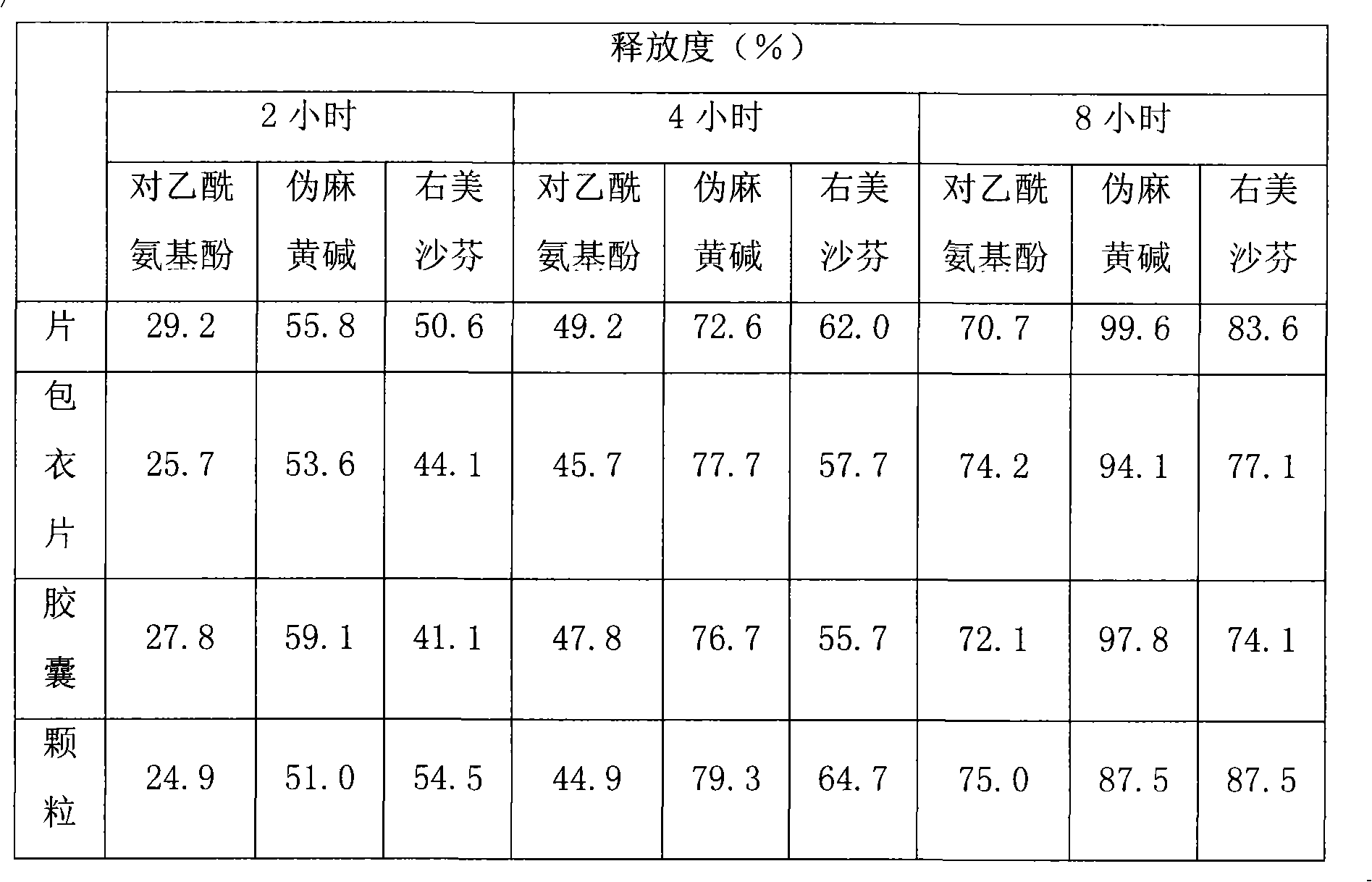
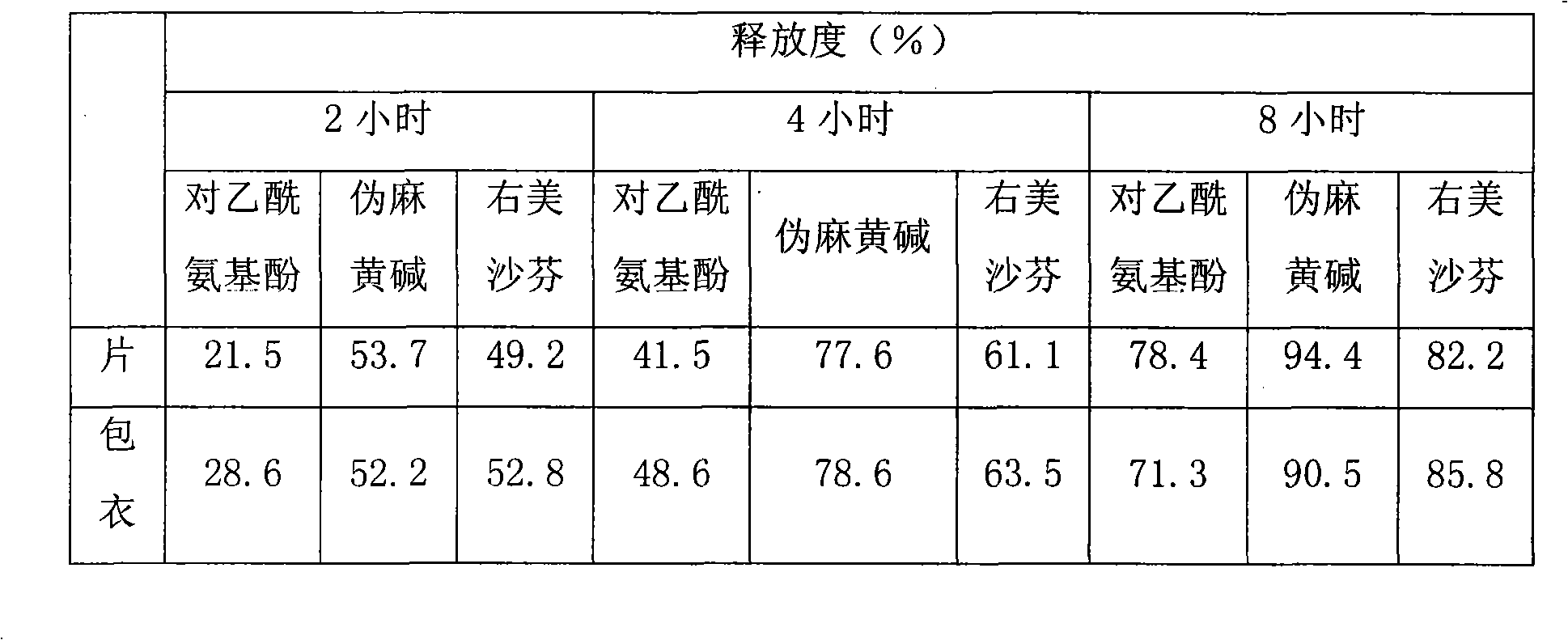
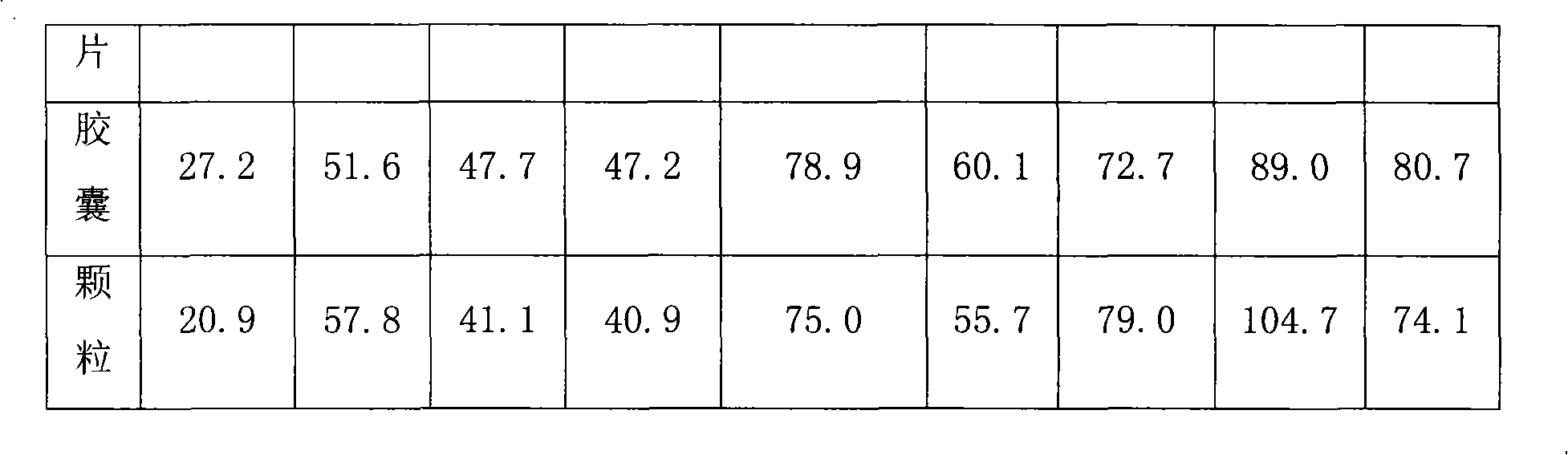
Compound slow release preparation of acetyl aminophenol, pseudoephedrine and dextromethorphan
A paracetamol, sustained-release preparation technology, applied in the field of medicine, can solve the problems of large fluctuations in blood drug concentration, easy to cause side effects, short half-life of dextromethorphan, etc. Concentration stabilization effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] prescription:
[0021] Acetaminophen
250g
90g
Hypromellose K4M
6g
Hypromellose E5
60g
36g
10% Povidone K 30 aqueous solution
Appropriate amount
[0022] Coating prescription:
[0023] Dextromethorphan Hydrobromide
30g
30g
Add to 1000ml
[0024] Preparation:
[0025] (1) Preparation of granules: Hypromellose K4M and hydroxypropylmethylcellulose E5 microcrystalline cellulose were passed through 80-mesh sieve respectively, fully mixed with equal increment method, and then mixed with para-acetyl Aminophenol and pseudoephedrine hydrochloride mixed evenly, with 10% povidone K 30 The aqueous solution is a soft material made of adhesive, wet granules are prepared through a 20-mesh sieve, dried at 60°C, granulated through a 20-mesh sieve, and set aside.
[0026] ...
Embodiment 2
[0036] prescription:
[0037] Acetaminophen
240g
50g
Hypromellose K4M
6g
Hypromellose E5
75g
45g
10% Povidone K 30 aqueous solution
Appropriate amount
[0038] Coating prescription:
[0039] Pseudoephedrine hydrochloride
50g
20g
Cellulose acetate
70.5g
water
Appropriate amount until completely dissolved
[0040] Preparation:
[0041] (1) Preparation of granules: Hypromellose K4M, hydroxypropylmethylcellulose E5, and microcrystalline cellulose were passed through 80 mesh sieves respectively, fully mixed with the method of equal increments, and then mixed with the same amount of increments. Mix acetaminophen and pseudoephedrine hydrochloride evenly with 10% povidone K 30 The aqueous solution is a soft material made of adhesive, wet granules are ...
Embodiment 3
[0053] prescription:
[0054] Acetaminophen
250g
pseudoephedrine sulfate
40g
20g
Hypromellose K100M
10g
72g
50g
10% Povidone K 30 aqueous solution
Appropriate amount
[0055] Coating prescription:
[0056] pseudoephedrine sulfate
50g
10g
Cellulose diacetate
35g
Appropriate amount until completely dissolved
[0057] Preparation:
[0058] (1) Preparation of granules: Hypromellose K100M, hydroxyethyl cellulose, and microcrystalline cellulose are passed through 80-mesh sieves respectively, fully mixed with equal increments, and then mixed with para-acetamide in equal increments. Phenol, dextromethorphan hydrobromide and pseudoephedrine sulfate were mixed evenly, and mixed with 10% povidone K 30 The aqueo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com