Preparation for treating allergic rhinitis and preparation method thereof
A technology for allergic rhinitis and preparations, which is applied in the field of drugs for the treatment of rhinitis. It can solve the problems of side effects, unsatisfactory safety, lack of sedative and anticholinergic effects, and achieve ideal safety, good consistency, and small side effects. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
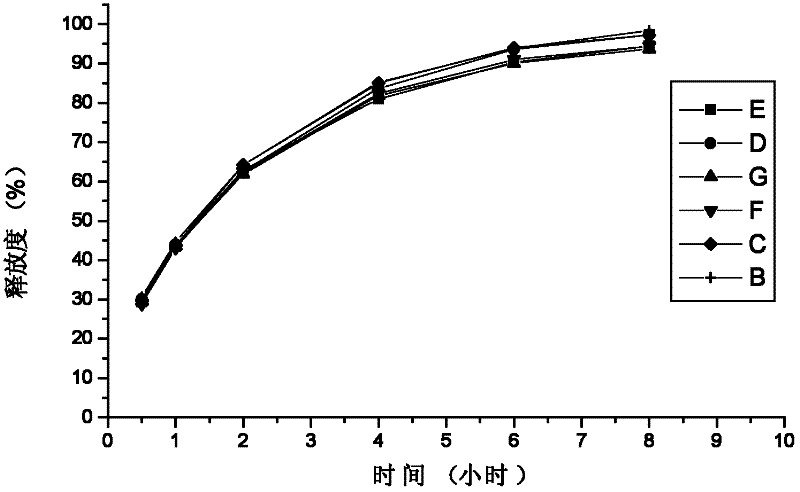
Embodiment 1
[0039] A sustained-release tablet for treating allergic rhinitis, prepared according to the following steps:
[0040] Chip composition
[0041]
[0042] Coating composition:
[0043]
[0044]Immediate release layer:
[0045] (1) Pulverize the above-mentioned levocetirizine hydrochloride and auxiliary materials, pass the main drug through a 100-mesh sieve, and pass the auxiliary materials through a 80-mesh sieve, and set aside.
[0046] (2) take the levocetirizine hydrochloride of above-mentioned amount and lactose, microcrystalline cellulose, carboxymethyl starch sodium and mix;
[0047] (3) wet granulation with 0.5% hypromellose (5CPS);
[0048] (4) Dry at 40°C-60°C, granulate, add the prescribed amount of magnesium stearate, mix well, detect the content of the intermediate product of levocetirizine hydrochloride, and calculate the weight of the immediate-release layer.
[0049] Slow release layer:
[0050] (1) Pulverize the above-mentioned pseudoephedrine hydrochl...
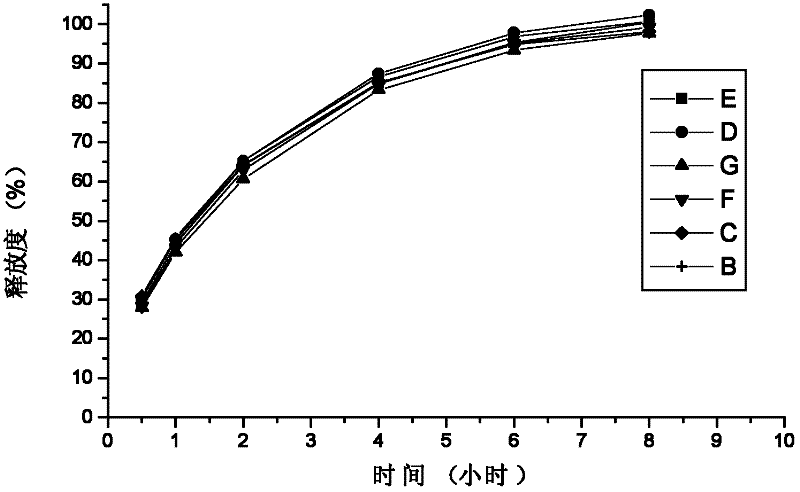
Embodiment 2
[0091] A preparation for treating allergic rhinitis, prepared according to the following steps:
[0092] Chip composition
[0093]
[0094]
[0095] Coating composition:
[0096]
[0097] Immediate release layer:
[0098] (1) Pulverize the above-mentioned levocetirizine hydrochloride and auxiliary materials, pass the main drug through a 100-mesh sieve, and pass the auxiliary materials through a 80-mesh sieve, and set aside.
[0099] (2) Take the above-mentioned amount of levocetirizine hydrochloride and lactose, microcrystalline cellulose, low-substituted hydroxypropyl cellulose and mix uniformly;
[0100] (3) wet granulation with 0.5% hypromellose (5CPS);
[0101] (4) Dry at a temperature of 40°C to 60°C, granulate, add the above-mentioned amount of magnesium stearate, mix well, detect the content of the intermediate product of levocetirizine hydrochloride, and calculate the weight of the immediate-release layer.
[0102] Slow release layer:
[0103] (1) Pulveri...
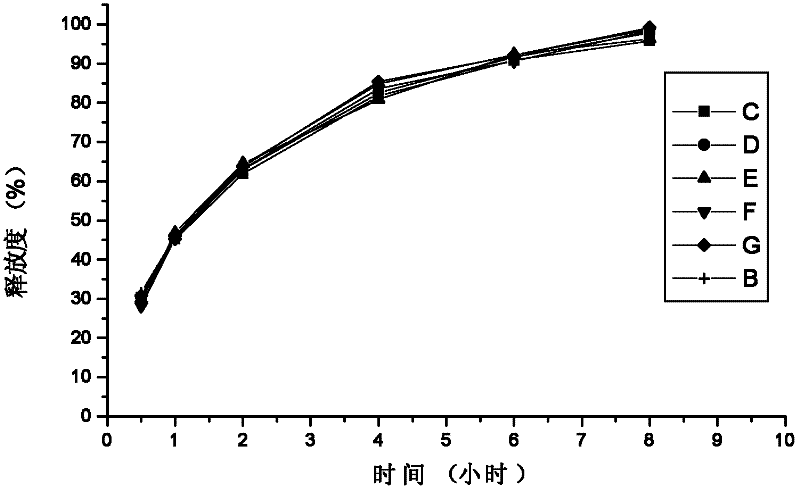
Embodiment 3
[0127]A sustained-release tablet for treating allergic rhinitis, prepared according to the following steps:
[0128] Chip composition
[0129]
[0130]
[0131] Coating composition:
[0132]
[0133] Immediate release layer:
[0134] (1) above-mentioned levocetirizine hydrochloride and adjuvant are pulverized, main ingredient is passed through 100 mesh sieve, adjuvant is passed through 80 mesh sieve, for subsequent use;
[0135] (2) Take the above-mentioned amount of levocetirizine hydrochloride and lactose, microcrystalline cellulose, low-substituted hydroxypropyl cellulose and mix uniformly;
[0136] (3) wet granulation with 0.5% hypromellose (5CPS);
[0137] (4) Dry at a temperature of 40°C to 60°C, granulate, add the above-mentioned amount of magnesium stearate, mix well, detect the content of the intermediate product of levocetirizine hydrochloride, and calculate the weight of the immediate-release layer.
[0138] Slow release layer:
[0139] (1) Pulverize t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com