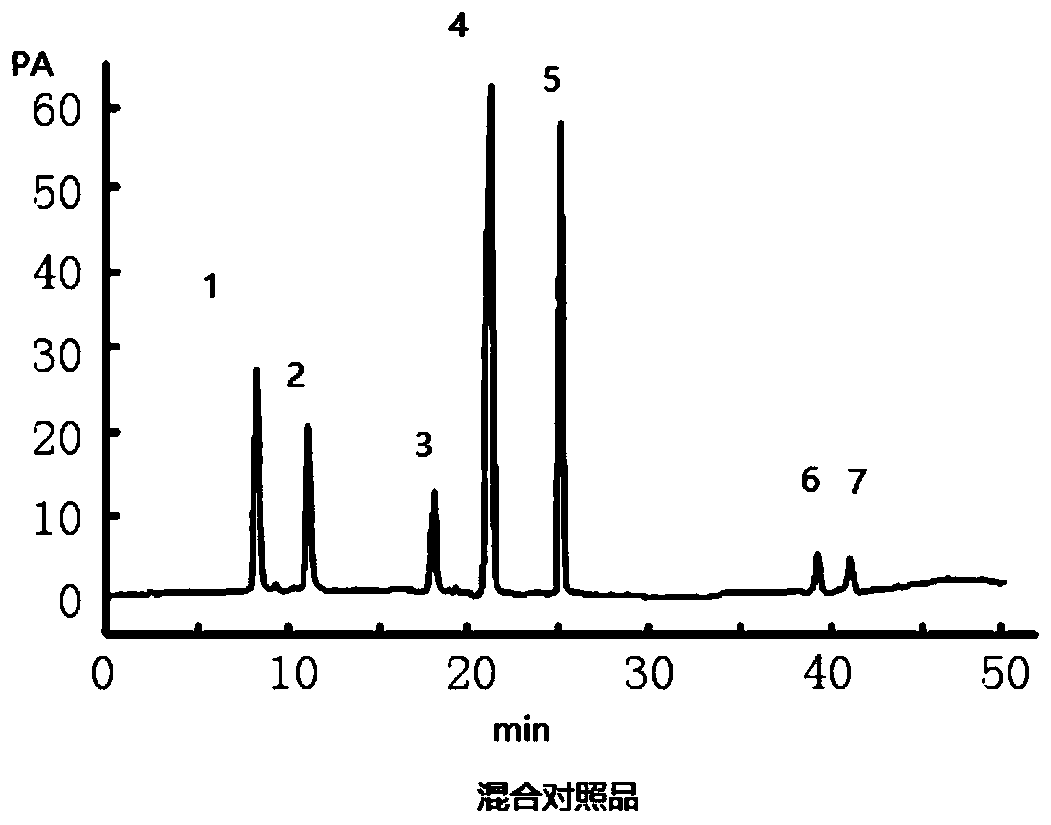
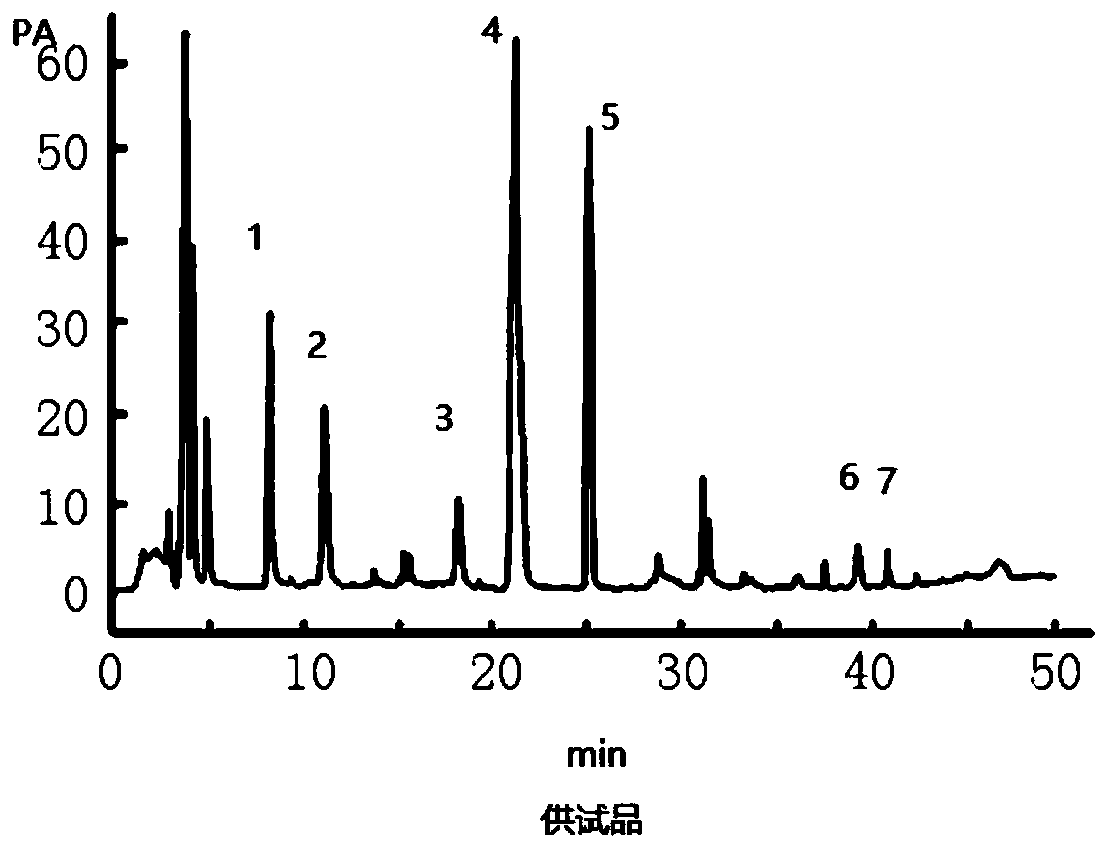
Method for simultaneously measuring seven index components in formula granules of Huagai powder for treatment of wind-cold-caused common cold and asthma by using high performance liquid chromatography
InactiveCN109856270ASimple processing methodFully extractedComponent separationPretreatment methodPseudoephedrine
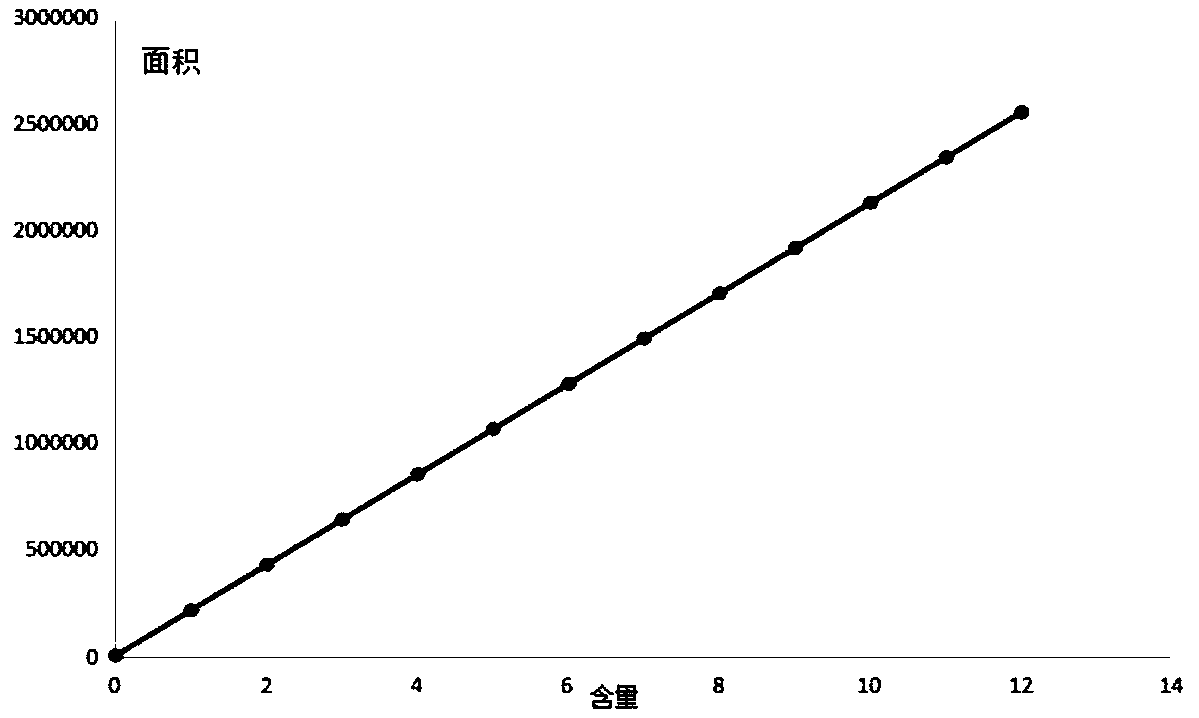
The invention discloses a method for simultaneously measuring seven index components in formula granules of Huagai powder for treatment of wind-cold-caused common cold and asthma by using high performance liquid chromatography. The seven index components include ephedrine hydrochloride, pseudoephedrine, amygdalin, glycyrrhizic acid, liquiritin, sanggenon C, and sanggenon D. The method comprises the following steps: step one, preparing a standard solution; step two, preparing a test sample solution; step three, carrying out liquid chromatogram separation; step four, performing a content calculation method. According to the invention, the pretreatment method of sample extraction is simple and the index components can be extracted fully. The method is performed accurately and rapidly with high sensitivity and low cost; and various methodological indexes can satisfy the actual detection demands. Seven measured compounds have high linearity in a standard curve linear range, wherein R2 is larger than 0.9990; the within-day precision relative standard offsets of all components are less than 1.0%; and the day-to-day precision relative standard offsets are less than 3.0%. The recovery ratesof the seven index components are in a range of 90.0% to 110.9%.
Owner:ZHEJIANG PHARMA COLLEGE +1
Detection of Methamphetamine Group Drugs
InactiveUS20080286816A1Inhibit bindingRisk arisesAnalysis using chemical indicatorsSerum albuminPseudoephedrineHydroxy compound
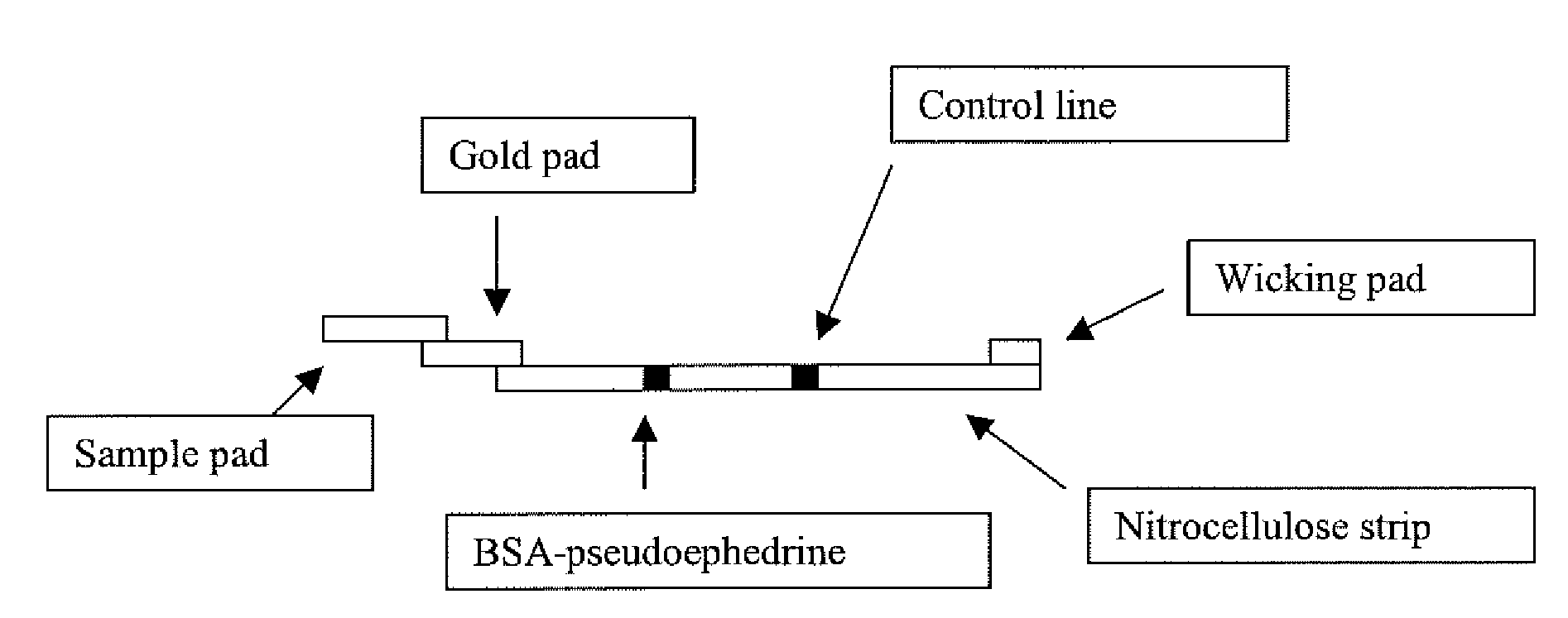
The present invention provides immunoassays which are highly specific for detection in biological samples of methamphetamine and other drugs of abuse of the methamphetamine group such as ecstasy and other ecstasy class drugs. More particularly, competitive assays are provided comprising: (a) contacting said sample with (i) a pseudoephedrine / carrier conjugate in which pseudoephedrine is linked via its hydroxyl group to the carrier and (ii) an antibody which is capable of binding both one or more drugs of the methamphetamine group and said conjugate; and (b) determining whether the binding of said antibody to said conjugate is reduced by the presence of said sample, a reduction in binding being indicative that the sample contains a methamphetamine group drug.
Owner:CONCATENO UK
Preparation methods for ephedrine or pseudoephedrine and for ephedrine or pseudoephedrine intermediate
ActiveCN106008183AWon't happenCutting costsOrganic compound preparationCarbonyl compound preparation by condensationBenzenePseudoephedrine
The invention discloses a preparation method for an ephedrine or pseudoephedrine intermediate. The preparation method comprises the following steps: with 2-chloropropionyl chloride and benzene as starting materials, carrying out the Friedel-Crafts reaction under the catalysis of Lewis acid so as to produce 2-chloro-1-phenyl-1-acetone; and reacting produced 2-chloro-1-phenyl-1-acetone with methylamine in an aprotic solvent so as to produce 2-methylamino-1-phenyl-1-acetone. The invention also discloses a preparation method for ephedrine or pseudoephedrine. According to the methods, phosphorus trichloride with severe pollution is not used anymore, and dangerous and expensive bromine is not used any longer; the Friedel-Crafts reaction and methylamination are carried out in the same solvent, so cost for public works is saved; and a safe, simple, cheap, green and novel process is provided for synthesis of ephedrine and pseudoephedrine.
Owner:ZHEJIANG APELOA KANGYU PHARMA +1
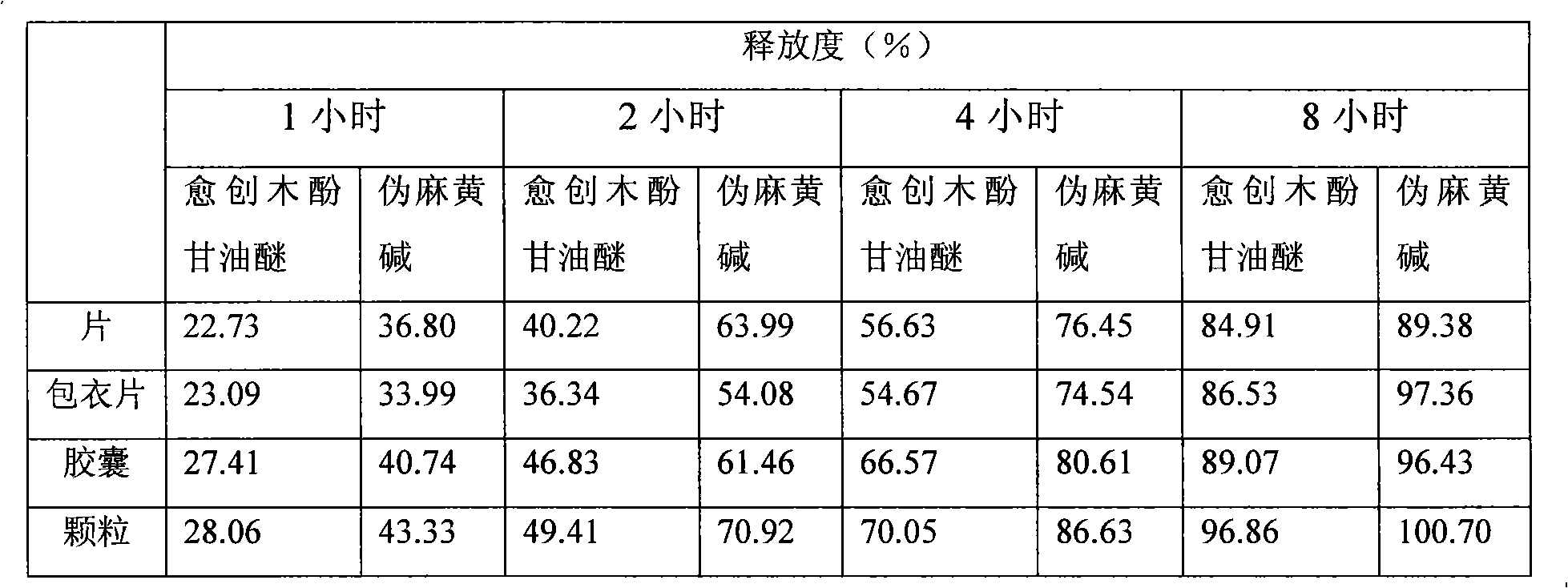
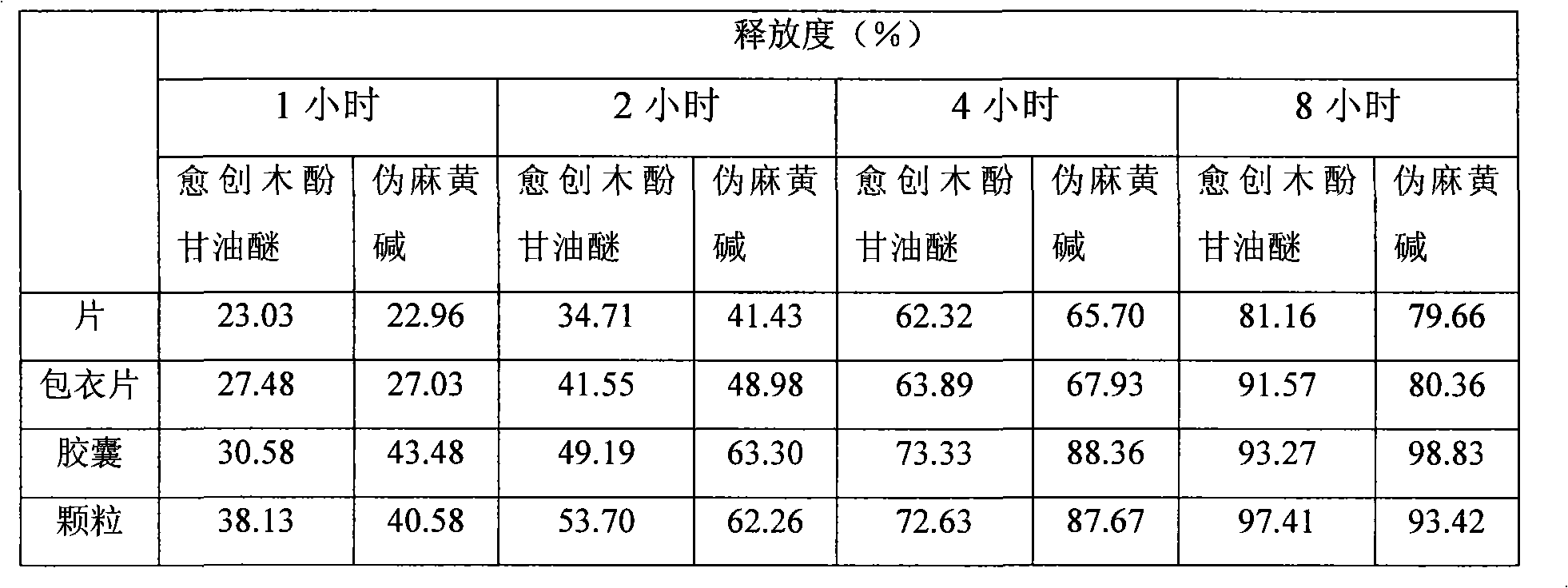
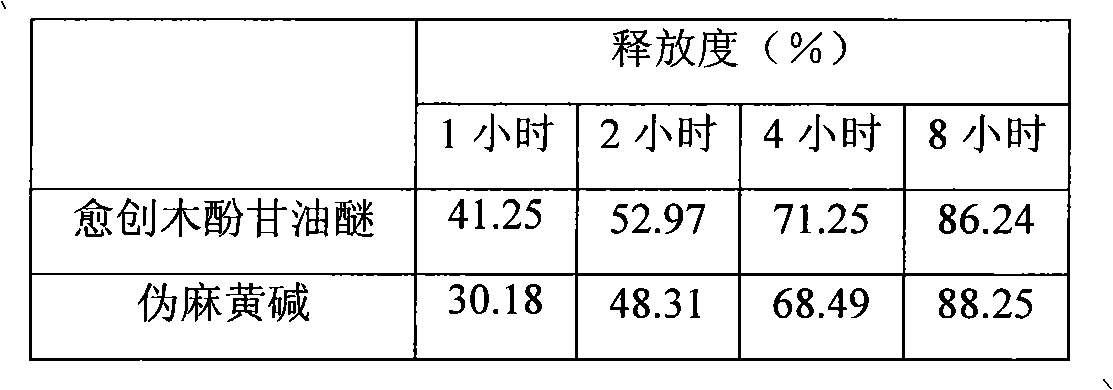
Glyceryl guaiacolate and pseudoephedrine compound sustained release preparation
ActiveCN101658507AStable blood concentrationImprove bioavailabilityEther/acetal active ingredientsAntiviralsActive componentPseudoephedrine
The invention relates to a sustained release preparation taking glyceryl guaiacolate and pseudoephedrine or physiologically acceptable salts thereof as active components, and provides a compound preparation which can overcome cold related symptoms and of which all the active components are slowly released.
Owner:北京科信聚润医药科技有限公司
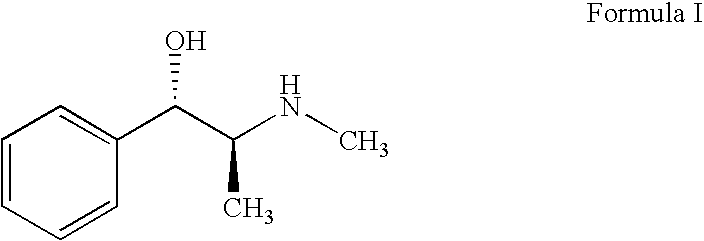
Chemical method for synthesizing ephedrine
ActiveCN101570492ASimple production processEasy to useOrganic compound preparationAmino-hyroxy compound preparationChemical synthesisOrganic acid
The invention relates to a chemical method for synthesizing ephedrine. In the technology, (+ / -)alpha-methylaminophenylpropanone hydrochloride is taken as a raw material and reduced to the mixtures of (+ / -)ephedrine and (+ / -)pseudoephedrine by a proper reducing agent; the (+ / -)ephedrine is separated, and the l-ephedrine or l-ephedrine hydrochloride is separated by using chiral organic acid as a resolving agent. The method enjoys simple technology, less equipment investment, less environment pollution, less hazardous and poisonous chemical reagents which are used and the like.
Owner:QINGHAI LAKE PHARMA COMPANY
Tablet Containing Cetirizine, Pseudoephedrine, and Naproxen Containing a Barrier Layer
In one aspect, the present invention features a tablet including: (i) a first drug layer including naproxen; (ii) a second drug layer including a decongestant (e.g., pseudoephedrine) wherein said second drug layer is a sustained release layer adapted to deliver a therapeutically effective amount of pseudoephedrine for a period of at least twelve hours; and (iii) a barrier layer that does not include naproxen, wherein the barrier layer is in contact with the first drug layer; and (iv) a third drug layer including cetirizine, wherein the third drug layer is in contact with the barrier layer and is not in contact with the first drug layer.
Owner:JOHNSON & JOHNSON CONSUMER COPANIES
Sustained release compound capsules and its preparation method
ActiveCN1593413AImprove complianceEffective plasma concentrationOrganic active ingredientsPharmaceutical delivery mechanismSustained release pelletsPseudoephedrine
The invention discloses a sustained release compound capsules and its preparation method, wherein each capsule comprises a desloratadine tablet and a plurality of pseudo ephedrine slow release particles, the active components include desloratadine 2.5mg-5mg, pseudo ephedrine 120mg-240mg. The compound slow release capsule can increase the biological availability and compliableness for the patients.
Owner:HAINAN PULIN PHARMA +2
Tablet Containing Coated Particles of Cetirizine, Pseudoephedrine, and/or Naproxen
In one aspect, the present invention features a tablet including a first drug layer and a second drug layer, wherein: (i) the first drug layer includes first drug particles including naproxen and third drug particles including cetirizine, where the first drug particles and / or the third drug particles are coated with an immediate release coating; and (ii) the second drug layer including pseudoephedrine, wherein said second drug layer is a sustained release layer adapted to deliver a therapeutically effective amount of pseudoephedrine for a period of at least twelve hours.
Owner:JOHNSON & JOHNSON CONSUMER COPANIES
Compound slow release preparation of acetyl aminophenol, pseudoephedrine and dextromethorphan
InactiveCN101596158AStable blood concentrationImprove bioavailabilityOrganic active ingredientsPharmaceutical delivery mechanismPseudoephedrineBULK ACTIVE INGREDIENT
The invention relates to a slow release preparation taking acetyl aminophenol, pseudoephedrine or physiologically acceptable slat thereof and dextromethorphan or physiologically acceptable slat thereof as active ingredients. The slow release preparation is characterized in that a releasing system comprises a tablet core and / or pill core capable of enabling a medicament to slowly release and a coating, wherein one part and / or all of the active ingredients exist in the tablet core and / or pill core, and the rest of the active ingredients exist in the coating.
Owner:COSCI MED TECH CO LTD
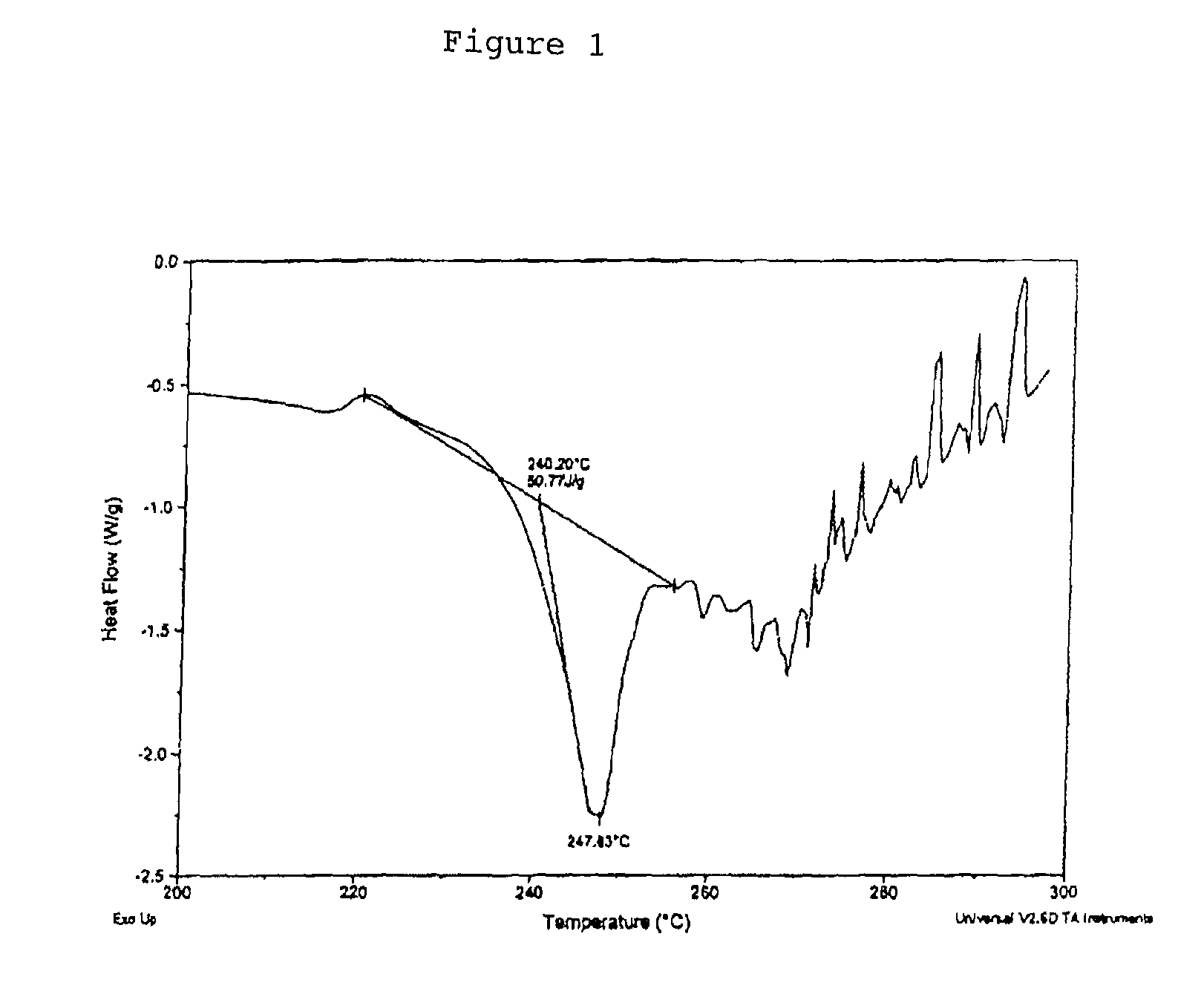
Osmotic device containing pseudoephedrine and an H1 antagonist
InactiveUS7147870B2Faster rateOvercome disadvantagesOsmotic deliveryDrageesDiseaseControlled release
The present invention provides an osmotic device containing controlled release pseudoephedrine in the core in combination with a rapid release H1 antagonist in an external coat. A wide range of H1 antagonist antihistamines, especially fexofenadine, can be used in this device. Particular embodiments of the invention provide osmotic devices having predetermined release profiles. One embodiment of the osmotic device includes an external coat that has been spray coated rather compression coated onto the device. The device with spray coated external core is smaller and easier to swallow than the similar device having a compression coated external coat. The device is useful for the treatment of respiratory congestion related disorders and allergy related disorders. The present devices provide PS and an H1 antagonist according to specific release profiles in combination with specific formulations.
Owner:OSMOTICA KERESKEDELMI & SZOLGALTATO
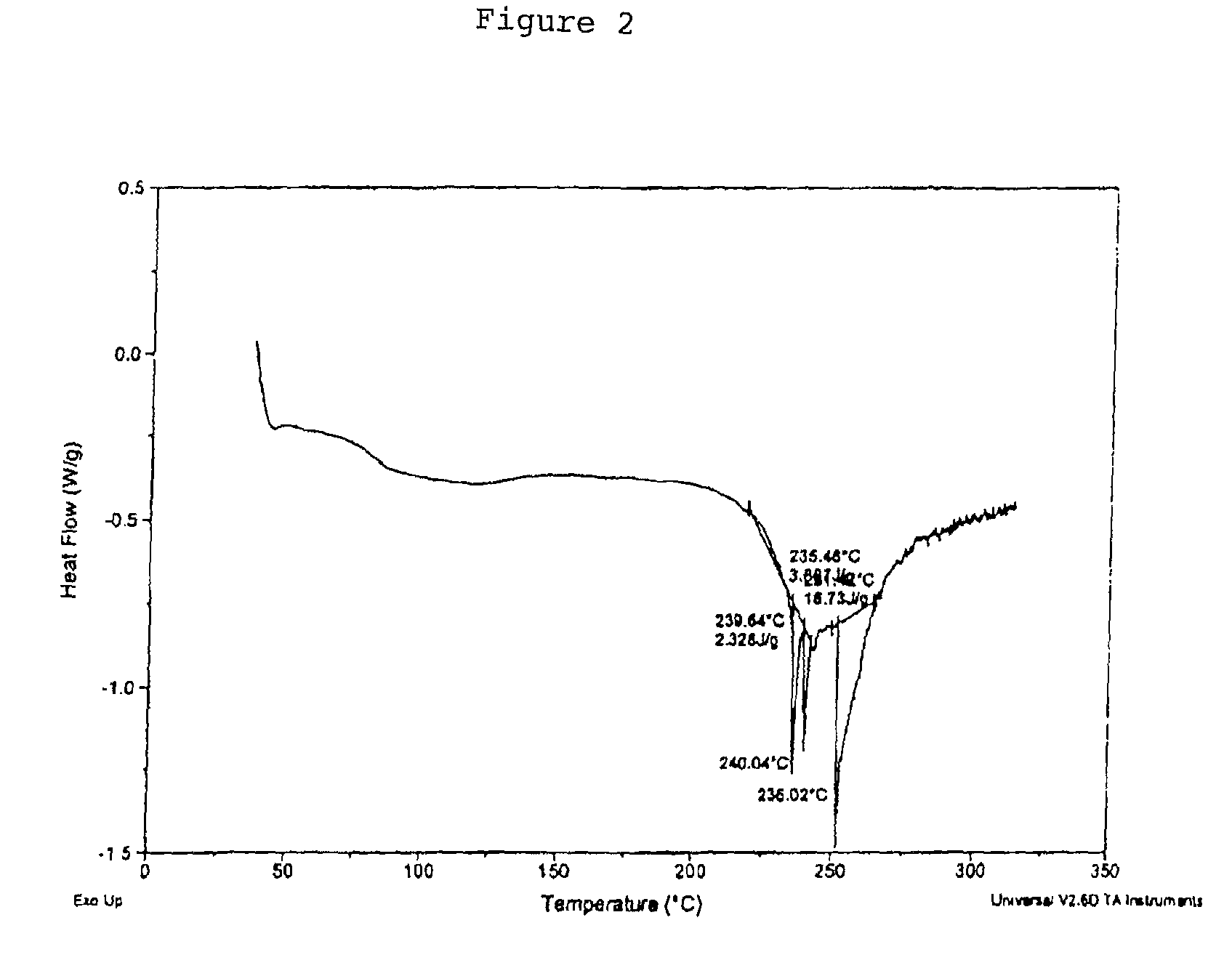
Safety of pseudoephedrine drug products
InactiveUS9421266B2Change the way of beingInhibit and prevent de-formulationOrganic active ingredientsDispersion deliveryOrganic acidPseudoephedrine
Drug substances comprising a amine containing pharmaceutically active compound, and at least one of an alditol acetal and an aromatic organic acid as an addition salt or an additive.
Owner:PISGAH LAB
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com