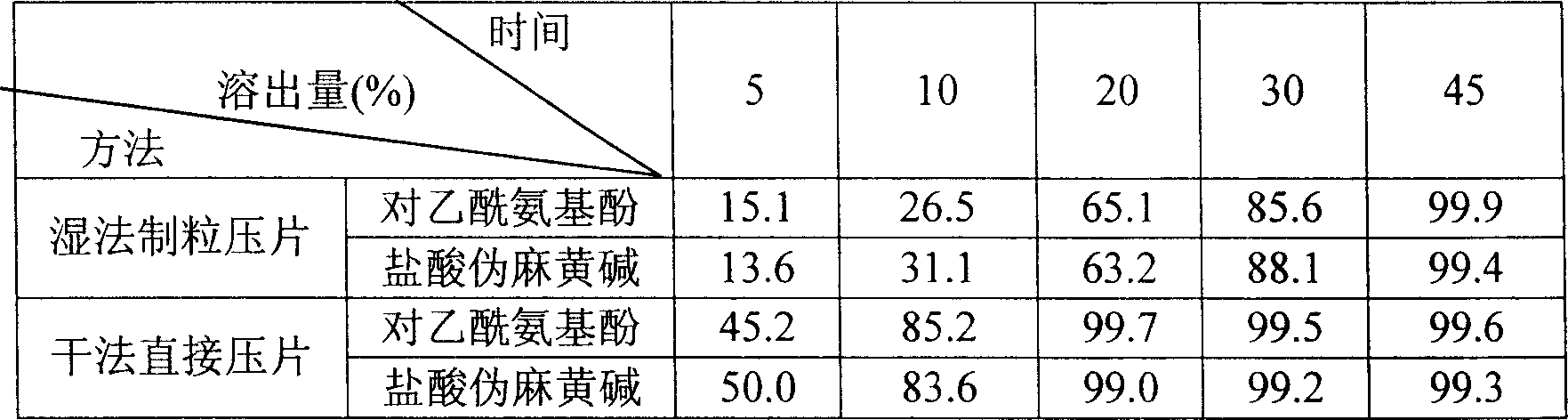
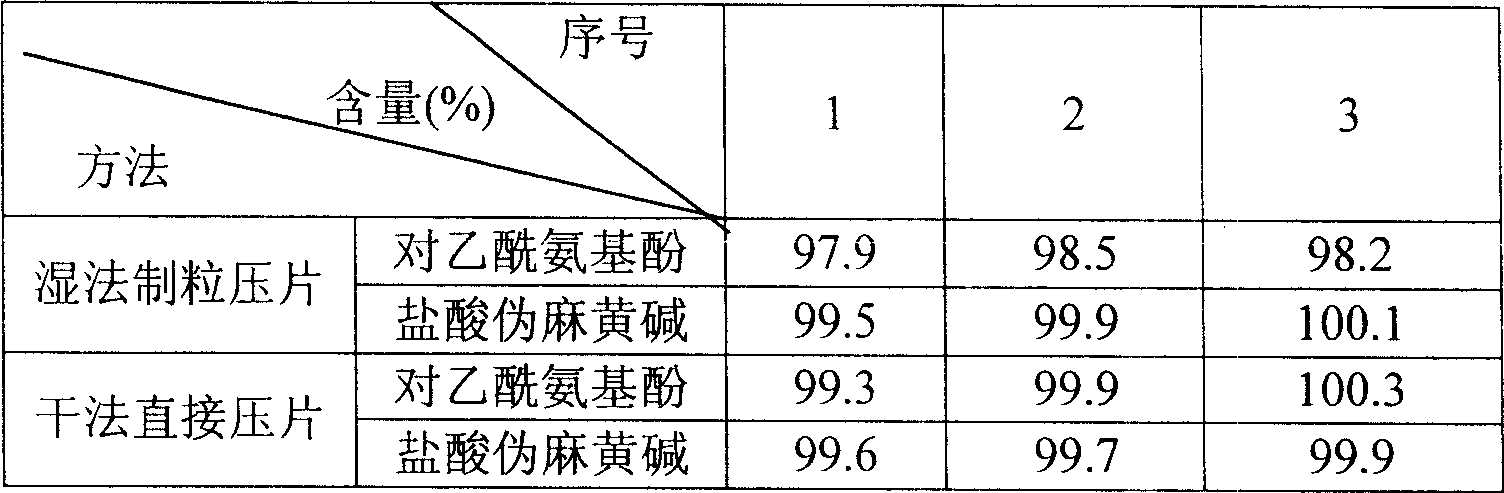
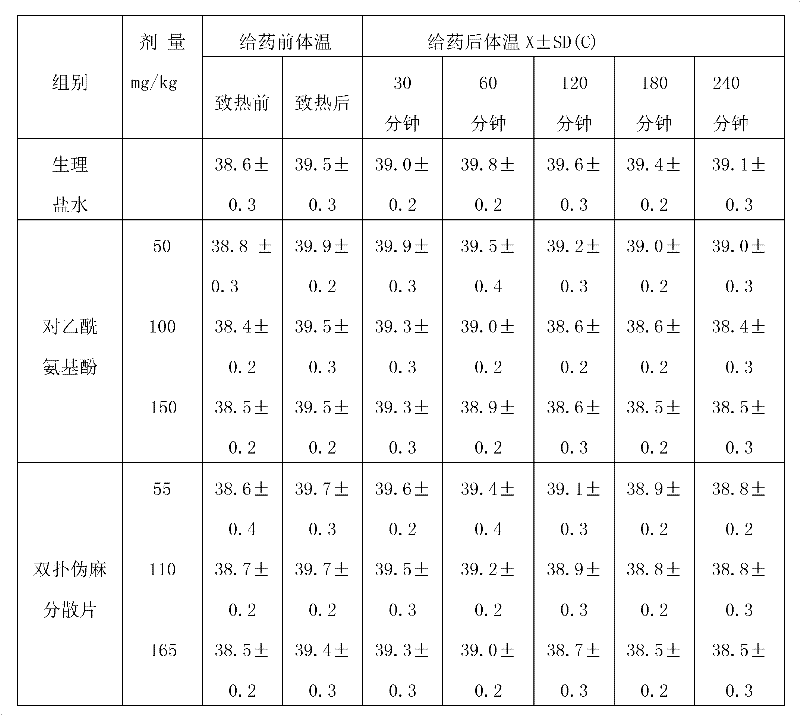
Patents
Literature
122 results about "Pseudoephedrine Hydrochloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
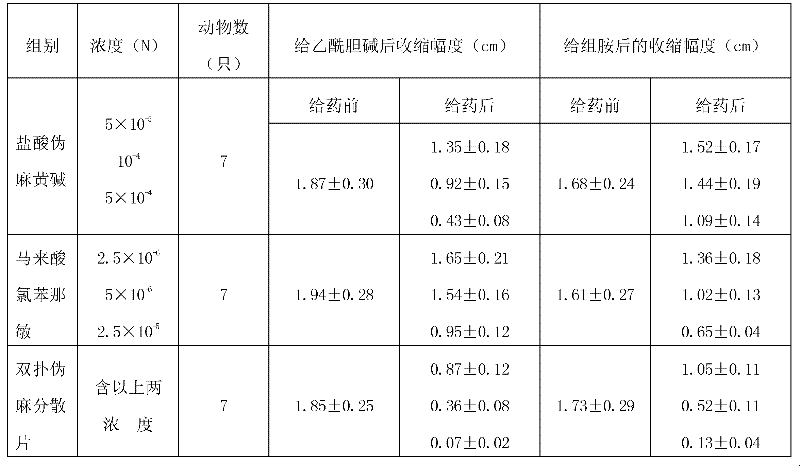
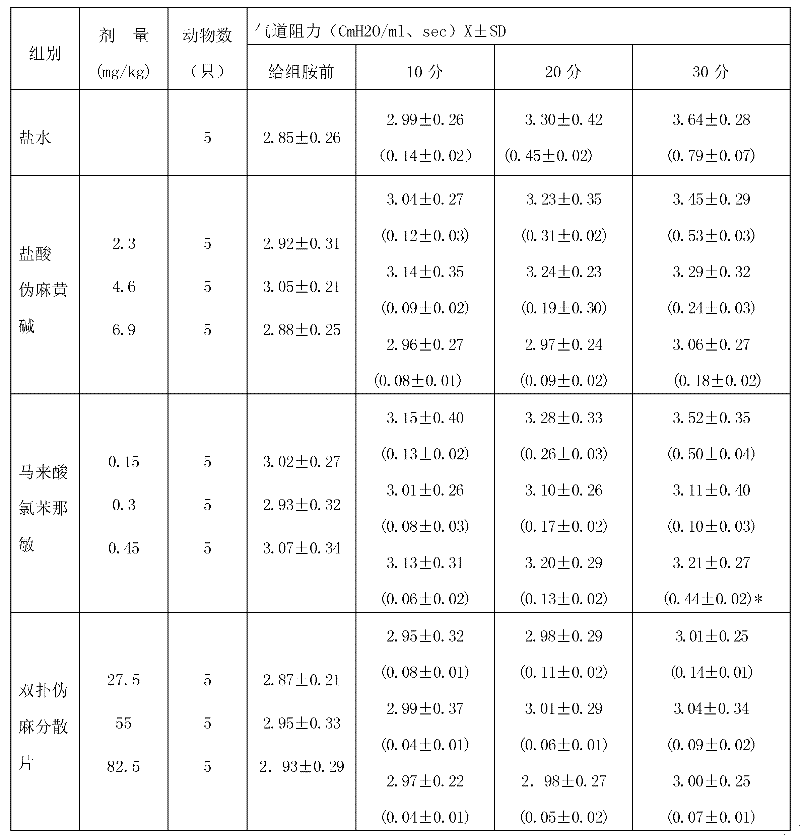
The hydrochloride salt form of pseudoephedrine, a phenethylamine and an diastereomer of ephedrine with sympathomimetic property. Pseudoephedrine hydrochloride displaces norepinephrine from storage vesicles in presynaptic neurones, thereby releasing norepinephrine into the neuronal synapses where it stimulates primarily alpha-adrenergic receptors. It also has weak direct agonist activity at alpha- and beta- adrenergic receptors. Receptor stimulation results in vasoconstriction and decreases nasal and sinus congestion.
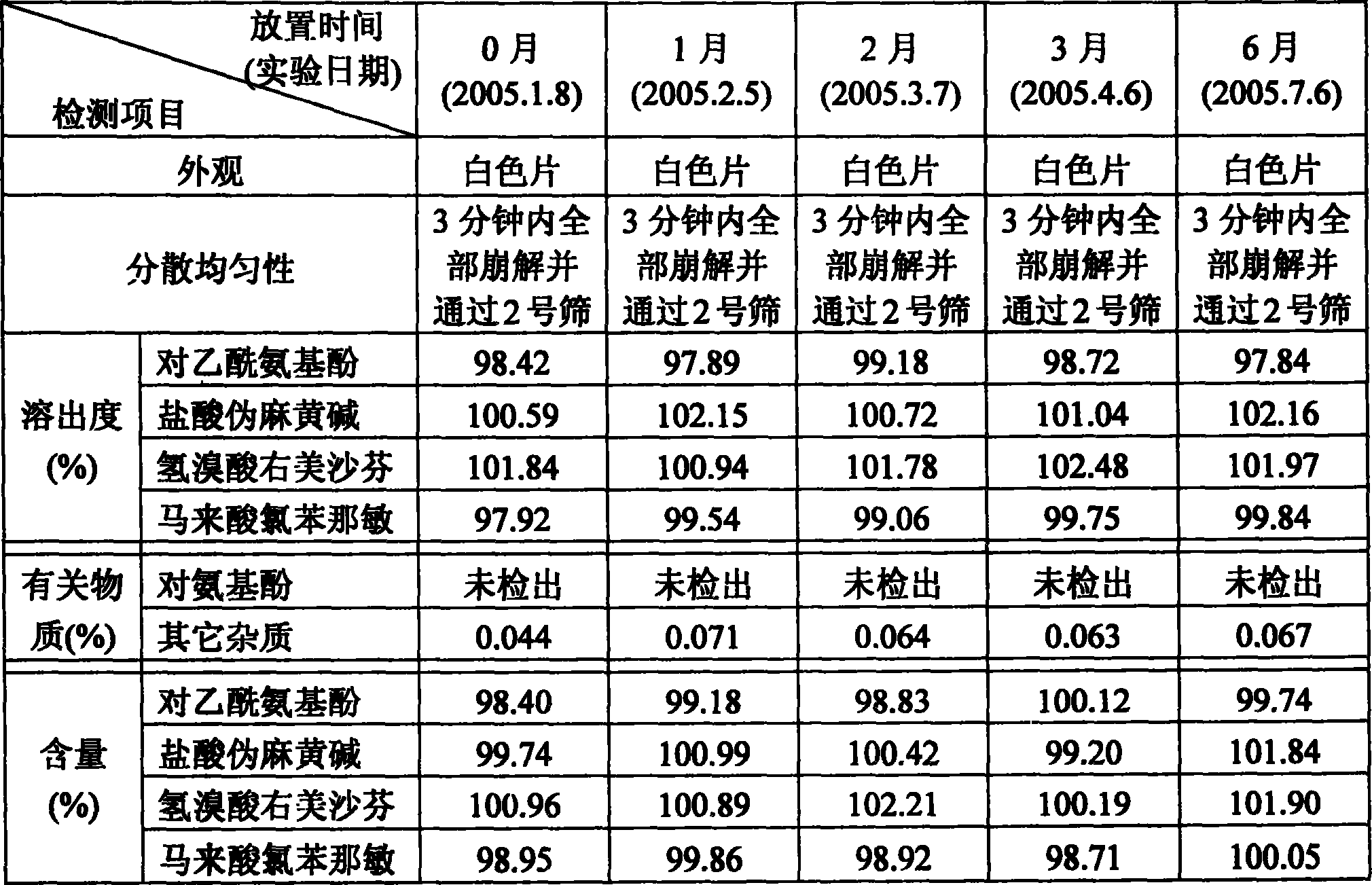
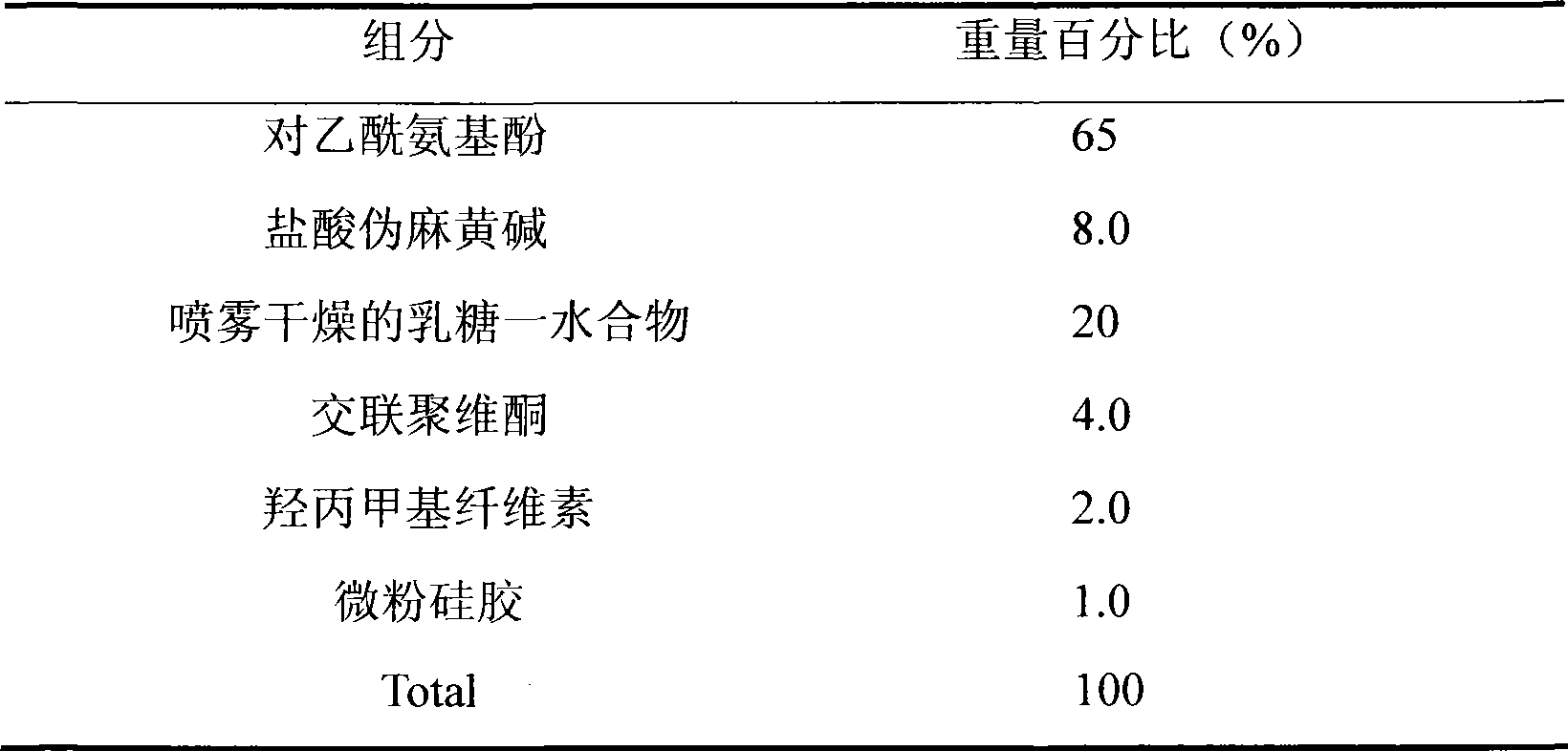
Oral disintegrant of compound paracetamol
InactiveCN1679525ASimple preparation processDisintegration has little adverse effectOrganic active ingredientsAntipyreticChlorphenamine maleateOrally disintegrating tablet
An oral disintegrating tablet of compound paracetamol is prepared from paracetamol, pseudoephedrine hydrochloride, dextromethorphan HBr, chlorphenamine maleate, filler, disintegrant, adhesive or moistening agent, lubricant, flavouring and pigment through direct tabletting.
Owner:FUDAN UNIV
Orally administrable pharmaceutical formulation
InactiveUS20060029661A1Low extractabilityMinimize abuse potentialOrganic active ingredientsBiocideOral medicationMedicine
The present invention relates to pharmaceutical formulations for oral administration through a soft gelatin capsule, wherein the pharmaceutical dosage form has pseudoephedrine hydrochloride as the active pharmaceutical ingredient. The active pharmaceutical ingredient, pseudoephedrine hydrochloride as an active is embedded in a suitable matrix, wherein said matrix composition is characterized by reducing the extractability of the pseudoephedrine hydrochloride.
Owner:M S STRIDES
Quality detection method of five-flavor manna medicine bath preparation
ActiveCN102707007ARaise quality standardsQuality is easy to controlComponent separationSilanesPseudoephedrine Hydrochloride
The invention discloses a quality detection method of a five-flavor manna medicine bath preparation. The five-flavor manna medicine bath preparation is made from raw materials of Juniperus formosana, ephedra, rhododendron anthopogonoide, myricaria and artemisia sieversiana according to a conventional method of pharmaceutics. On the basis of the primary standard, the thin layer chromatography of ephedra and rhododendron anthopogonoide is revised, the thin layer chromatography of Juniperus formosana and artemisia sieversiana is added. Under the simple and convenient condition of mobile phase, octyl silane bonding silica gel or phenyl bonding silica gel is used as filler, and simultaneously, the contents of ephedrine hydrochloride and pseudoephedrine hydrochloride in the ephedra are detected. The invention also provides a method for measuring the content of hyperoside in five-flavor manna preparation rhododendron anthopogonoide, thus ensuring the safety, effectiveness and controllability of product quality. By the quality detection method, the quality standard of the existing five-flavor manna medicine bath preparation is improved correspondingly.
Owner:JINHE TIBETAN MEDICINE
Anti-cold medicine soft capsule and its preparing method
ActiveCN1615866AThe appearance is clear and transparentReasonable compositionAntiinfectivesCapsule deliveryDispersed mediaDextromethorphan Hydrobromide
The present invention provides a soft anti-cold medicine capsule and its preparation process and belongs to the field of medicine technology. The content consists of active medicine component and stuffing, the active medicine component includes acetaminophen, pseudoephedrine hydrochloride and dextromethorphan hydrobromide, and the stuffing includes dispersing medium, surfactant, suspension assistant and microemulsifier. The present invention has fast acting and obvious curative effect on cold syndrome, and the soft capsule is clear and transparent.
Owner:CSPC OUYI PHARM CO LTD
Compound acrivastine sustained release tablets, and preparation method thereof
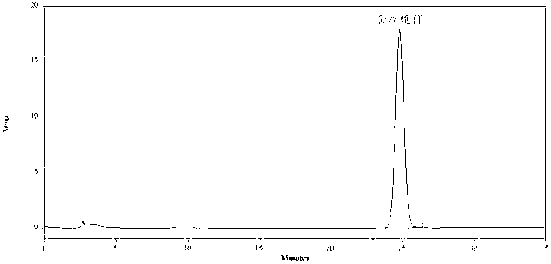
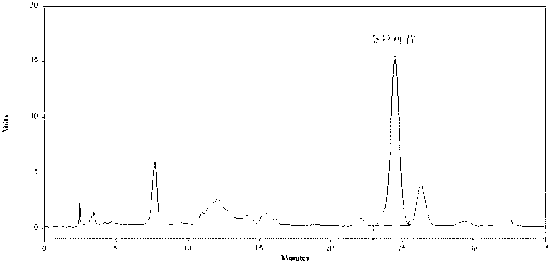
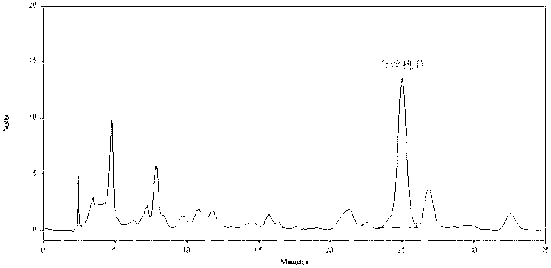
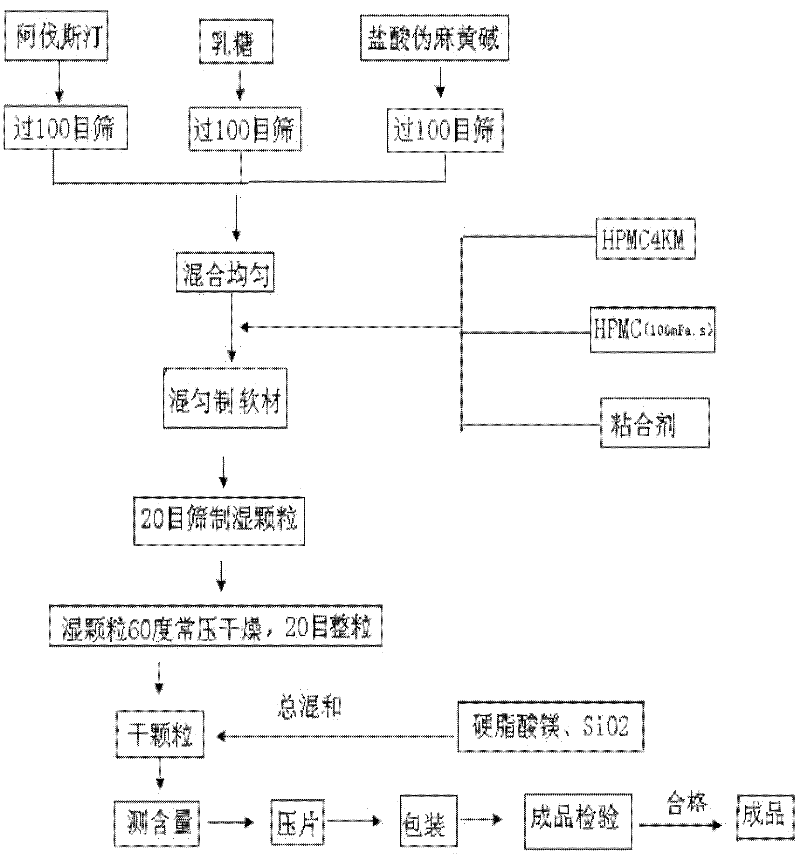
ActiveCN102247368ALong duration of actionModerationOrganic active ingredientsPill deliveryAdhesivePseudoephedrine Hydrochloride
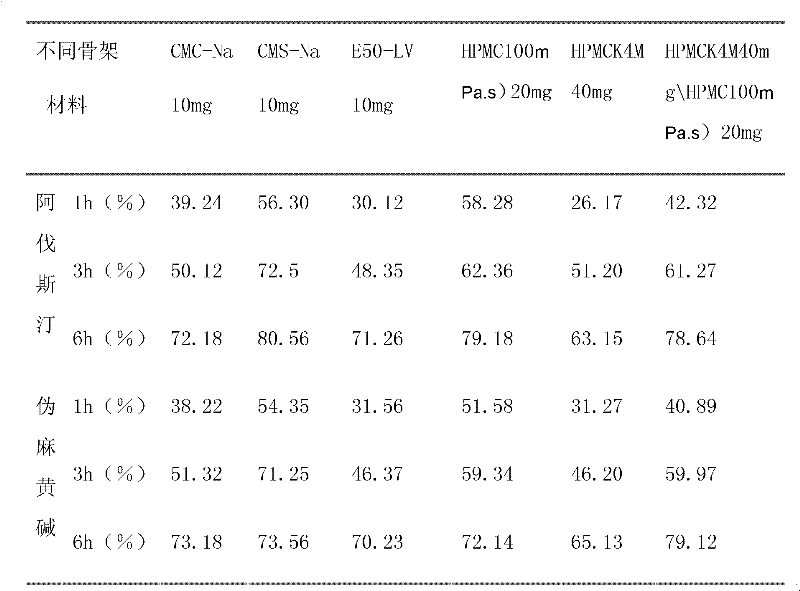
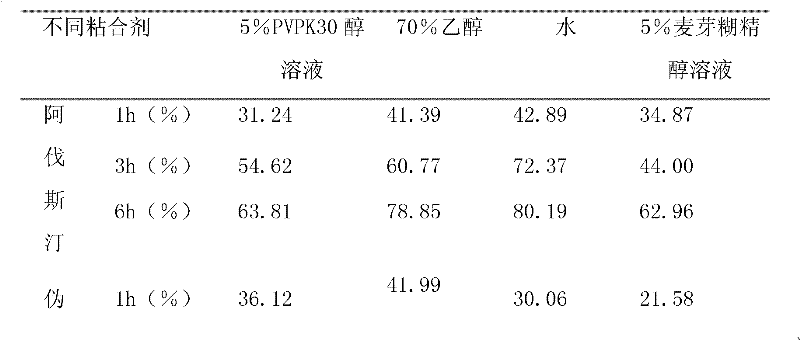
The invention relates to compound acrivastine sustained release tablets, which are prepared from formulated raw materials of, by mass: 14 to 18 parts of acrivastine, 118 to 122 parts of pseudoephedrine hydrochloride, 38 to 42 parts of hydroxypropyl methylcellulose K4M, 18 to 22 parts of hydroxypropyl methylcellulose (100mPa.s), 98 to 102 parts of lactose, 1 to 3 parts of magnesium stearate, and 1to 3 parts of SiO2. The preparation method comprises the steps that: the effective components acrivastine and pseudoephedrine hydrochloride, and the filling material are weighed, sieved and mixed; the framework material hydroxypropyl methylcellulose and an adhesive are added to the mixture; a soft material is prepared and sieved, such that wet granules are prepared; the granules are dried and shaped; a lubricant is added to the granules; all the materials are well mixed and are compressed into tablets according to certain requirements; the tablets are packed, such that finished products are obtained. Compared to common tablets, the sustained release tablets provided by the invention has advantages of long functioning duration, which is 12 hours; mild function; slow release; and less and light adverse reaction.
Owner:ANHUI YONSENT PHARMA
Chinese ephedra medicinal material, and content determination method of three alkaloids in preparation thereof
ActiveCN103512998AExtend your lifeSimplify flushing proceduresComponent separationMethylephedrine hydrochloridePseudoephedrine Hydrochloride
The invention relates to a Chinese ephedra medicinal material, and a content determination method of three alkaloids in a preparation thereof. The method has the characteristics that: a common reversed-phase column and a non-buffer salt which is a mobile phase of acetonitrile-methanol-0.1% phosphoric acid with a volume ratio of 1-1.5:4-4.2:94.5-95.0 are adopted; at 207+ / -2nm, contents of ephedrine hydrochloride, pseudoephedrine hydrochloride, and ephedrine methyl hydrochloride in the Chinese ephedra medicinal material and the preparation thereof are simultaneously determined. A sample is only processed through water-containing methanol ultrasonic extraction and alumina impurity removing, such that a near-colorless transparent solution is obtained. The method is simple, fast, reproducible, and accurate. According to the quantitative chromatogram of the three alkaloids, a baseline is stable, peak separation is good, and the peak appearing is completed within 20min. Compared with a traditional method with a buffer-salt solution, a specific chromatographic column, and unconventional organic phase ratio and flow rate, with the method provided by the invention, instrument and chromatographic column service lives are prolonged, cost is reduced, and detection conditions are communized. The method is suitable for popularization and application of basic units.
Owner:丰宁满族自治县七环旅游开发有限公司
Compound drug for curing colds and preparation technology thereof
ActiveCN101700245AControl uptakeImprove efficacyOrganic active ingredientsAntipyreticImmediate releasePseudoephedrine Hydrochloride
The invention relates to a compound drug for curing colds and a preparation technology thereof. The compound drug consists of active components of Ibuprofen, pseudoephedrinehydrochloride, chlorphenamine maleate with effective doses and proper amount of pharmaceutic adjuvants, which is characterized by preparing lbuprofen with a partial effective dose and pseudoephedrinehydrochloride with an effective dose into a sustained-release preparation; preparing ibuprofen with a residual effective dose and chlorphenamine maleate with an effective dose into an immediate-release preparation; and preparing the sustained-release preparation and the immediate-release preparation into various drug preparations according to conventional methods. The invention adopts the sustained-release technology and the immediate-release technology, effectively controls absorption and use of the three different drug active components in vivo, enables the blood concentration of the three drug active components to achieve effective curative concentration and coincidence in decreasing time, thus enhancing the pesticide effect of the compound drug in the invention cooperatively and improving bioavailability of the compound drug in the invention.
Owner:TIANSHENG PHARMA GROUP
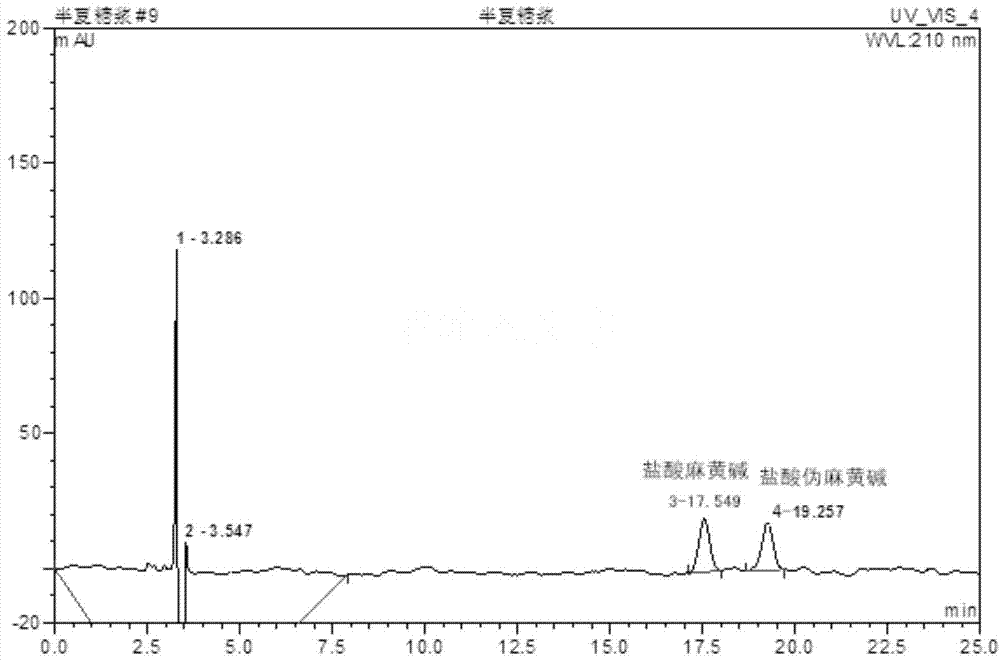
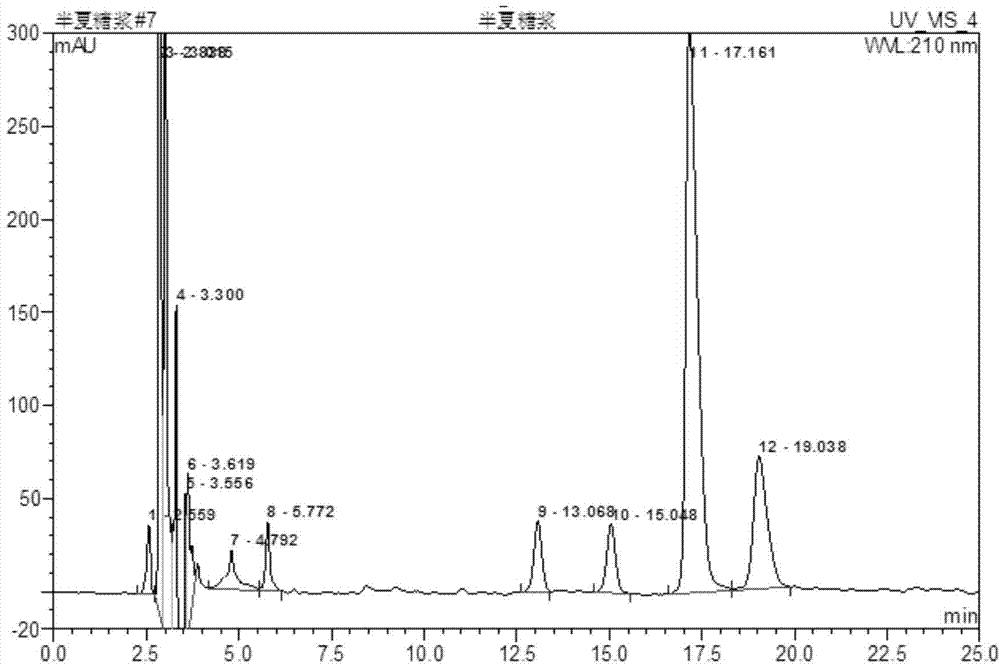
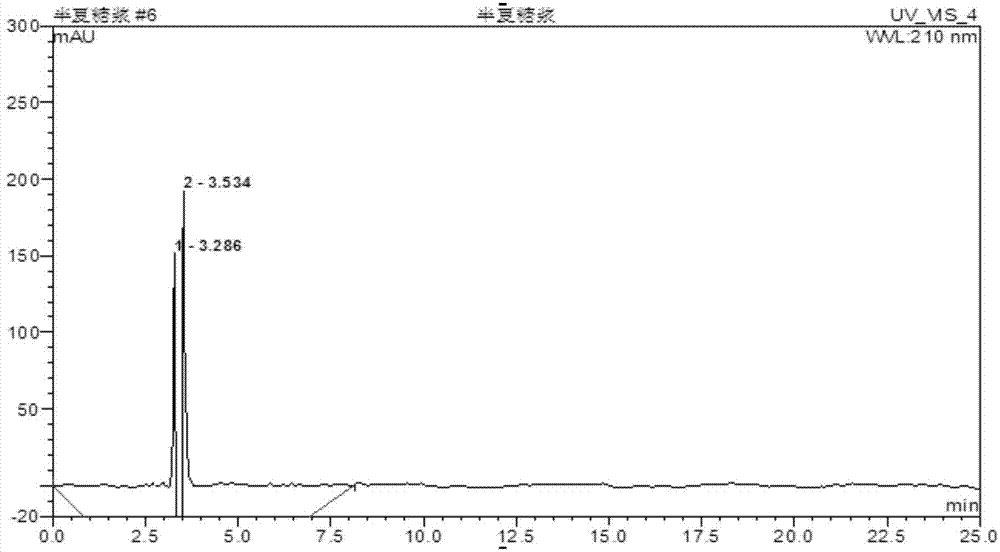
Method for determining content of active ingredients such as ephedrine hydrochloride and pseudoephedrine hydrochloride in pinellia ternata syrup
InactiveCN104764820AImprove stabilityImprove accuracyComponent separationPseudoephedrine HydrochlorideBULK ACTIVE INGREDIENT
The invention discloses a method for determining the content of active ingredients such as ephedrine hydrochloride and pseudoephedrine hydrochloride in pinellia ternata syrup. The method comprises the following steps of: preparation of reference solution, preparation of to-be-tested sample solution and high-performance liquid chromatographic determination; and if every 1mL of product contains more than or equal to 30 micrograms of ephedrine hydrochloride and pseudoephedrine hydrochloride in total, the product is qualified. The method adopts the high-performance liquid chromatography to determine the content of the ephedrine hydrochloride and the pseudoephedrine hydrochloride which are used for investigating the quality of the pinellia ternata syrup product; and compared with the existing standard in absence of a content determining item, the method can control the internal quality of medicines directly by detecting the content of ephedrine hydrochloride and pseudoephedrine hydrochloride; in the method, the separating effect for ephedrine hydrochloride and pseudoephedrine hydrochloride is good, the stability and the accuracy are high, and the repeatability is good, so that the quality of the pinellia ternata syrup product can be well controlled.
Owner:HUBEI DUANZHENG PHARMA CO LTD
Detection method of Wuweiganlu preparation
ActiveCN102645493AQuality improvementStable quality detection methodComponent separationPreparing sample for investigationPseudoephedrine HydrochlorideJuniperus formosana
The invention provides a detection method of a Wuweiganlu preparation. The method comprises the following steps of: performing microscopic identification of the microscopic characteristics of juniperus formosana and myricaria in the Wuweiganlu preparation; performing thin-layer chromatography identification of rhododendron anthopogonoide, juniperus formosana and artemisia sieversiana in the Wuweiganlu preparation; and measuring the content of ephedrine hydrochloride and pseudoephedrine hydrochloride in ephedra and the content of artemisetin in artemisia sieversiana in the preparation by a high performance liquid chromatography. The detection method provided by the invention has the advantages of good reproducibility and stability, high precision, strong specificity, clear spot color, high separation degree, accurate content and the like, and is simple to operate; and by creating a reliable quality detection method with strong specificity, the quality of the Wuweiganlu preparation can be effectively controlled so that the quality of the Wuweiganlu preparation is stable, safe and controllable.
Owner:TIBET QIZHENG TIBETAN MEDICINE
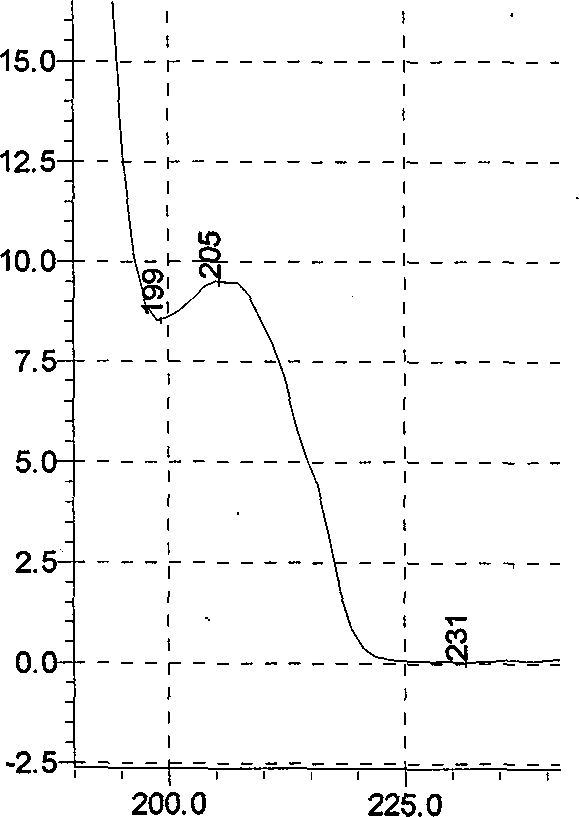
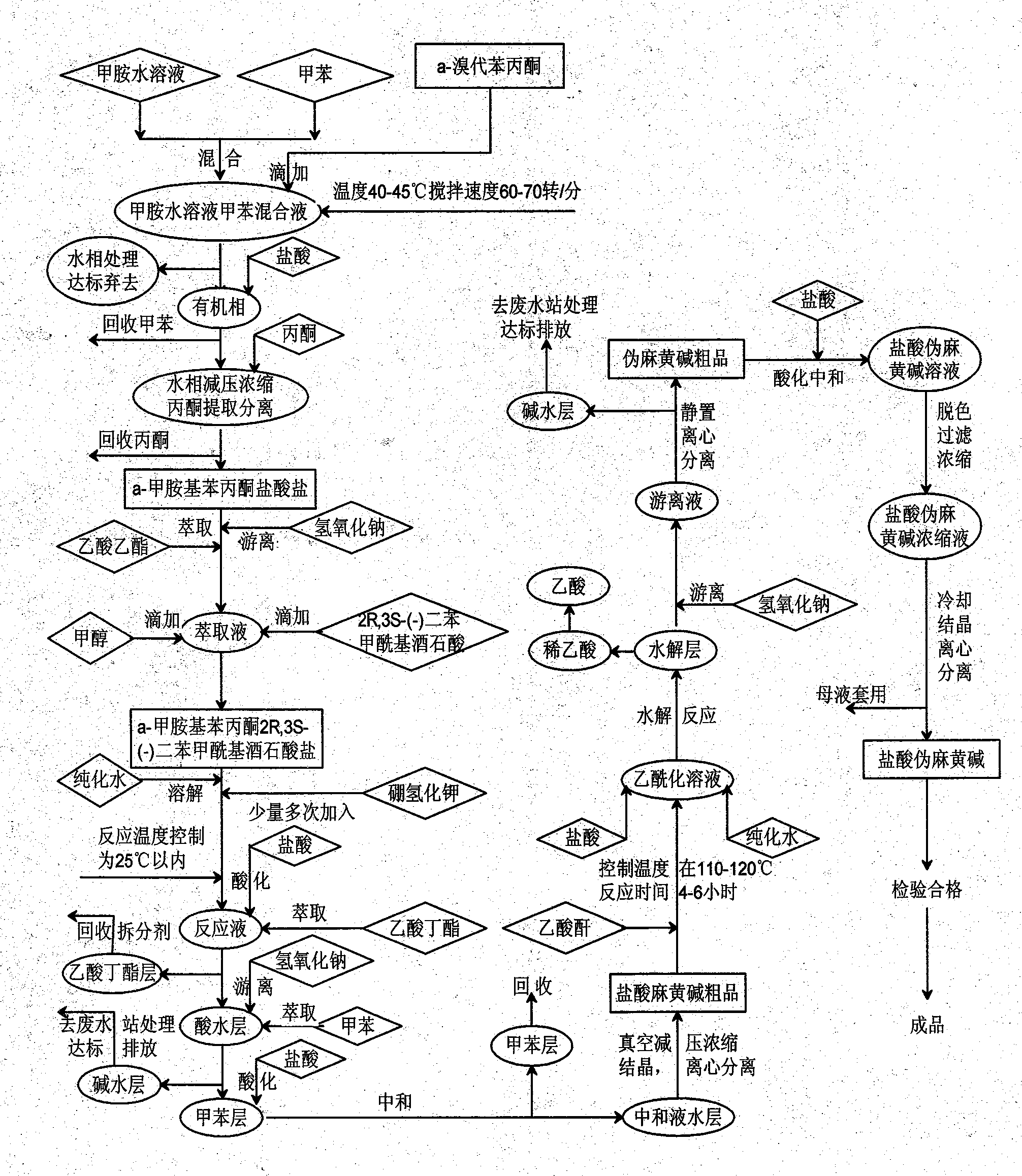
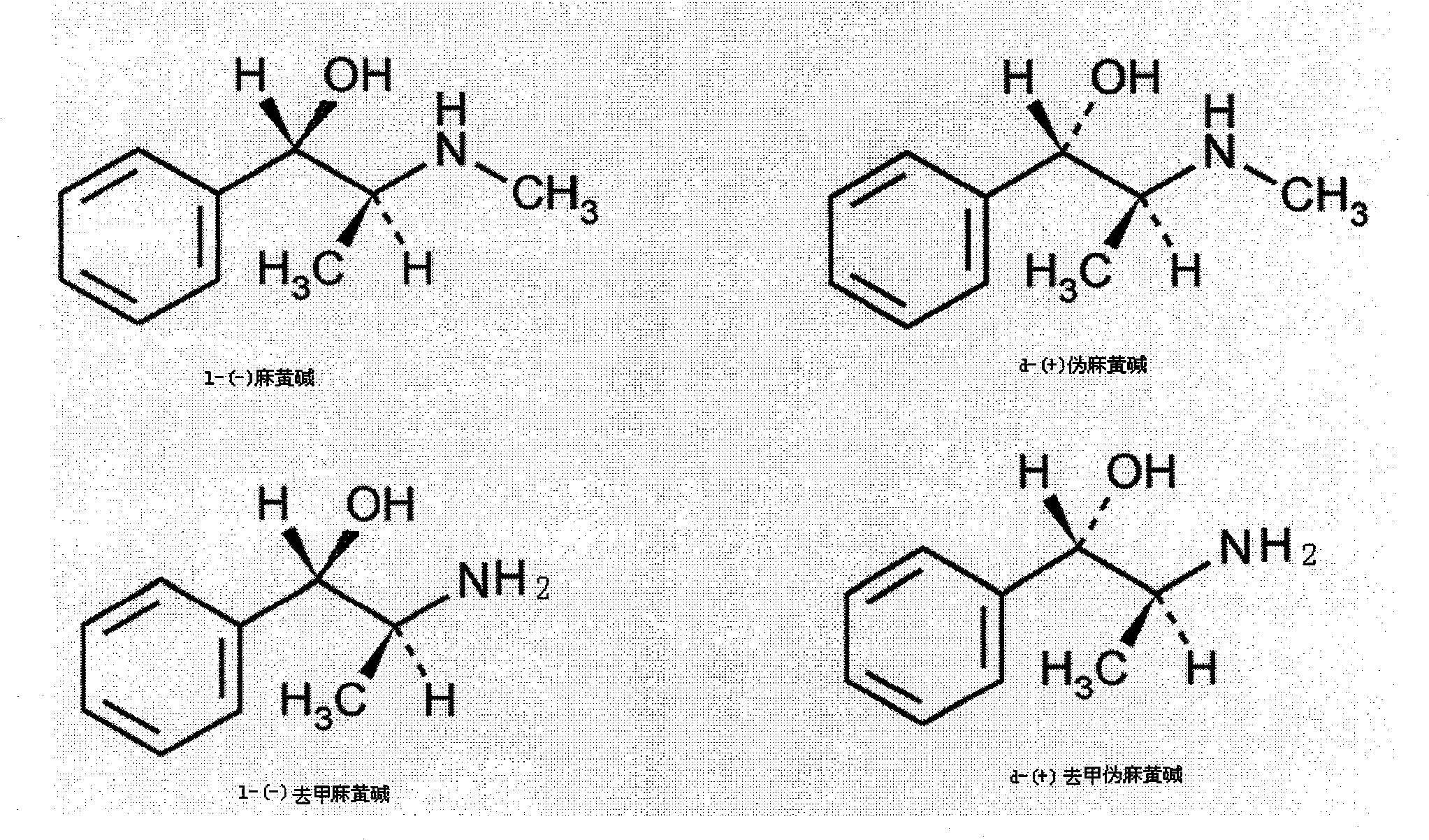
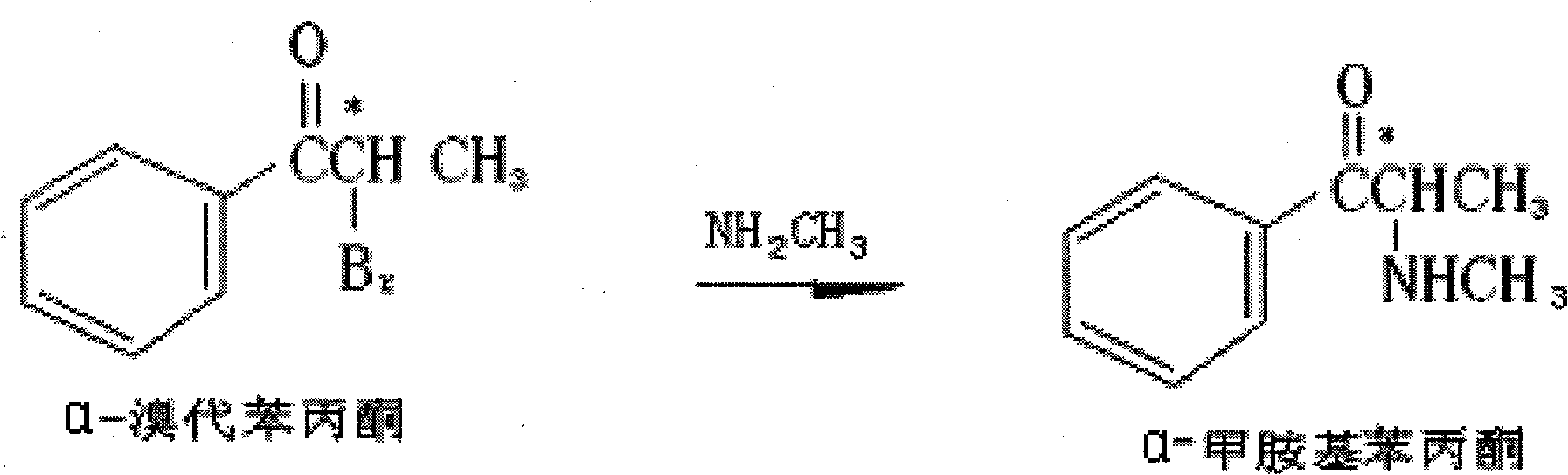
Preparation method of L-(-)-ephedrine chloride and d-(+)-pseudoephedrine hydrochloride
InactiveCN101870660AMild reaction conditionsRaw materials are easy to getOrganic compound preparationOptically-active compound separationPseudoephedrine HydrochlorideKetone
The invention relates to a method for preparing L-(-)-ephedrine chloride and d-(+)-pseudoephedrine hydrochloride by taking alpha-bromophenyl ethyl ketone as a raw material through steps of methylamination, resolution, reduction, acylation, acidolysis, hydrolysis and the like. The method has the advantages of mild reaction conditions, available raw material, small equipment investment, simple three wastes treatment, high yield and the like. The ephedrine chloride and the pseudoephedrine hydrochloride which are prepared by the invention belong to beta2 adrenoceptor agonists, and the preparations (tablets and capsules, such as new contac capsules produced by Tianjin Smith Kline & French laboratories Ltd.) of the ephedrine chloride and the pseudoephedrine hydrochloride release norepinephrine by mainly stimulating sympathetic nerve ending after being orally taken to take the sympathomimetic nerve effect in an indirect way. The L-(-)-ephedrine chloride and the d-(+)-pseudoephedrine hydrochloride are used for the adjuvant therapy of cold clinically and can soothe nasal mucosa congestion caused by the cold, allergic rhinitis, rhinitis and nasosinusitis.
Owner:QINGHAI LAKE PHARMA COMPANY
Medicinal composition containing ibuprofen
ActiveCN101028258AGood curative effectOrganic active ingredientsAntipyreticPseudoephedrine HydrochlorideCurative effect
A composite medicine containing ibuprofen, which may be the free acid of ibuprofen, salt of ibuprofen, their combination, or the combination of ibuprofen and pseudoephedrine hydrochloride, is disclosed.
Owner:SHIJIAZHUANG PHARMA GRP NBP PHARMA CO LTD
Quality control method of concentrated-type oral liquid for treating cough and asthma of children
InactiveCN107894488AStrong specificityEasy to separateComponent separationPediatric coughPseudoephedrine Hydrochloride
The present invention relates to a quality control method of traditional Chinese medicine oral liquid, in particular to a quality control method of children's Kechuanling oral liquid. Alkaline and pseudoephedrine hydrochloride content detection. The quality control method has high accuracy, good stability, and good reproducibility; it improves the current situation that there is no accurate quality control method for Xiaoer Kechuanling Oral Liquid, and ensures the accuracy and comprehensiveness of the quality control of Xiaoer Kechuanling Oral Liquid ; Ensure the quality of Kechuanling oral liquid for children, thereby ensuring the safety and effectiveness of medication for patients.
Owner:HARBIN KANGLONG PHARM CO LTD
Rationed fenmameimin drops
InactiveCN1436530AReasonable passive medicationEasy to acceptAntiinfectivesHeterocyclic compound active ingredientsPEG 400Dextromethorphan Hydrobromide
The present invention is one kind of medicine named Fenmameimin, and the medicine is prepared with four kinds of medicine material including acetaminophenol, pseudoephedrine hydrochloride, dextromethorphan hydrobromide and Chlorpheniramine; four kinds of solvent including polyglycol 400, glycerin, propylene glycol and water; as well as corrective, preservative, coloring agent, etc. During the preparation, rationing pump is used for rationing pump out mechanically. The medicine Fenmameimin is one kind of liquid preparation and has the advantages of fast absorption, accurate administration, convenient administration especially for infant and good taste.
Owner:黄振华
Dispersion tablets for treating and preventing upper respiratory tract infection
InactiveCN101112362AImprove medication complianceSolve the taste problemAntiviralsPharmaceutical non-active ingredientsDrug compoundPseudoephedrine Hydrochloride
The present invention discloses a drug compound which has good taste, contains acetaminophen, chlorpheniramine maleate, cloperastini hydrochloride, pseudoephedrine hydrochloride, caffeine and bromelain and can be dispersed uniformly and is used for curing the upper respiratory tract Infection; the invention further contains fillers, disintegrating agents, flow agents, lubricants, sweeteners and other pharmaceutical excipients, and the single dose thereof is the dispersible tablet form.
Owner:BEIJING D VENTUREPHARM TECH DEV
Dispersible tablet for treating cold and its preparing process
InactiveCN1850083AOrganic active ingredientsAntiinfectivesCross-linkLow-substituted hydroxypropylcellulose
The present invention relates to a dispersion tablet for curing common cold and its preparation process. Said invention is formed from main medicine including paracetamol, pseudoephedrine hydrochloride, dextromethorphan and chlorpheniramine maleate and auxiliary medicine including avicel, low-substituted hydroxypropyl cellulose, povidone K30, cross-linked povidone, aspartame, aspartame, micropowder silica gel and magnesium stearate.
Owner:江西聚仁堂药业有限公司
Water soluble medicament sustained-release tablets and preparation method thereof
InactiveCN101829068ASmall toxicityImprove complianceNervous disorderPharmaceutical delivery mechanismCaptoprilDuodenal juice
The invention discloses water soluble medicament sustained-release tablets, which comprise the following components in part by weight: water soluble medicament 1-30, octadecanol 5-70, Eudragite L 100-55 2-50, talcpowder 2-30 and lactose 2-30. The water soluble medicament may be galanthamine hydrobromide, captopril, metoprolol tartaric acid or pseudoephedrine hydrochloride. In the invention, the octadecanol serving as a hydrophobic auxiliary material and the Eudragite L 100-55 sreving as a water soluble polymer auxiliary are adopted, so the release speed of the medicament can be controlled properly. The Eudragite L 100-55 is insoluble in gastric juice, but dissolves in duodenal juice to make high-viscosity sticky liquid, and thus the release and diffusion speed of the medicament can be reduced. The talcpowder which is a hydrophilic matter insoluble in water plays a porogen role in the sustained-release tablets. In the invention, the release speed of the medicament is regulated by regulating the mixing ratio of the medicament to the auxiliary material. Thus, the release speed of an active medicament is controlled.
Owner:XUZHOU PHOTOSYNTHETIC BIOLOGICAL NUTRIMENT
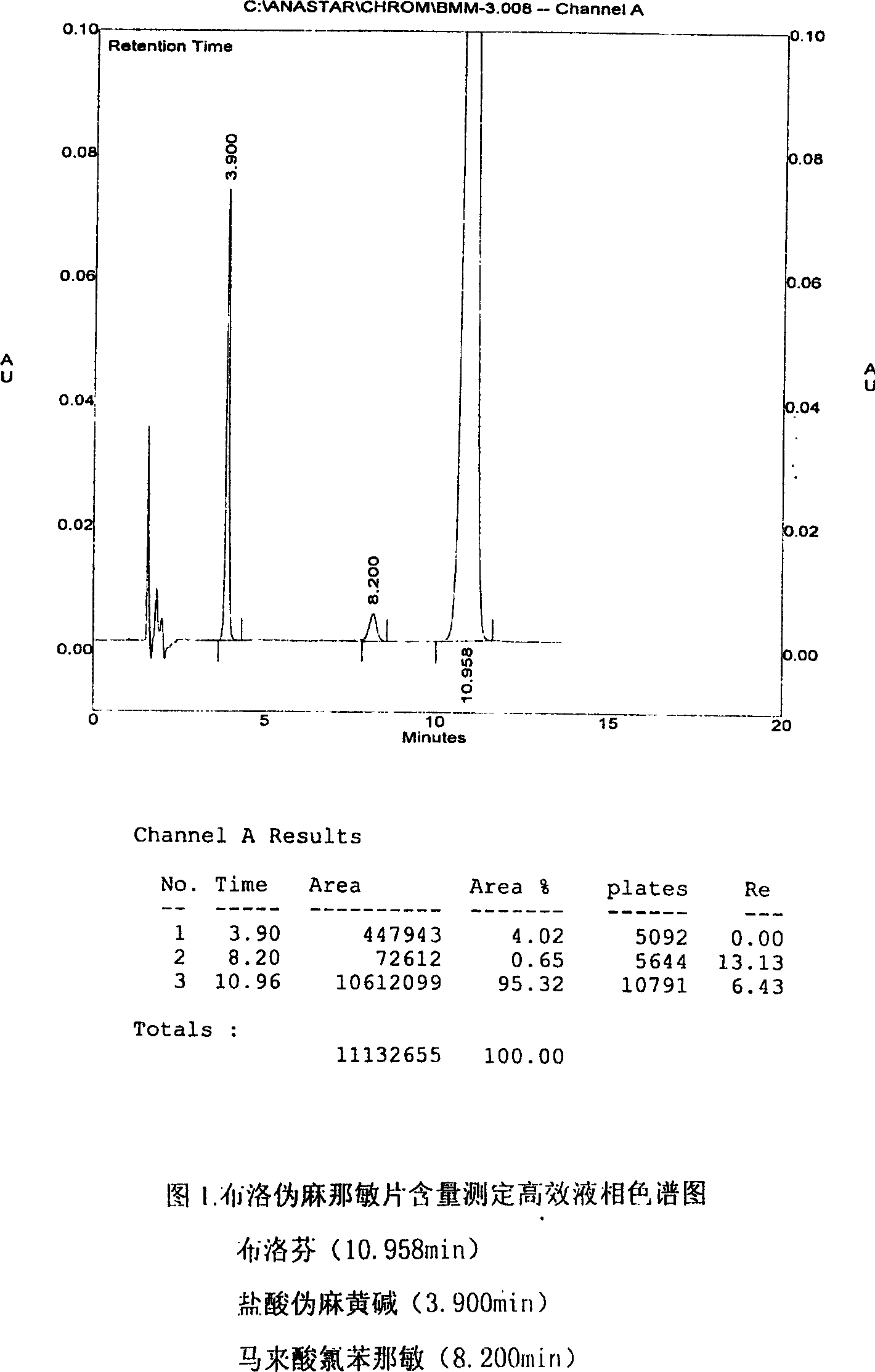
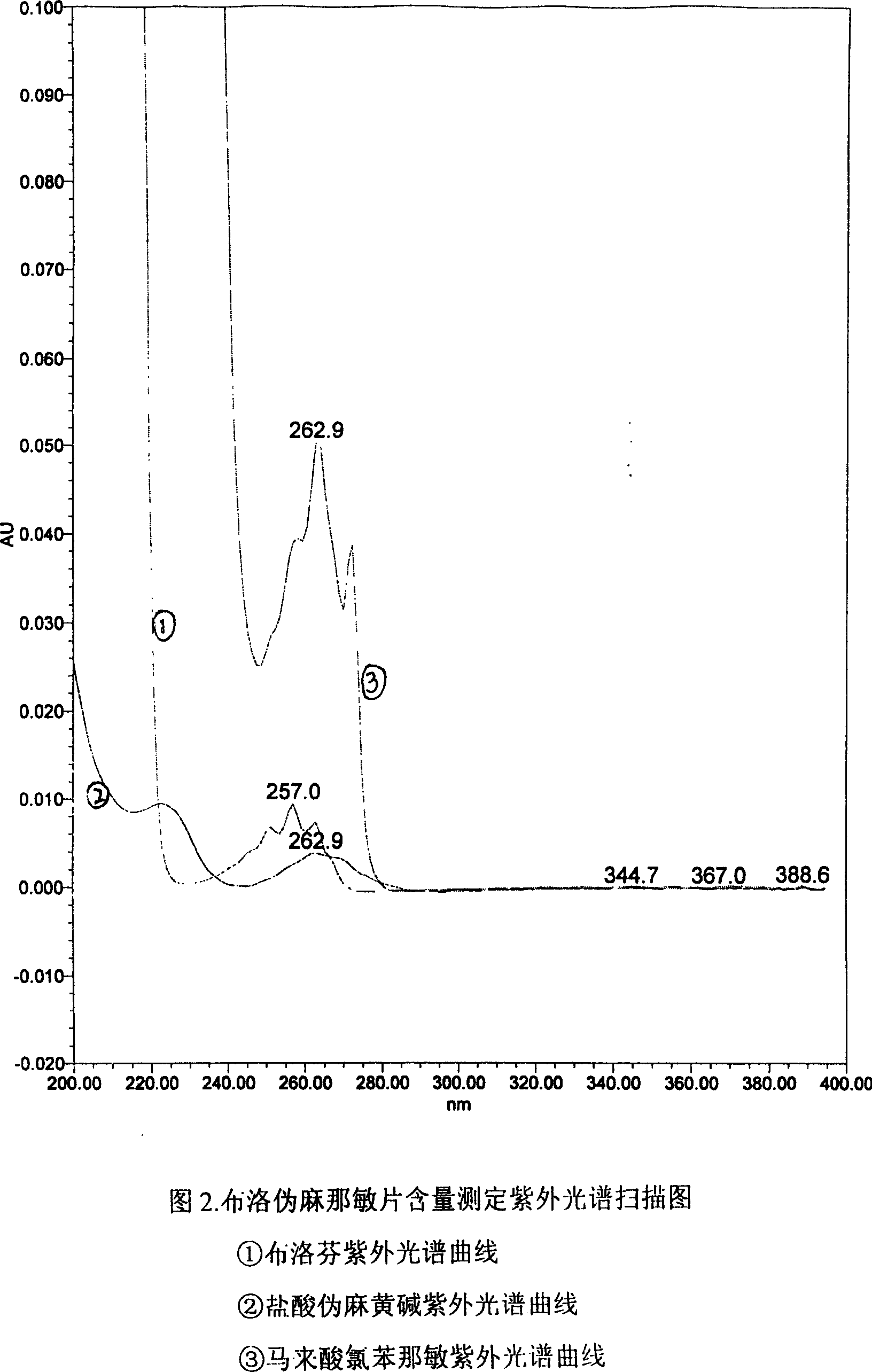
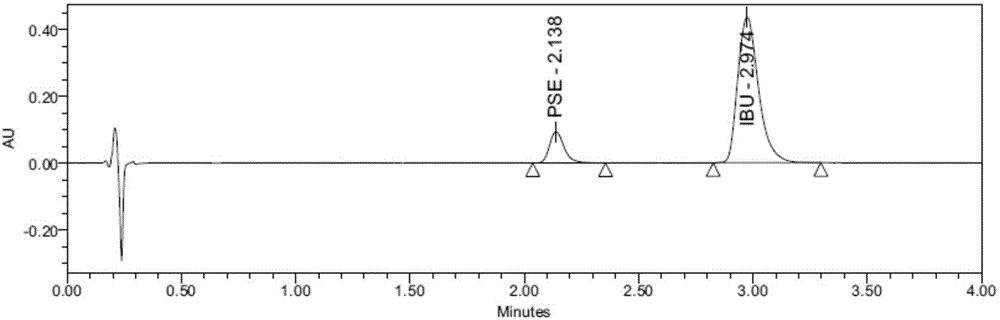
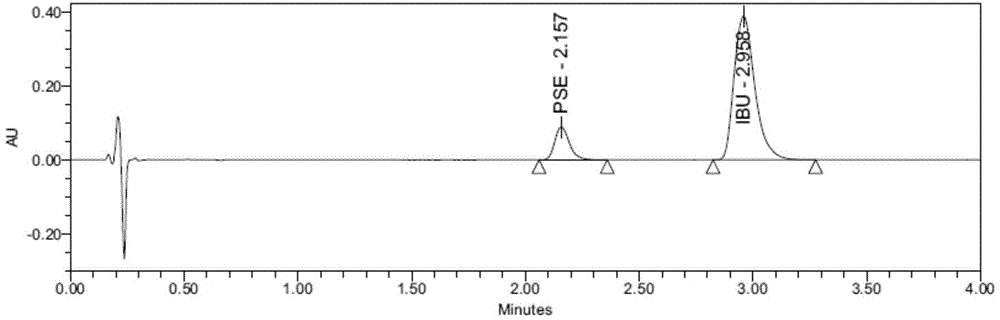
Content detecting method for Ibuprofen, chlorphenamine maleate and Pseudoephedrine Hydrochloride compound preparation
InactiveCN1888891AThe detection method is accurateEasy to operateComponent separationSpecial data processing applicationsColloidal silicaPhosphate
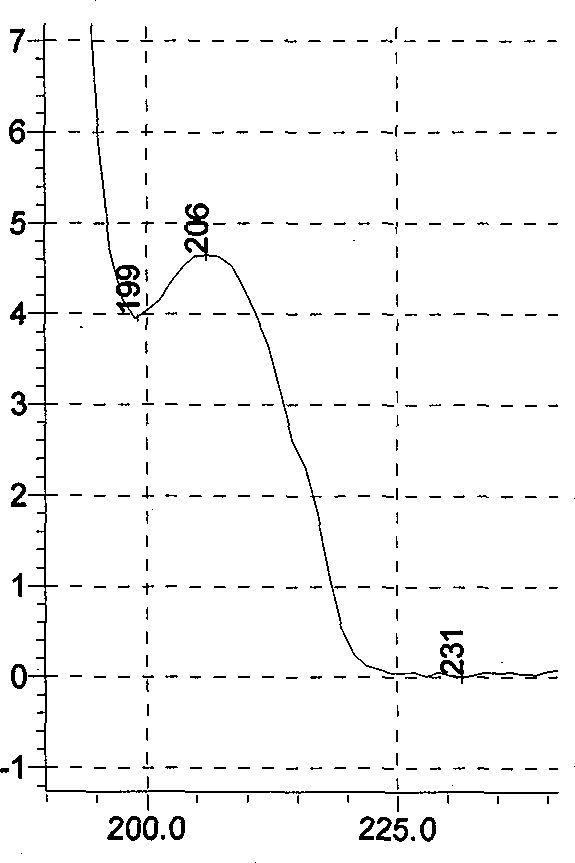
A content detecting method for pro-pseudo-chlorpheniramine compound preparation uses octadecyl silance bonding colloidal silica as packing agent. The mobile phase is monopotassium phosphate, methanol and triethyl-amine and the detecting wavelength is 215-225nm. Dissolve suitable pro-pseudo-chlorpheniramine preparation in the need testing solution with phosphate buffer solution under ultrasonic sound condition. Dissolve suitable ibuprofen, hydrochloric acid pseudoephedrine and chlorphenamine Maleate in the comparison solution with phosphate buffer solution under ultrasonic sound condition. Take each 10-20 mul of the need testing solution and the comparison solution accurately and inject into liquid chromatograph spectrometer and record chromatogram graph. Use peak acreage to calculate the content to ibuprofen, hydrochloric acid pseudoephedrine and chlorphenamine Maleate with the external standard method. It can confirm the content to three kind of component in compound preparation by using one mobile phase with the sententious and fast virtues to inspect the stability to the production and control the procreative quality of industrialization.
Owner:SHANDONG INST OF PHARMA IND
Soft capsule composition containing acetaminophen
A composite soft capsule contains proportionally the active component chosen from paracetranol and one or more of pseudoephedrine hydrochloride, chlorpheniramine maleate, dextromethorphen hydrobromate, bagodryl hydrochloride and coffin, polyethanediol, sodium (or potassium) acetate, and polyvidone.
Owner:ZHEJIANG WANLIAN PHARMA IND +1
Buluoweima slow-release tablet and its preparing method
ActiveCN1868466AEnhanced inhibitory effectAnti-inflammatoryOrganic active ingredientsAntipyreticAlcoholAcrylic resin
A slow-release Buluoweima tablet for treating cold and its complecations (headache, fever, throat pain, etc) is composed of the color-layer particles prepared from ibuprofen, pseudoephedrine hydrochloride, acrylic resin B, hydroxypropyl methylcellulose, lactose, iron oxide, and talk powder, and the white-layer particles prepared from libuprofen, acrylic resin B, hydroxypropyl methylcellulose, talc powder and alcohol. Its preparing process is also disclosed.
Owner:哈尔滨格拉雷药业有限公司
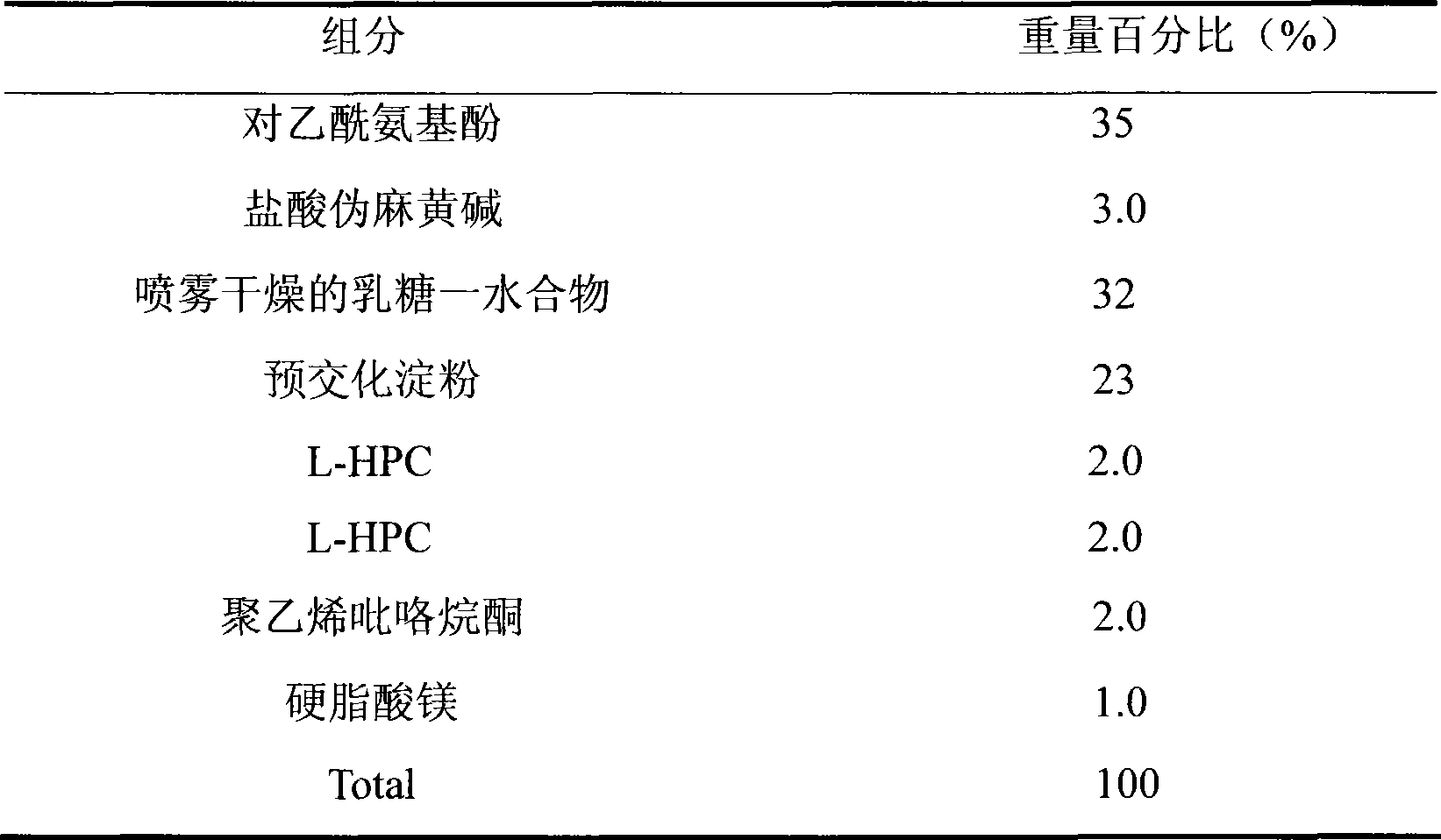
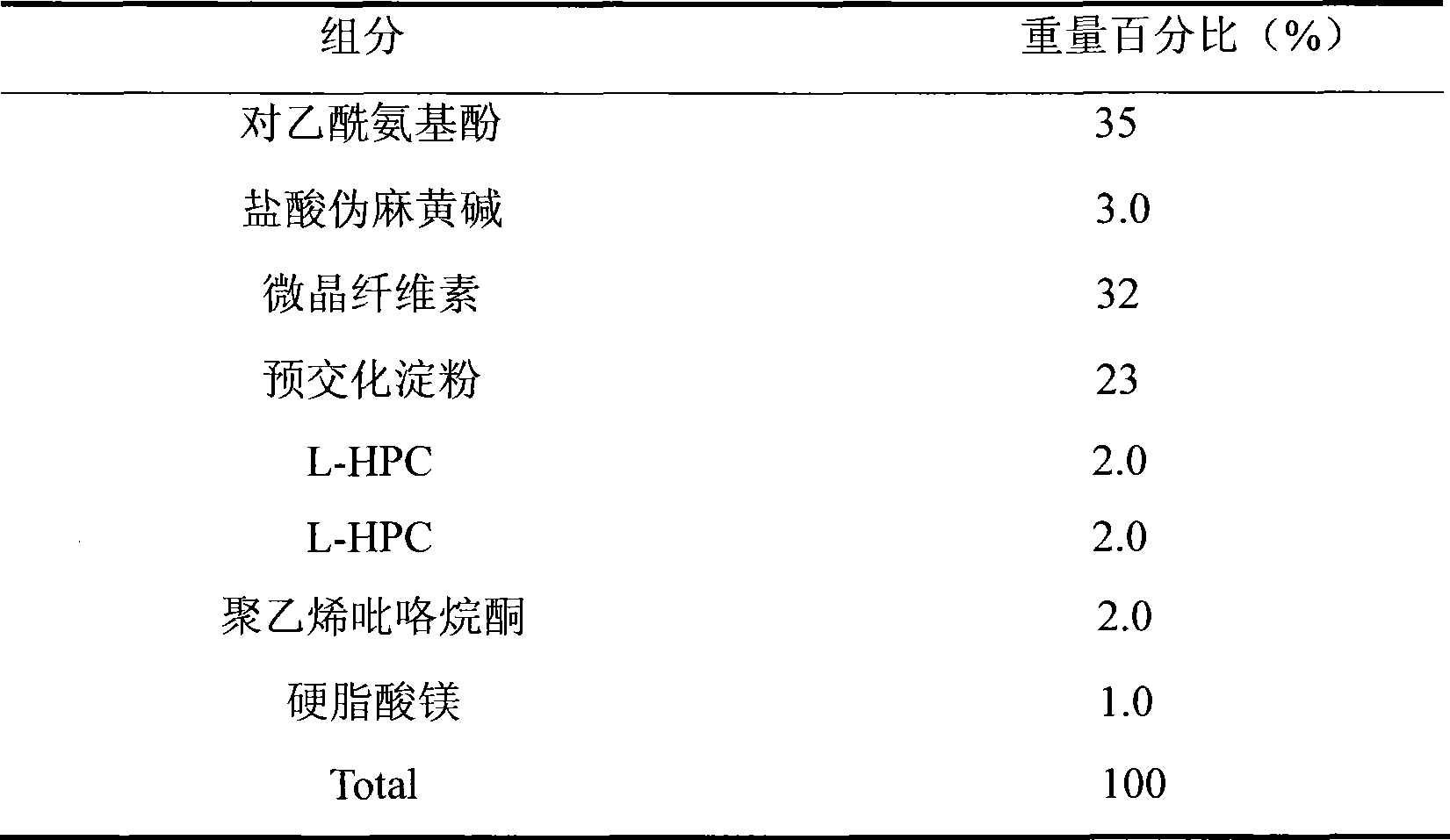
Medicament composition containing paracetamol and pseudoephedrine hydrochloride and preparation method thereof
InactiveCN101467988AImprove liquidityGood compressibilityOrganic active ingredientsAntiviralsPseudoephedrine HydrochlorideAcetaminophen
The invention discloses a stable pharmaceutical composition and method for preparing same. The composition contains acetaminophen, pseudoephedrine hydrochloride and pharmaceutically acceptable accessories capable of improving disadvantages such as bad fluidity and compressibility, and electrostatic absorption during the production of acetaminophen which is suitable for industrialized production.
Owner:北京德众万全医药科技有限公司
Chemical mixture and its use
InactiveCN1491652AQuick effectLittle side effectsOrganic active ingredientsAntiinfectivesDexamethasoneChemical mixtures
The present invention relates to a kind of chemical mixture and its application. The chemical mixture of five components including acetaminophen, ABOB, anisodamine, dexamethasone and pseudoephedrine hydrochloride is used in the medicinal treatment of cold with excellent effect, no bad reaction, and no side effect.
Owner:张善良
Preparation method of compound capsule for treating cold
ActiveCN102349900AControl uptakeImprove efficacyOrganic active ingredientsAntipyreticChlorphenamine maleateBlood concentration
The invention relates to a preparation process of a compound capsule for treating cold. The compound capsule contains effective dose of active components, namely ibuprofen, pseudoephedrine hydrochloride and chlorphenamine maleate as well as a proper amount of pharmaceutic adjuvant. The preparation process comprises the following steps of: preparing partial effective dose of ibuprofen and pseudoephedrine hydrochloride into a sustained release preparation; preparing allowance of ibuprofen and effective dose of pseudoephedrine hydrochloride into a rapid release preparation; and preparing the sustained release preparation and the rapid release preparation into various medicinal preparations. According to the preparation process disclosed by the invention, a sustained release technology and a rapid release technology are adopted to effectively control the absorption action of three different medical active components in the body, and thus the blood concentrations of the three different medical active components achieve the effective treatment concentration which is consistent with the disappeared time, further the medical effect of the compound medicament is synergically enhanced and the bioavailability of the compound medicament disclosed by the invention is improved.
Owner:TIANSHENG PHARMA GROUP
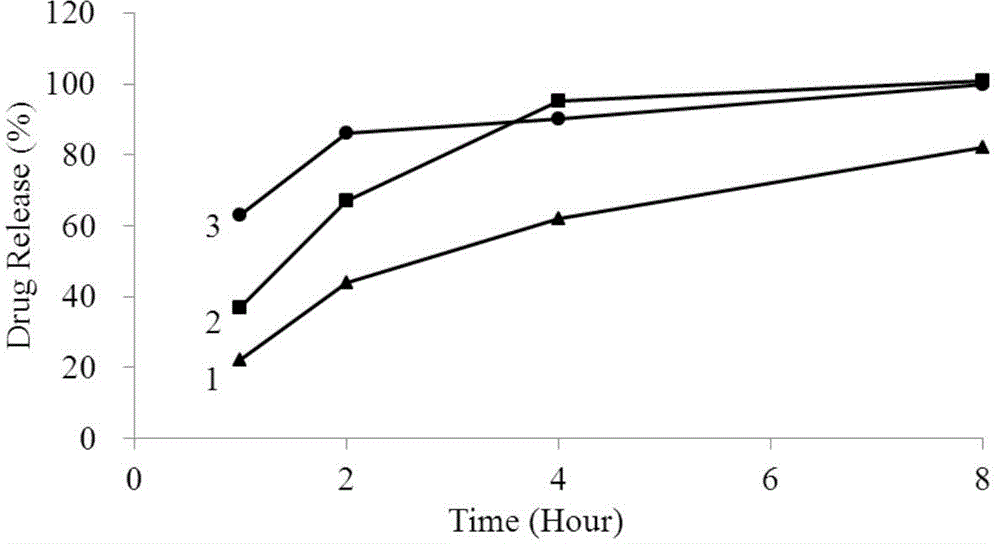
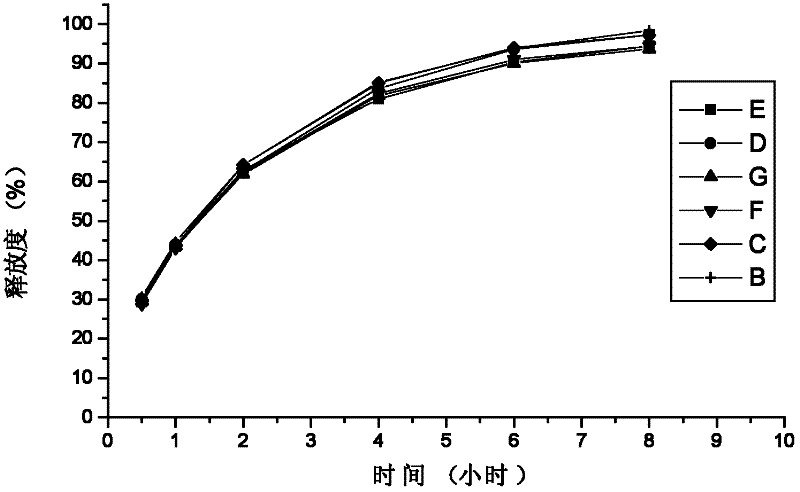
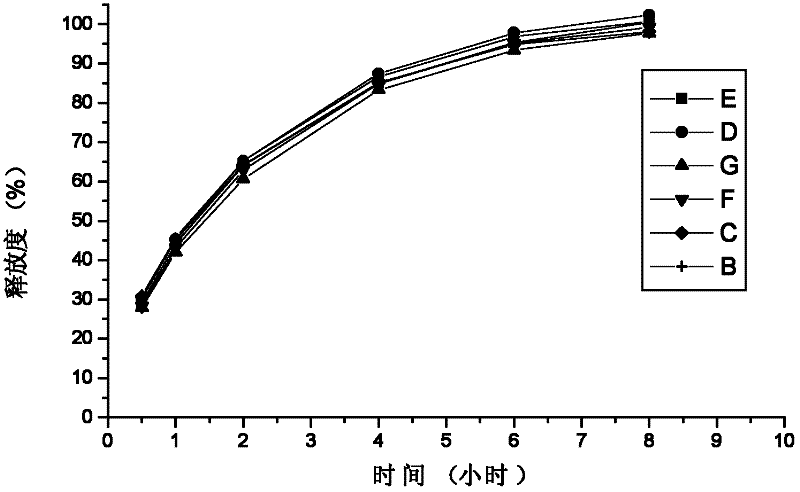
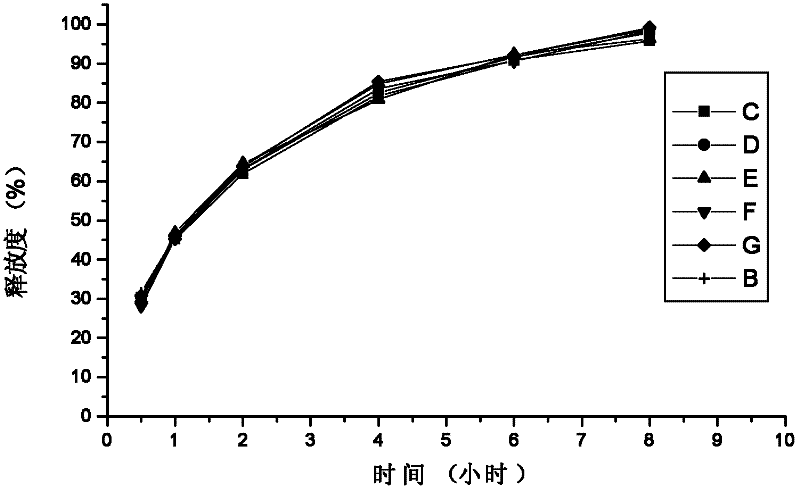
Method for investigation of release degree of ibuprofen-pseudoephedrin hydrochloride sustained-release preparation
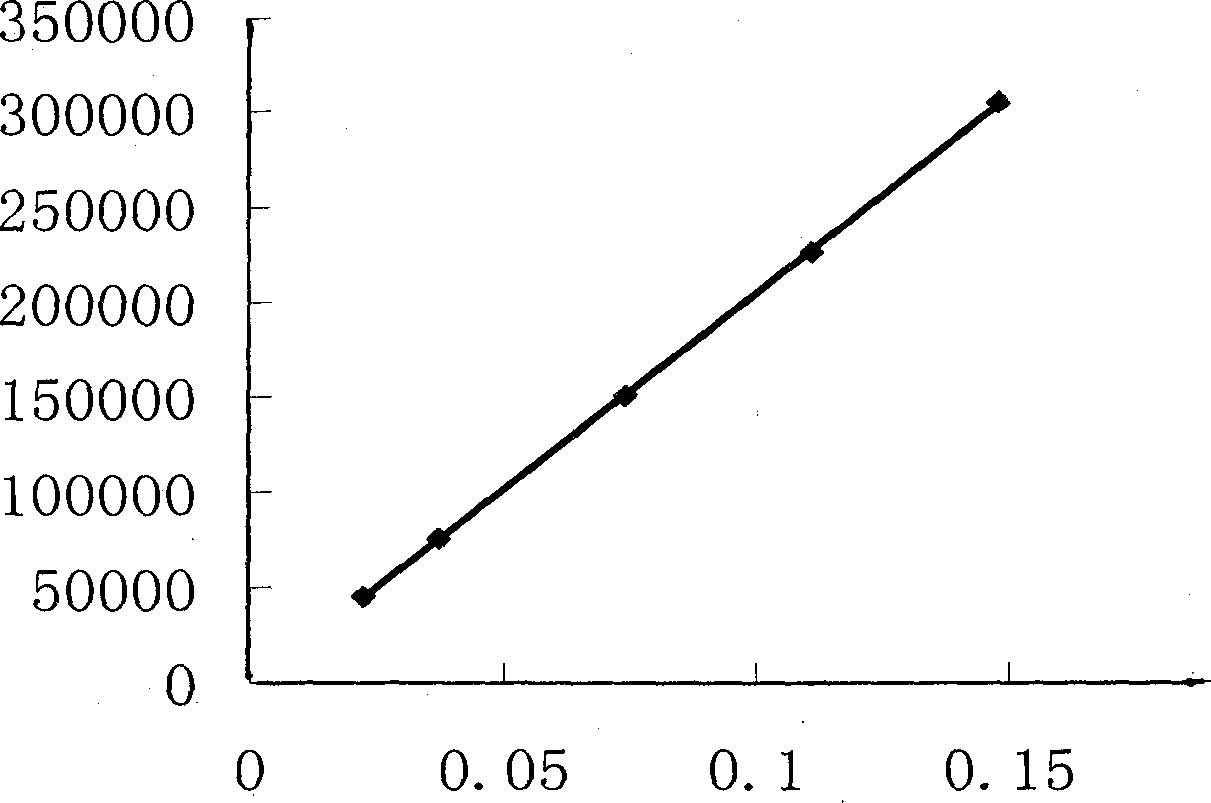
ActiveCN104133014AOvercoming precisionOvercome the deficiency of low specificityComponent separationPhosphateSustained Release Capsule
The invention discloses a method for investigation of a release degree of an ibuprofen-pseudoephedrin hydrochloride sustained-release preparation. A phosphate buffer solution added with lecithin is used as a release medium, an ibuprofen-pseudoephedrin hydrochloride sustained-release preparation is put into a rotation basket, the release medium is added into a dissolution cup, and a temperature of the release medium and a rotation rate of the rotation basket are controlled so that the ibuprofen-pseudoephedrin hydrochloride sustained-release preparation can release the compound ingredients in the release medium under the control. A comparison between release degrees of the ibuprofen-pseudoephedrin hydrochloride sustained-release preparation in a release medium without lecithin and in the release medium with lecithin proves that after use of an appropriate amount of lecithin in the phosphate buffer solution, release degrees of pseudoephedrine hydrochloride and ibuprofen in the ibuprofen-pseudoephedrin hydrochloride sustained-release capsule can be controlled in a limited range. The invention provides the method for investigation of a release degree of the compound sustained-release preparation by a surfactant. The method can provide important technical basis for finally establishing quality standards, can provide prompting information for prescription design, production control and clinical administration and has obvious social and economic benefits.
Owner:广州法尔麦兰药物技术有限公司 +1
Paracetamol and pseudoephedrine hydrochloride tablets made by dry powder direct tabletting
ActiveCN101143138ADissolution rate is fastReduce the impactOrganic active ingredientsAntipyreticAnalgesics drugsWhole body
The invention relates to an antipyretic and analgesic and a nonsteroid anti-inflammation analgesic drug and concretely relates to a paracetamol and pseudoephedrine hydrochloride tablet, which is obtained by directly tablet forming dry powder, which is used for remedying the cold. The paracetamol and pseudoephedrine hydrochloride tablet consists of the components of the following weight portions of 500 portions of paracetamol, 30 portions of pseudoephedrine, 48 portions of microcrystalline cellulose, 5.3 portions of vapor phase silicon dioxide and 48 portions of pre-gelatinized starch. The invention is fit for remedying the symptoms caused by the cold of fever, headache, the ache of the whole body and limbs, nasal obstruction, rhinorrhea, sneezes etc.
Owner:上海裕信生物制药有限公司
Medicine for treating child influenza and preparation method thereof
InactiveCN102499920ASignificant effectImprove securityOrganic active ingredientsAntipyreticPseudoephedrine HydrochlorideMagnesium stearate
The invention relates to a medicine for treating child influenza, which takes acetaminophen, pseudoephedrine hydrochloride and chlorphenamine maleate as main materials, and takes microcrystalline cellulose, starch, aspartame, sodium starch glycolate, magnesium stearate, 60% ethanol and 2% sodium starch glycolate aqueous solution as auxiliary materials. A preparation method is as follows: the acetaminophen, the pseudoephedrine hydrochloride and the chlorphenamine maleate are crushed into fine powder for spare use; the chlorphenamine maleate is uniformly mixed with the acetaminophen and the pseudoephedrine hydrochloride through a equivalent incremental method; parts of the microcrystalline cellulose, the starch, the aspartame and the sodium starch glycolate are weighed to be uniformly mixed, are crushed to sieve, and are mixed again, the 60% ethanol and the 2% sodium starch glycolate aqueous solution are added to the prepared mixture, are completely stirred to make a soft material, and wet particles are produced after the sieving; the produced wet particles are dried at 75 DEG C and granulated; after the granulation, the residual auxiliary materials of the microcrystalline cellulose, the starch, the sodium starch glycolate and the magnesium stearate are added again to mix uniformly; and dispersible tablets are pressed and packaged. The medicine has the advantages of obvious curative effect, good safety, convenience in medicine taking and suitable taste for children.
Owner:山西皇城相府药业股份有限公司
Cold resistance compound rimantadine preparation
InactiveCN1689565AEffective preventionEffective therapeuticOrganic active ingredientsAntiviralsPseudoephedrine HydrochlorideCold resistance
The present invention relates to cold resisting compound rimantadine preparation. The preparation is composition containing rimantadine hydrochloride, acetaminophen, pseudoephedrine hydrochloride, chlorpheniramine and its pharmaceutically acceptable salt. The composition is used in treating and remitting cold.
Owner:ZHEJIANG APELOA KANGYU PHARMA +1
Orally disintegrating tablet containing paracetamol, pseudoephedrine hydrochloride and dextromethorphan hydrobromide and preparation method thereof
InactiveCN101756918AImprove liquidityOrganic active ingredientsAntiviralsFluidized bedDextromethorphan Hydrobromide
The invention relates to a compound orally disintegrating tablet containing paracetamol, pseudoephedrine hydrochloride and dextromethorphan hydrobromide and a preparation method thereof. The preparation method is characterized by comprising the following steps of: uniformly mixing and dissolving the three kinds of active substances, uniformly mixing the three kinds of active components by adopting a spray drying technology of a fluidized bed to process so that mixed powder is obtained, and then pelletizing, coating and tabletting so that the orally disintegrating tablet is obtained. The orally disintegrating tablet has the advantages of simple process and uniform quality.
Owner:CHONGQING PHARMA RES INST
Preparation for treating allergic rhinitis and preparation method thereof
InactiveCN102302497AFlat surfacePromote absorptionOrganic active ingredientsRespiratory disorderSide effectClinical efficacy
The invention relates to a preparation for treating allergic rhinitis. The preparation is prepared by pressing a quick release layer and a sustained-release layer into bilayer tablets and coating, wherein the quick release layer consists of the following components in percentage by mass: 1 to 10 percent of levocetirizine hydrochloride, 1 to 15 percent of disintegrating agent, 0.5 to 3 percent of lubricating agent, 0 to 8 percent of adhesive and the balance of filler; the sustained-release layer consists of the following components in percentage by mass: 20 to 30 percent of pseudoephedrine hydrochloride, 50 to 60 percent of sustained-release framework material, 0.5 to 3 percent of lubricating agent, 0 to 8 percent of adhesive and the balance of filler; and the mass ratio of the levocetirizine hydrochloride to the pseudoephedrine hydrochloride is 1:48. The sustained-release tablets for treating the allergic rhinitis have bright and clean surfaces, and the release behavior of the main medicine pseudoephedrine hydrochloride of the sustained-release tablets has high consistency with that of American marketed medicine cetirizine hydrochloride and pseudoephedrine hydrochloride sustained-release tablets through detection; and the dissolution rate of the main medicine levocetirizine hydrochloride of the sustained-release tablets in 30 minutes is over 90 percent, so that the sustained-release preparation for treating the allergic rhinitis has high bioavailability, a good absorption effect, comprehensive and effective clinical curative effects, small side effects and ideal safety.
Owner:TIANSHENG PHARMA GROUP
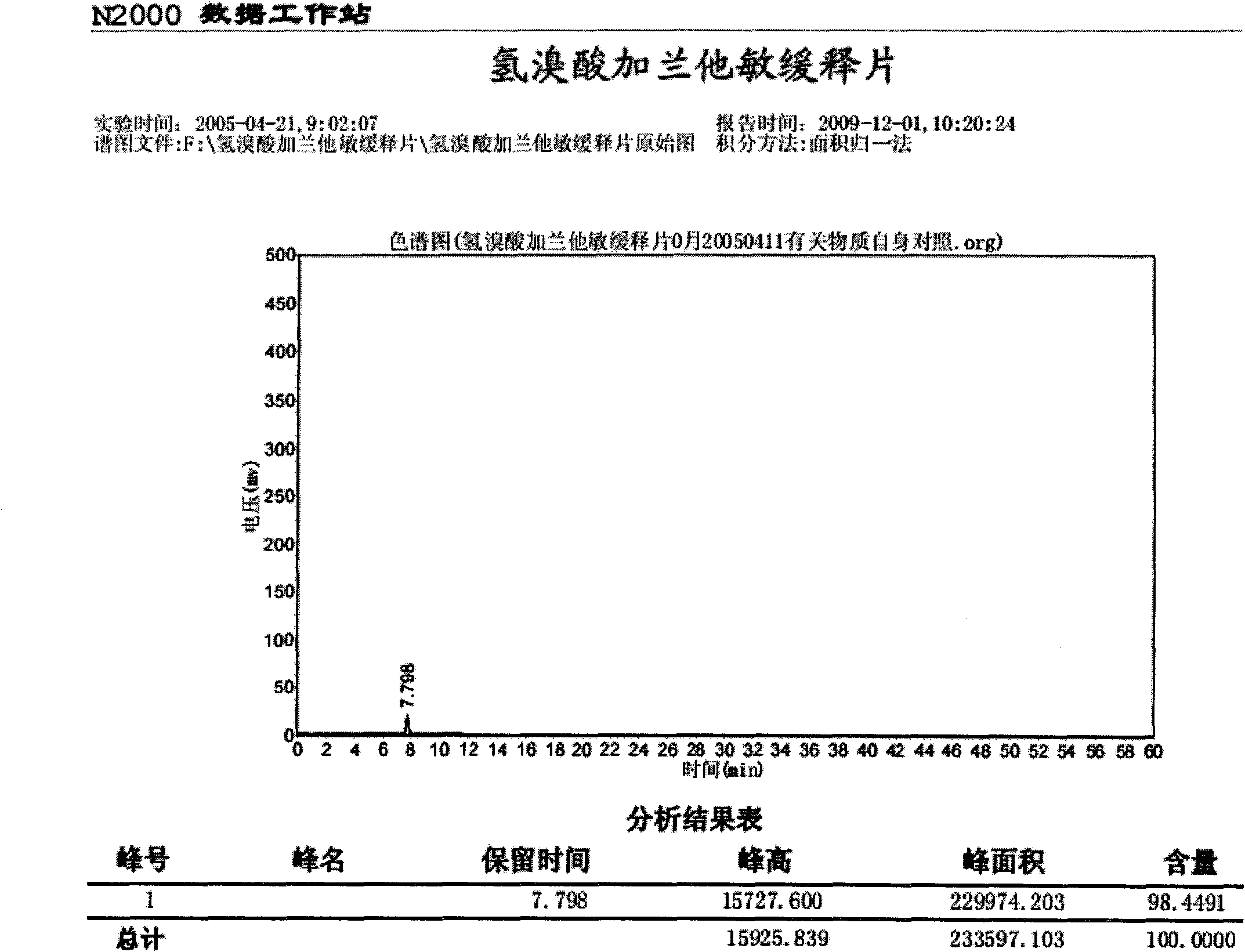
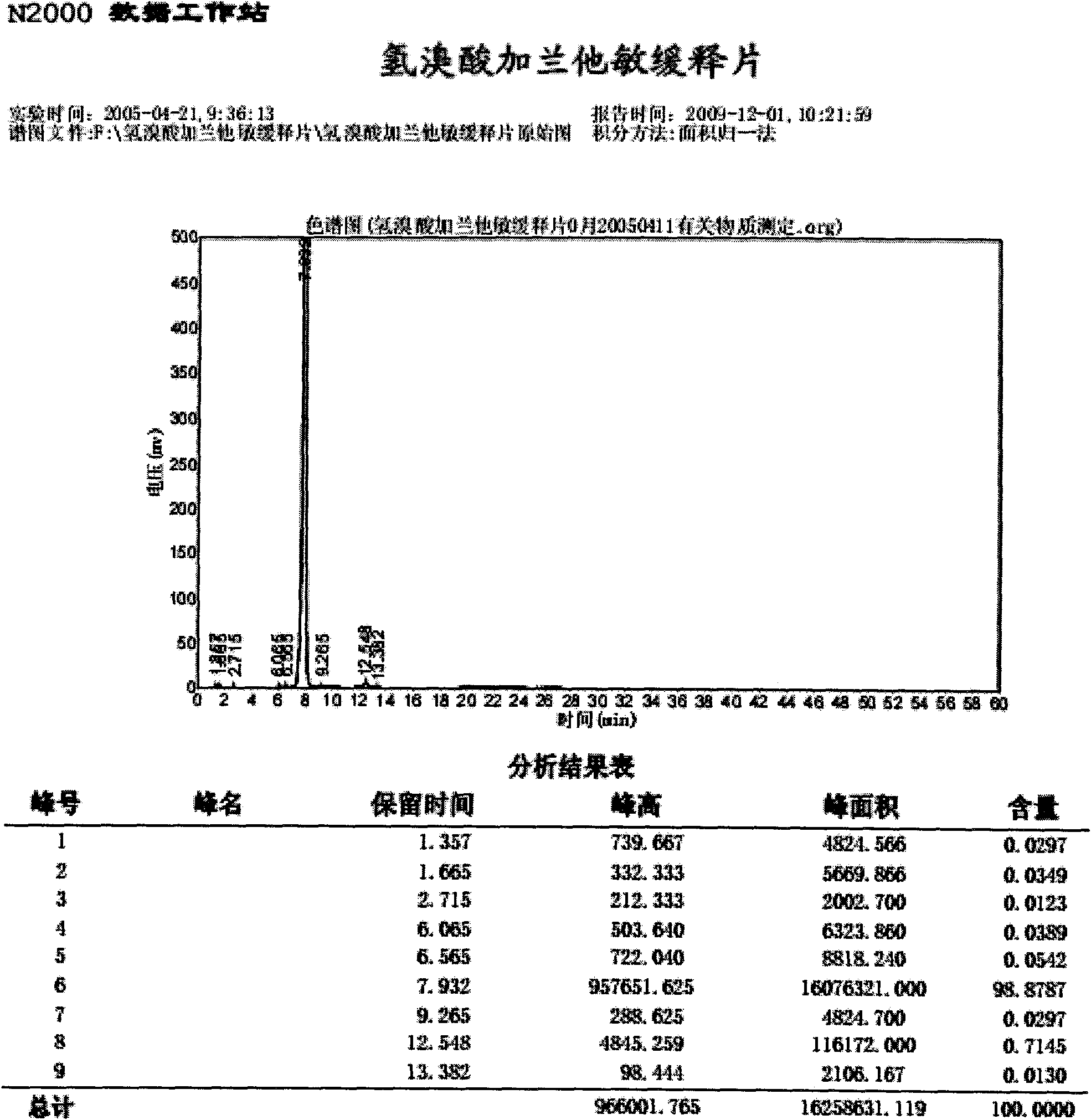
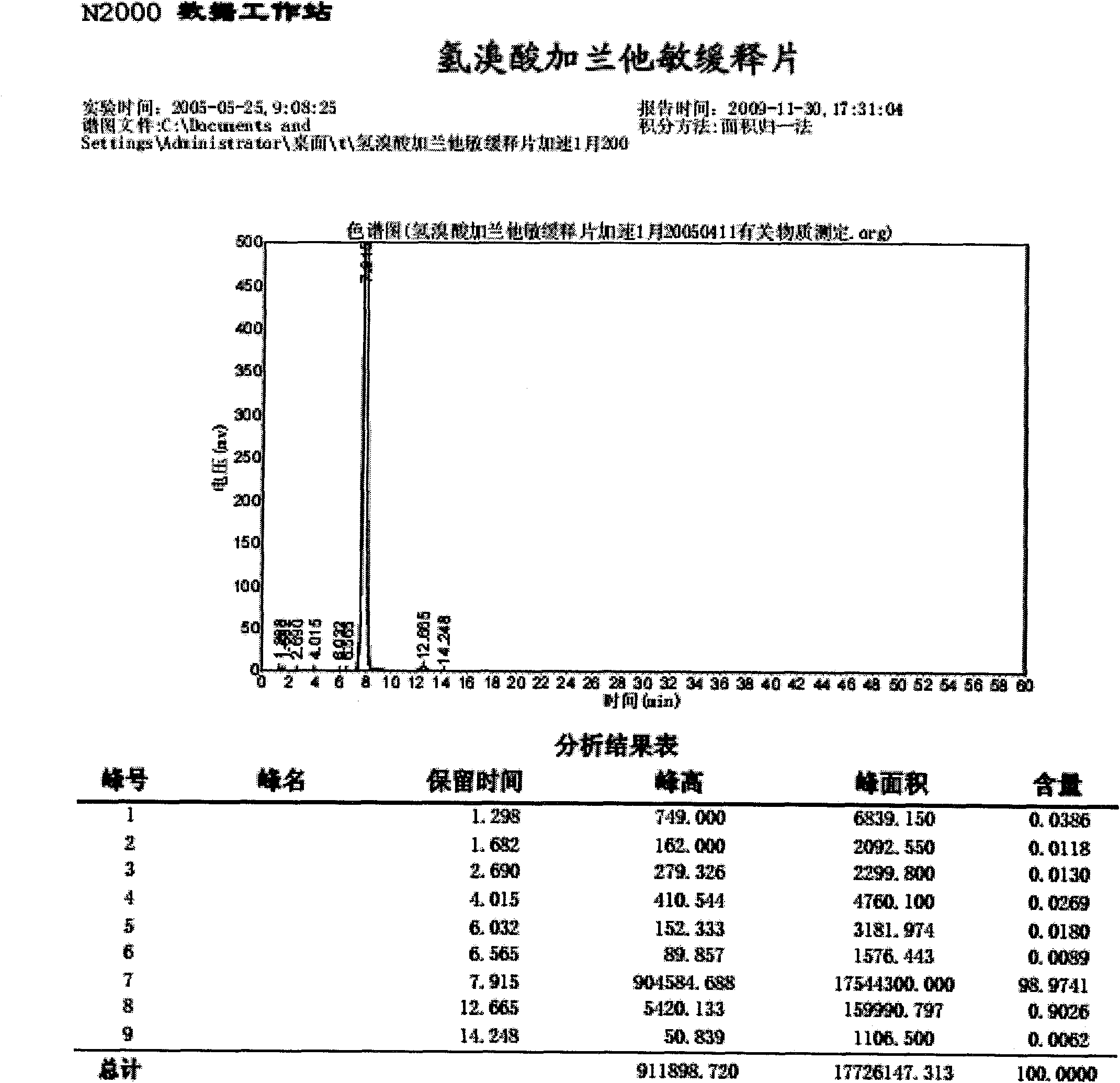
Preparation method of compound pseudoephedrine hydrochloride capsule
The invention relates to a preparation method of a compound pseudoephedrine hydrochloride capsule. The preparation method provided by the invention is characterized by comprising the following steps of detecting chloroacetic acid content of sodium carboxymethyl starch, when the weight of chloroacetic acid is 0 to 0.05% of that of sodium carboxymethyl starch, mixing active ingredients and auxiliary materials well, carrying out granulation, drying, sieving and loading to obtain the compound pseudoephedrine hydrochloride capsule. Through the preparation method provided by the invention, it is avoided that chlorphenamine maleate and chloroacetic acid of the compound pseudoephedrine hydrochloride capsule undergo a chemical reaction in storage to produce an impurity shown in the formula I and thus chlorphenamine maleate content is reduced. The preparation method provided by the invention reduces factors causing preparation destabilization and improves preparation safety.
Owner:SINOVA LABS +1
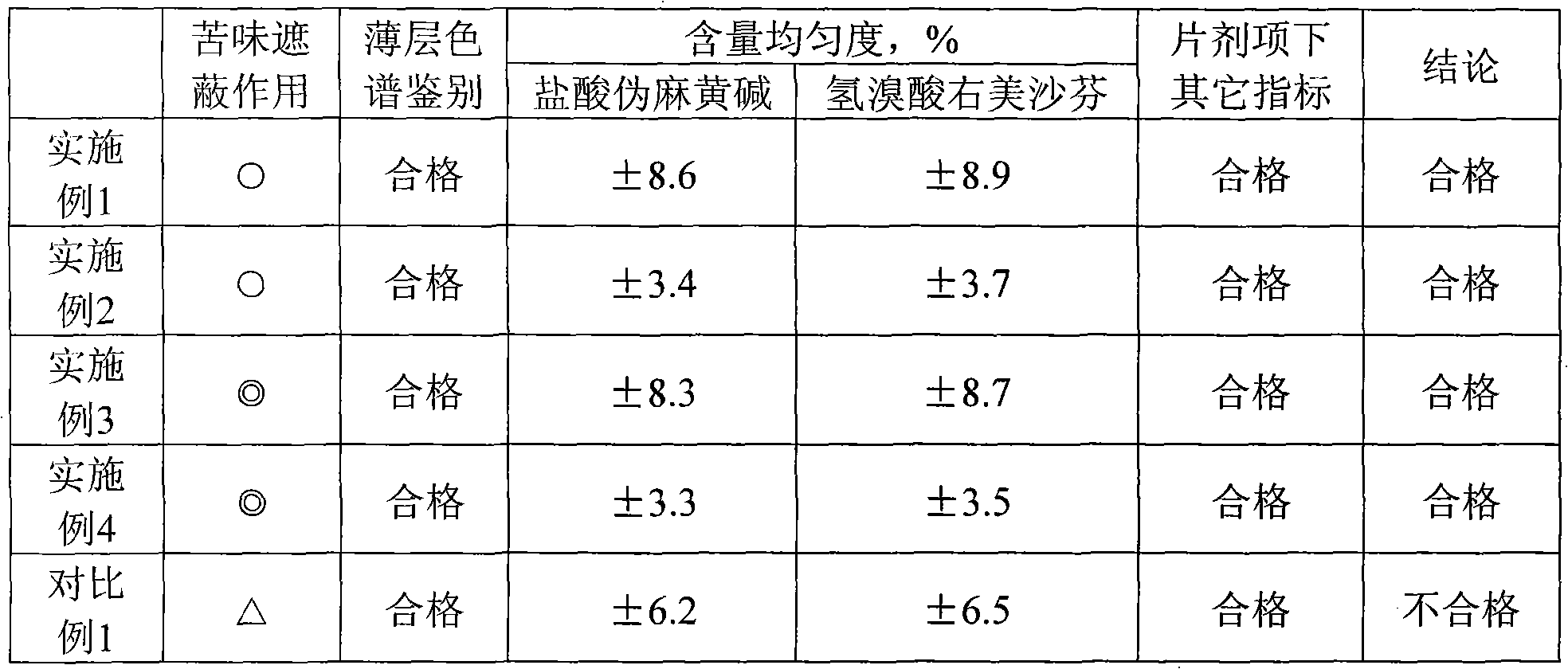
Preparation method and quality test method of pseudoephedrine hydrochloride and dextromethorphan hydrobromide chewable tablets
ActiveCN101810617AControllable bitternessGood content uniformityOrganic active ingredientsComponent separationDextromethorphan HydrobromidePseudoephedrine Hydrochloride
The invention discloses a preparation method and a quality test method of pseudoephedrine hydrochloride and dextromethorphan hydrobromide chewable tablets. The preparation method is characterized in that: medicaments are dispersed in sodium alga acid uniformly first; then auxiliary materials are added, and the mixture is granulated; and finally, the grains are coated with a coating material to isolate the bitterness of medicaments. The quality test method comprises thin layer chromatography and a content measurement method, has high specificity and ensures the preparation process of the invention has high controllability.
Owner:JIANGXI HERBI SKY CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com