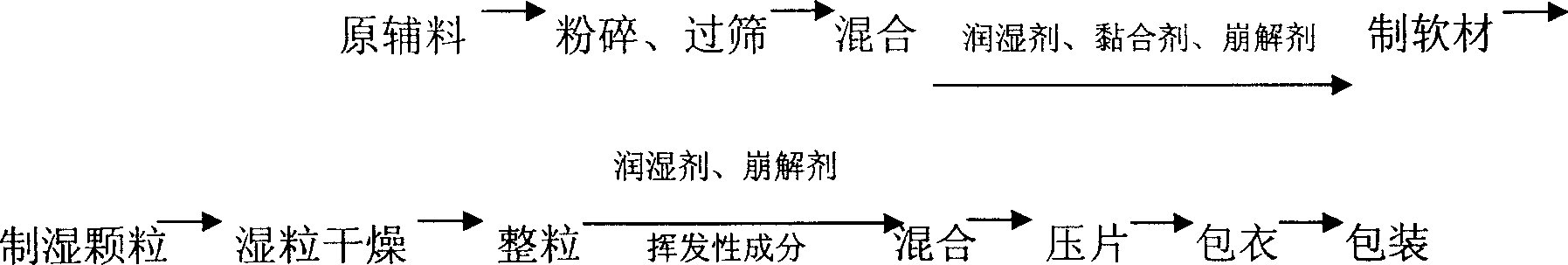
Paracetamol and pseudoephedrine hydrochloride tablets made by dry powder direct tabletting
A technology of direct compression of dry powder and paracetamol, which is applied in the directions of medical preparations containing active ingredients, pill delivery, organic active ingredients, etc., can solve the problems of poor fluidity and compressibility, etc., and achieve the effect of stable storage process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] Weigh 500g of paracetamol, 30g of pseudoephedrine hydrochloride, 48g of microcrystalline cellulose, 5.3g of fumed silicon dioxide, and 48g of pregelatinized starch, and add the above-mentioned components in stages according to the principle of equal mixing, and stir until Mix evenly, and then directly compress into tablets, making a total of 1000 tablets.
Embodiment 2
[0014] Weigh 5000g of acetaminophen, 300g of pseudoephedrine hydrochloride, 480g of microcrystalline cellulose, 53g of fumed silica, and 480g of pregelatinized starch, and add the above-mentioned components in small to many portions according to the principle of equal mixing, and stir until mixed Evenly, and then directly compressed into tablets, a total of 10,000 tablets were made.
Embodiment 3
[0016] Weigh 1000g of paracetamol, 60g of pseudoephedrine hydrochloride, 96g of microcrystalline cellulose, 10.6g of fumed silicon dioxide, and 96g of pregelatinized starch, and add the above-mentioned components in stages from less to more according to the principle of equal mixing, and stir until Mix evenly, and then directly compress into tablets, making a total of 2000 tablets.
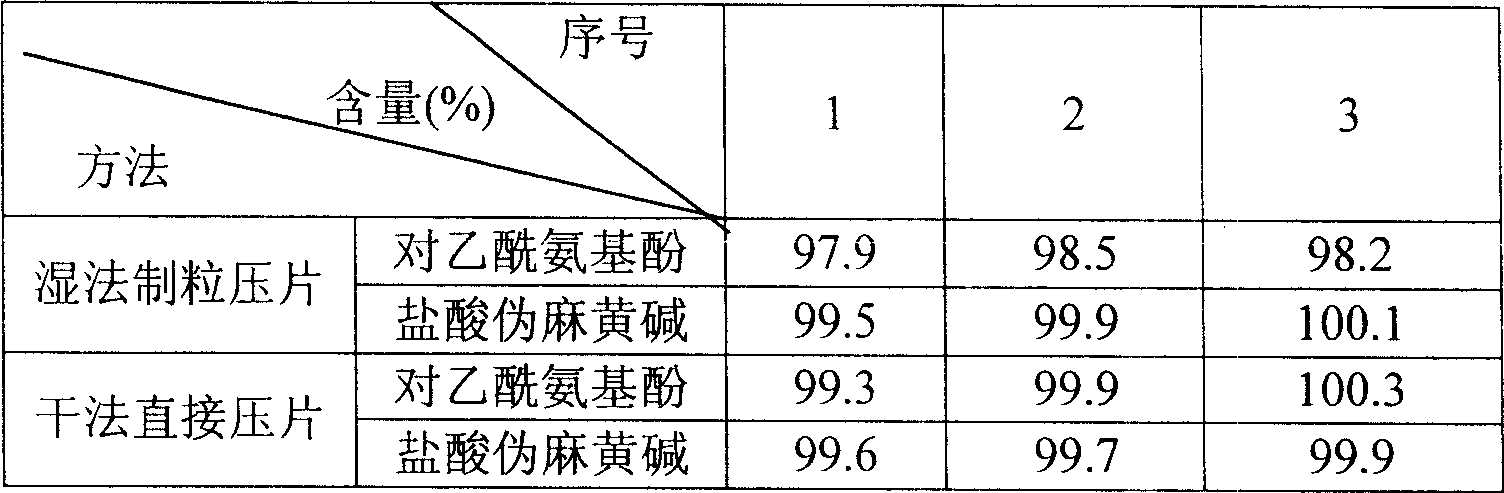
[0017] Two, effect embodiment.
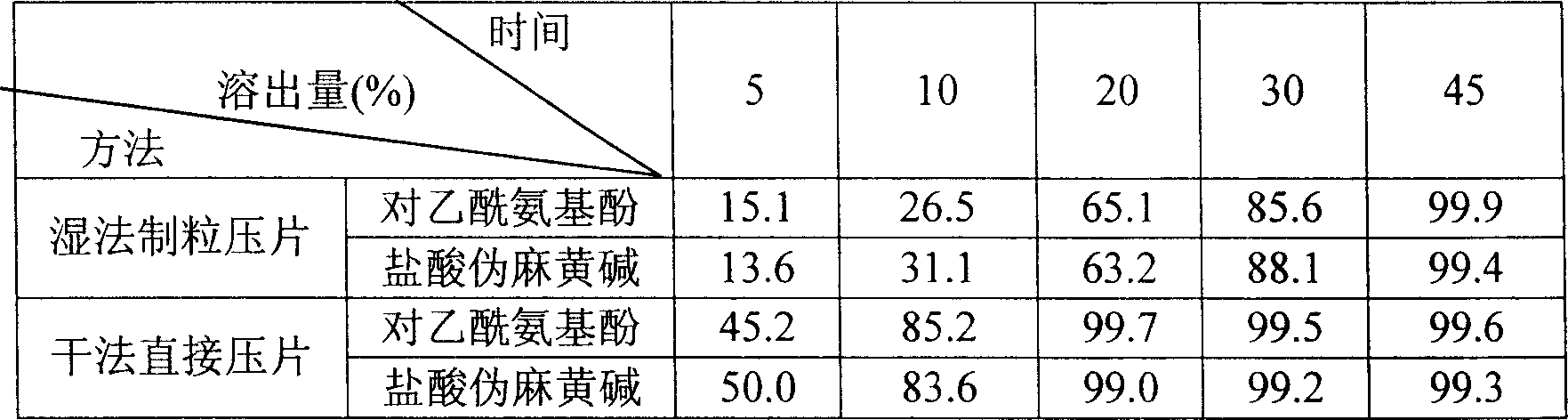
[0018] 1. Dissolution rate comparison.
[0019] Measuring method: use RCZ-6C type drug dissolution apparatus, according to dissolution determination method (Chinese Pharmacopoeia 2005 edition two appendix X C first method), with 1000ml water as solvent, 37 ℃, rotating speed is 100r.min -1 , sampling time is 45min, get solution and filter in right amount, get continued filtrate 20 μ l, measure according to the method under assay item, calculate the stripping amount of acetaminophen and pseudoephedrine hydrochloride in every tablet.
[0020] Sampling and analysis o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com