Chinese ephedra medicinal material, and content determination method of three alkaloids in preparation thereof
A determination method and technology of alkaloids, which are applied in the directions of measuring devices, material separation, analysis materials, etc., can solve problems such as the harm of the service life of instruments and chromatographic columns, and increase the operation steps of chromatographic columns. The effect of expansion and cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
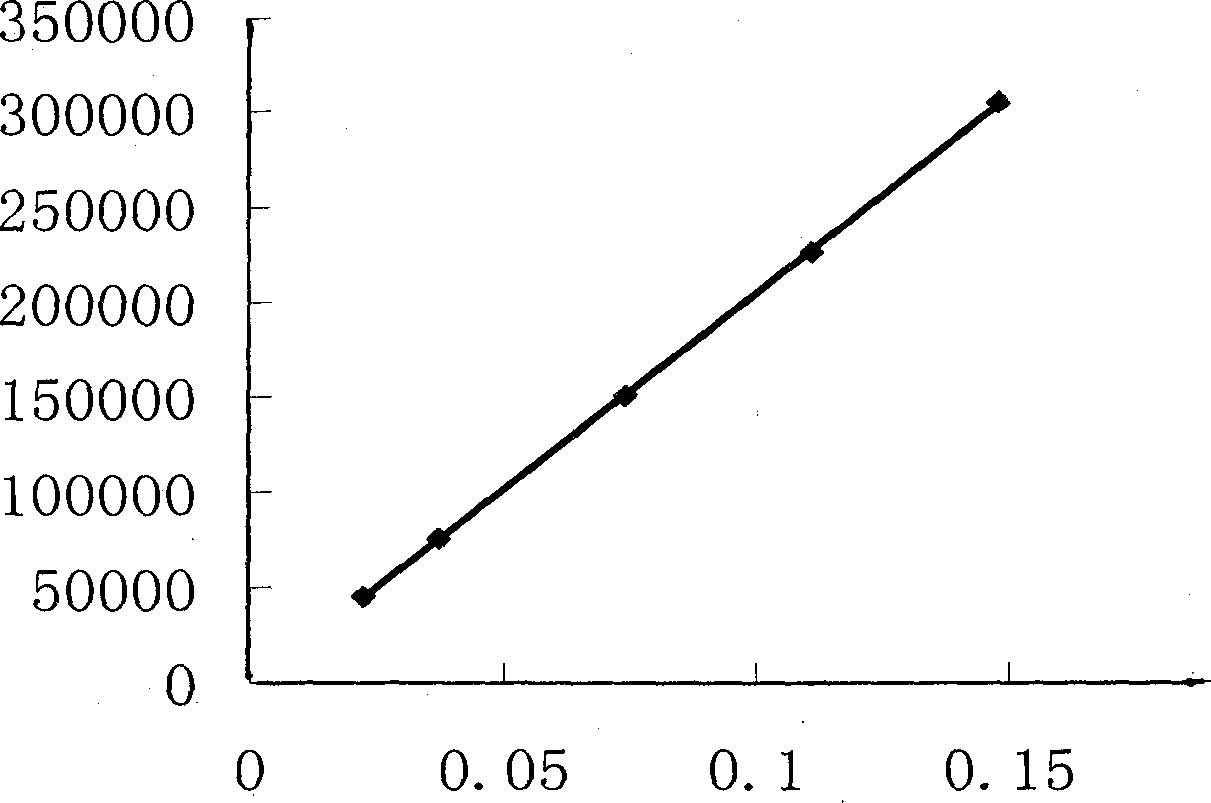
[0065] (1) Chromatographic conditions and system suitability test: Octadecylsilane bonded silica gel is used as filler; acetonitrile-methanol-0.1% phosphoric acid with volume ratio of 1-1.5:4-4.2:94.5-95.0 is used as mobile phase; Column temperature: 40°C; detection wavelength is 207nm; calculated based on ephedrine hydrochloride peak, it should not be lower than 3000;
[0066] (2) Preparation of reference substance solution Take an appropriate amount of ephedrine hydrochloride, pseudoephedrine hydrochloride and methylephedrine hydrochloride, accurately weighed, add hydrochloric acid-70% methanol solution with a volume ratio of 1: 200, and make every 1ml of ephedrine hydrochloride A solution of 30-15 μg, pseudoephedrine hydrochloride 10-5 μg, and methylephedrine hydrochloride 6-3 μg was used as the reference solution;
[0067](3) Preparation of the test solution: Take 0.2-0.3 g of ephedra medicinal material fine powder, accurately weighed, or 0.5-0.8 g of ephedra preparation, ...
Embodiment 2
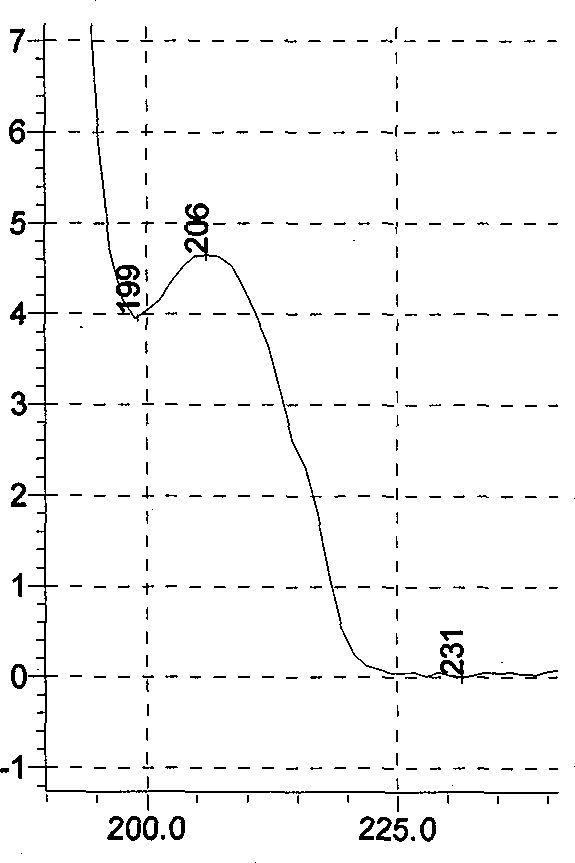
[0070] (1) Chromatographic conditions and system suitability test: Octadecylsilane bonded silica gel is used as filler; acetonitrile-methanol-0.1% phosphoric acid with a volume ratio of 1:4.2:94.8 is used as mobile phase; column temperature: 40°C; The detection wavelength is 207nm; calculated according to the peak of ephedrine hydrochloride, it should not be lower than 3000;
[0071] (2) Preparation of the reference substance solution Take appropriate amounts of ephedrine hydrochloride, pseudoephedrine hydrochloride and methylephedrine hydrochloride respectively, accurately weigh them, add hydrochloric acid-70% methanol solution with a volume ratio of 1:200, and make every 1ml of ephedrine hydrochloride A solution of 30 μg of alkali, 10 μg of pseudoephedrine hydrochloride, and 5 μg of methylephedrine hydrochloride is obtained;
[0072] (3) Preparation of the test solution Take 20 tablets of Maxing cough tablets, remove the coating, accurately weigh, grind finely, pass through ...
Embodiment 3
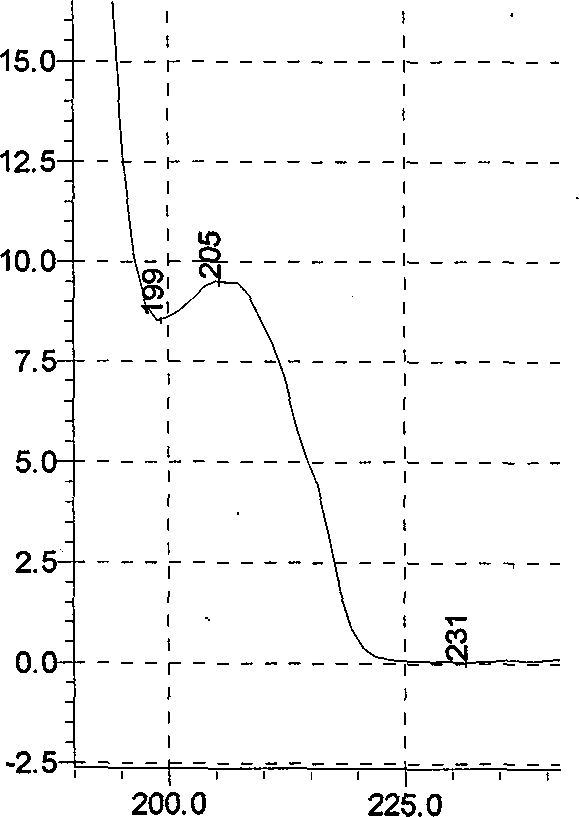
[0075] (1) Chromatographic conditions and system suitability test: Octadecylsilane bonded silica gel is used as filler; acetonitrile-methanol-0.1% phosphoric acid with a volume ratio of 1:4:95 is used as mobile phase; column temperature: 40°C; The detection wavelength is 207nm; calculated according to the peak of ephedrine hydrochloride, it should not be lower than 3000;
[0076] (2) Preparation of the reference substance solution Take appropriate amounts of ephedrine hydrochloride, pseudoephedrine hydrochloride and methylephedrine hydrochloride respectively, accurately weigh them, add hydrochloric acid-70% methanol solution with a volume ratio of 1:200, and make every 1ml of ephedrine hydrochloride A solution of 15 μg of alkali, 5 μg of pseudoephedrine hydrochloride, and 3 μg of methylephedrine hydrochloride is obtained;
[0077] (3) Preparation of the test solution: Take 20 pieces of Jinma Xing cough tablets, remove the coating, accurately weigh, grind, pass through a 50-mes...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The inside diameter of | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com