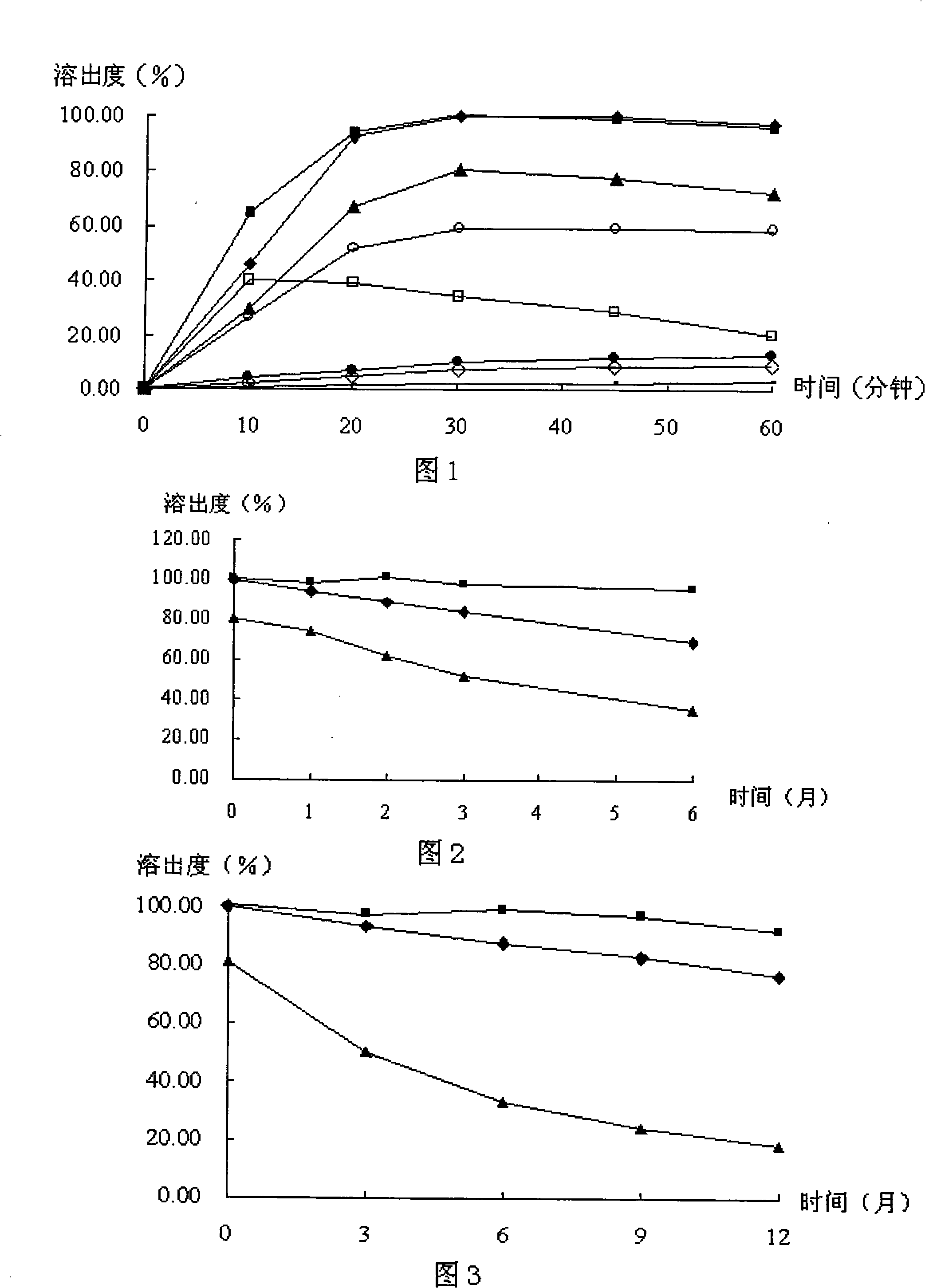
Nimodipine capsule containing semi-solid combination and preparation
A technology for nimodipine capsules and nimodipine solids, which is applied in the field of pharmaceutical preparations, can solve the problems of reduced dissolution rate, low dissolution rate, easy aging, etc., and achieves the effects of increased dissolution rate, fast dissolution rate, and easy aging.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1: prepare nimodipine capsule according to the following steps:
[0025] a. the preparation of nimodipine solid dispersion: take copovidone S630 and Eudragit E100 as carrier, get 3 parts of micronized nimodipine, 5 parts of Eudragit E100 and 2 parts of copovidone S630 (by weight ), after uniform mixing, add to the feeding hopper of the twin-screw melting extruder, set the temperature in the first zone to 115°C, the temperature in the second, third, and fourth zones are all 130°C, and the number of revolutions is 36 rpm, and the extrudate is placed in ( -70) ℃ refrigerator quenching, crushing, passing through a 120-mesh sieve to obtain nimodipine solid dispersion.
[0026] b. The preparation of nimodipine capsules: get semi-solid matrix copovidone S630 / 72g, polyoxyethylene sorbitan fatty acid ester (Tween 80) / 96g, semi-solid matrix polyethylene glycol 400 / 360g, polyethylene glycol Diol 6000 / 8.4g, heat and stir at 90°C, add nimodipine solid dispersion powder / 1...
Embodiment 2
[0028] Embodiment 2: prepare nimodipine capsule according to the following steps:
[0029] a. Preparation of Nimodipine Solid Dispersion: Take copovidone S630 as carrier, get 2 parts of micronized nimodipine and 8 parts of copovidone S630 (by weight), after uniform mixing, add twin-screw melt extrusion In the feeding hopper of the machine, set the temperature of the first zone to 115°C, the temperature of the second, third, and fourth zones to be 125°C, and the number of rotations to be 30 rpm, and put the extruded product in a (-30)°C refrigerator to quench, crush, and pass through 100 mesh sieve to obtain nimodipine solid dispersion.
[0030] b. Preparation of Nimodipine Capsules: Take sorbitan fatty acid lipid (Span 60) / 120g, semi-solid matrix polyethylene glycol 400 / 300g, glycerin / 72g, polyethylene glycol 8000 / 12g, and heat at 80°C After stirring evenly, add nimodipine solid dispersion powder / 120g, continue to stir until uniform, cool down to 60°C, and quantitatively fill...
Embodiment 3
[0031] Embodiment 3: prepare nimodipine capsule according to the following steps:
[0032]a. Preparation of Nimodipine Solid Dispersion: Take Acrylic Resin No. 4 as carrier, get 3 parts of micronized Nimodipine, 7 parts of Acrylic Resin No. 4 (by weight), after uniform mixing, add single-screw melt extrusion In the feeding hopper of the machine, set the temperature of the first zone to 105°C, the temperature of the second, third, and fourth zones to be 135°C, and the number of rotations to be 24 rpm. The extruded product is cooled at room temperature, crushed, and passed through a 100-mesh sieve to obtain Ni Modipine solid dispersion.
[0033] b. Preparation of Nimodipine Capsules: Take poloxamer 188 / 120g, glycerin 300g, heat and stir at 75°C until uniform, add nimodipine solid dispersion powder / 120g, continue to stir until uniform, cool to 70°C, The liquid-filled capsule filling machine quantitatively fills the contents into cellulose capsules, each capsule contains about 45...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com