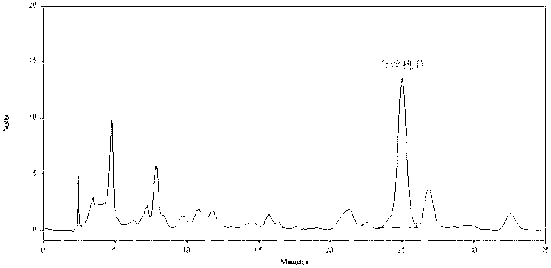
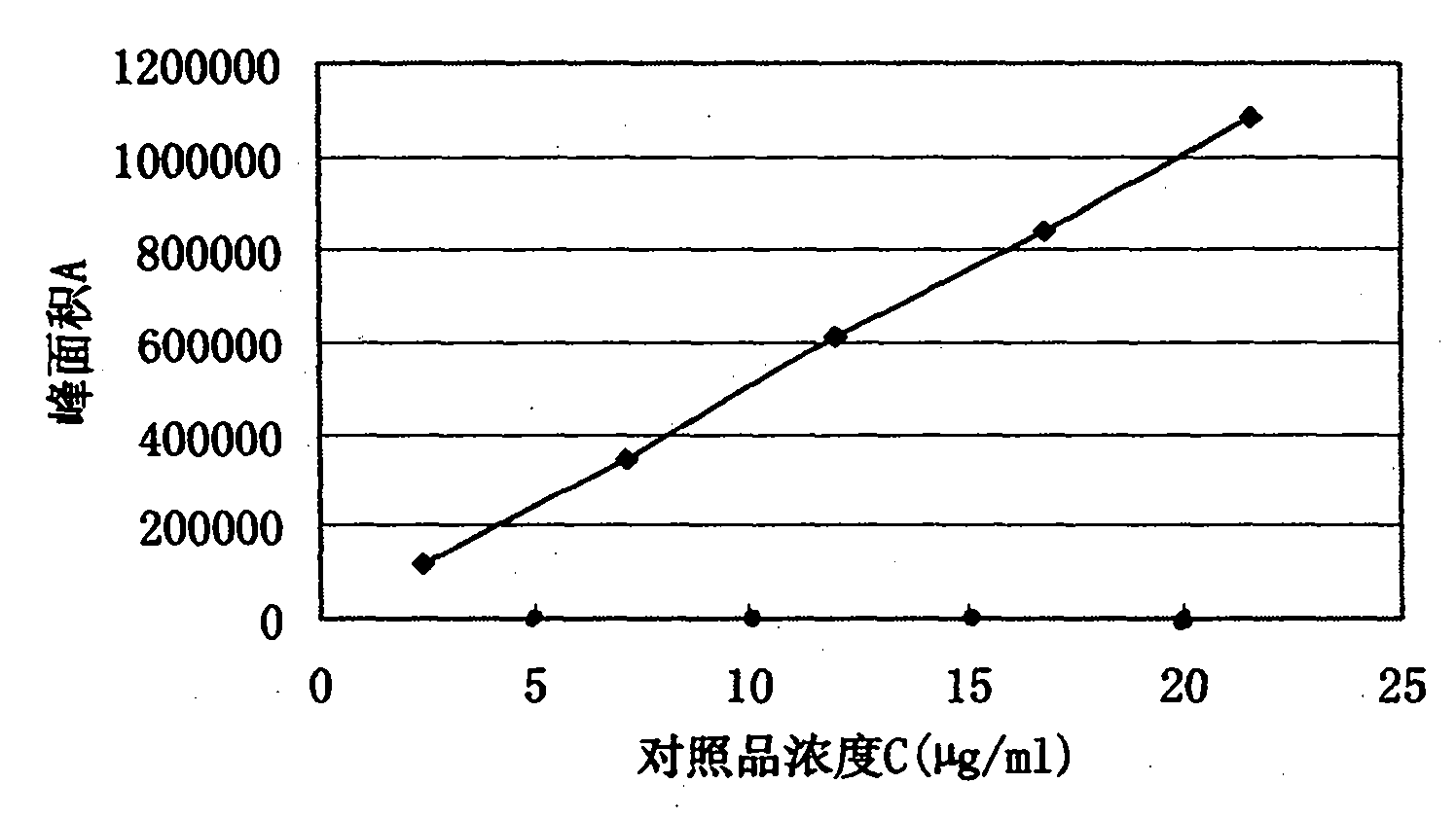
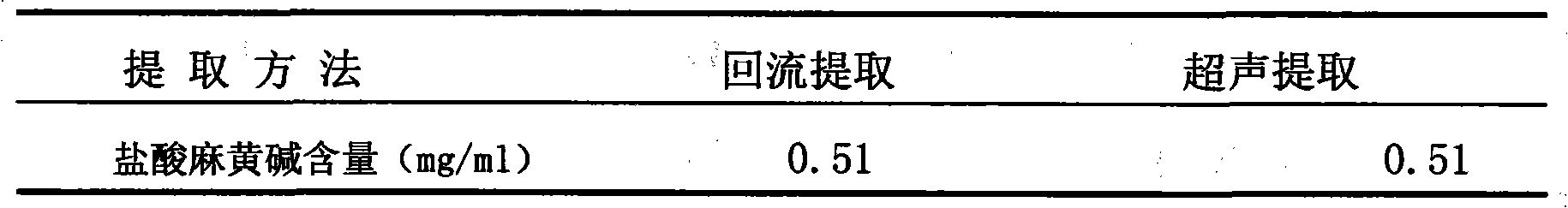
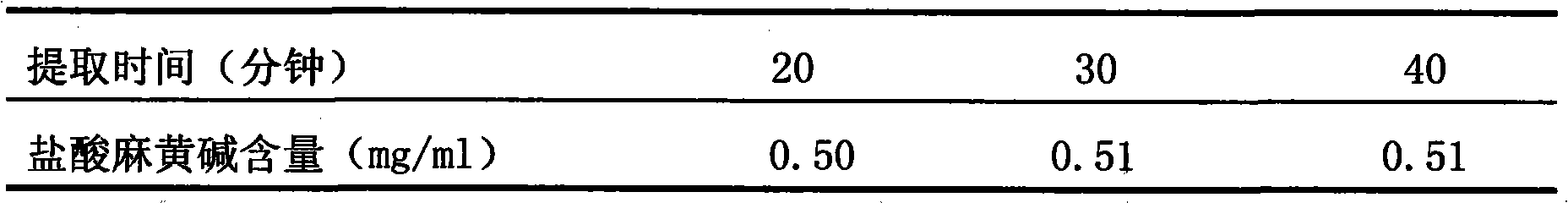
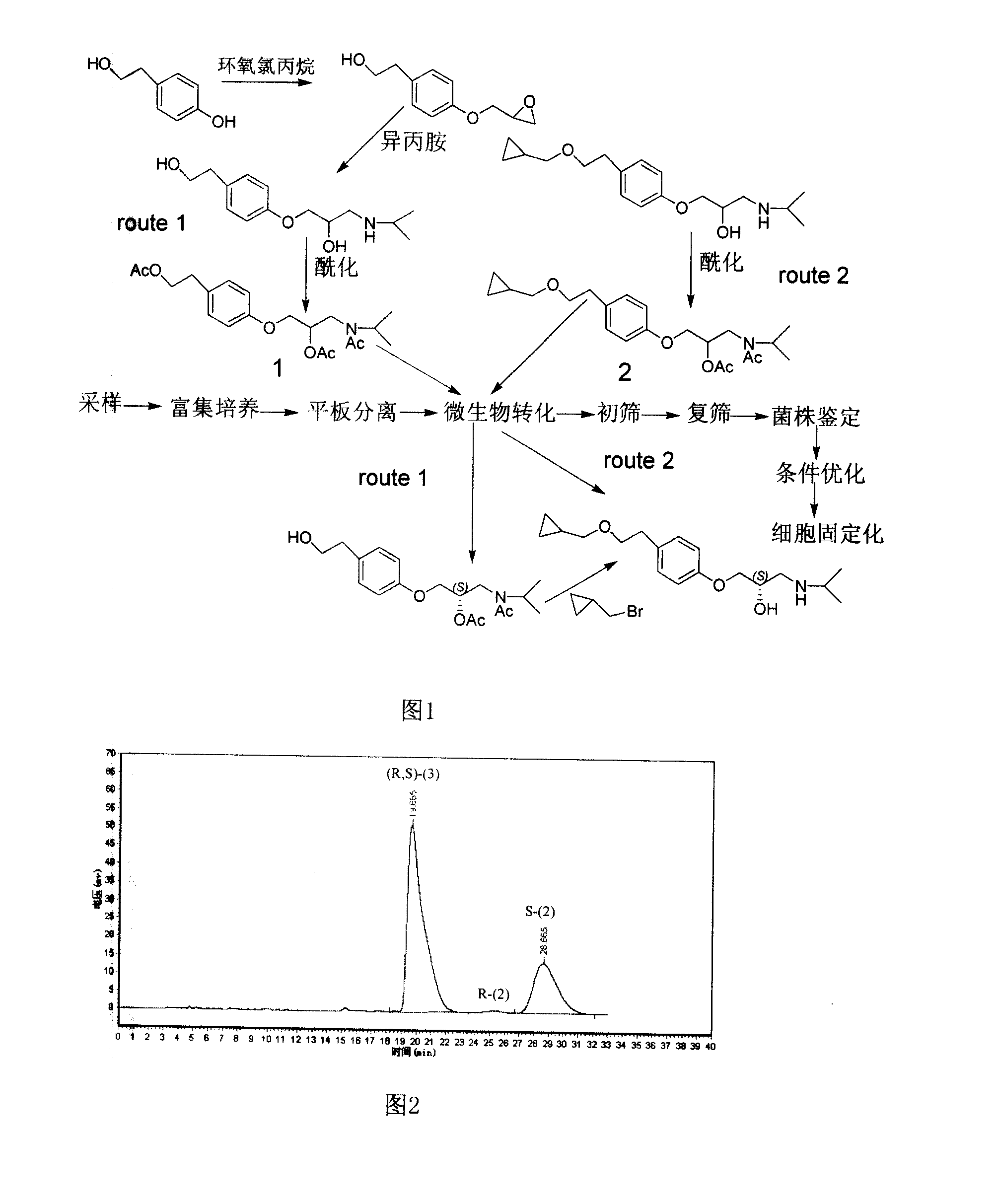
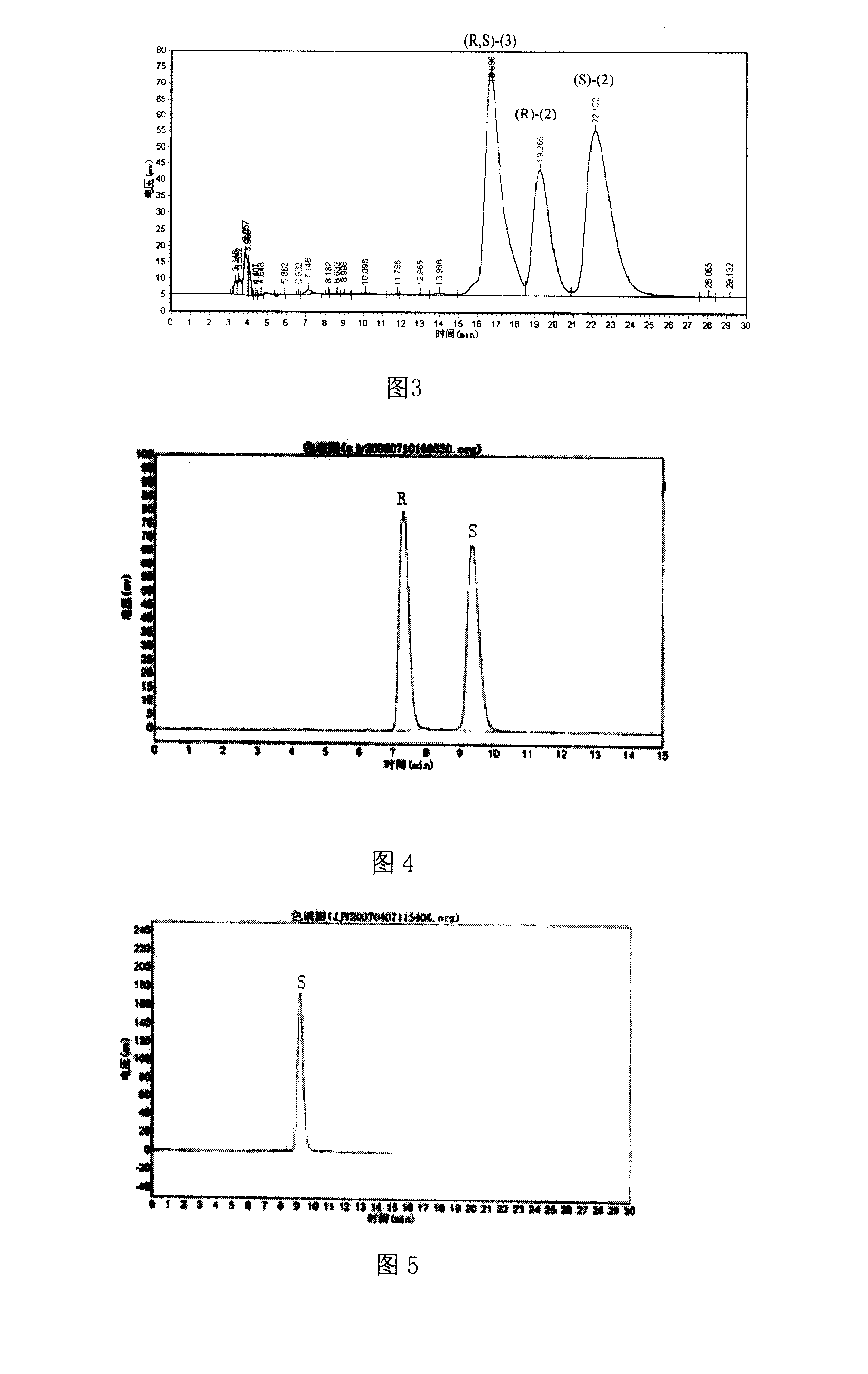
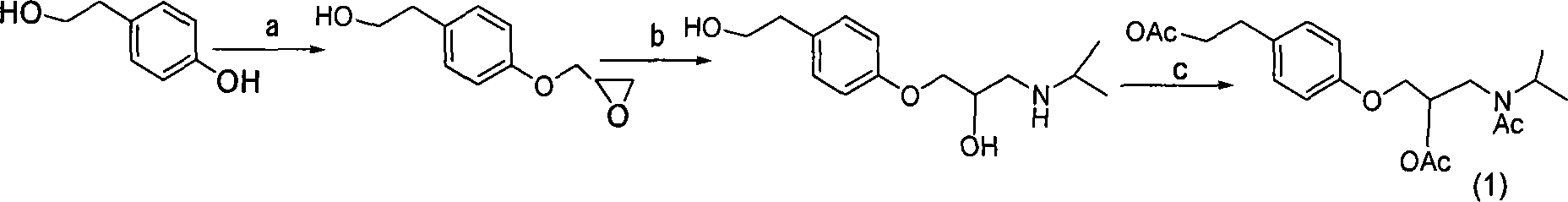
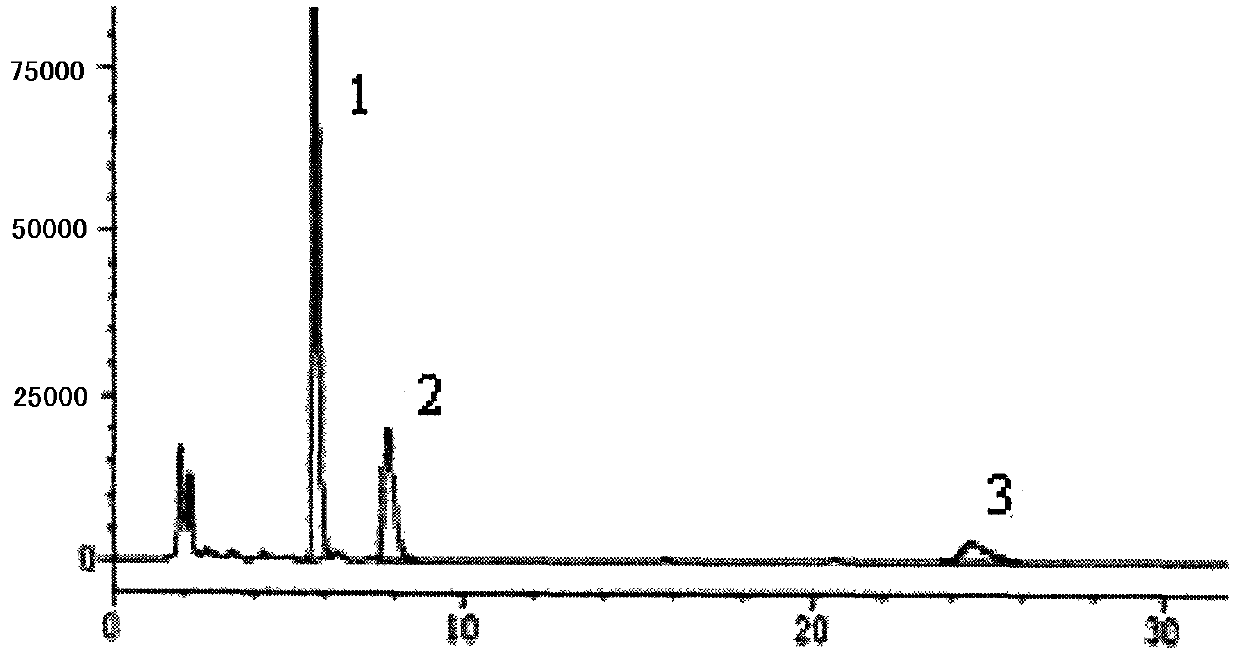
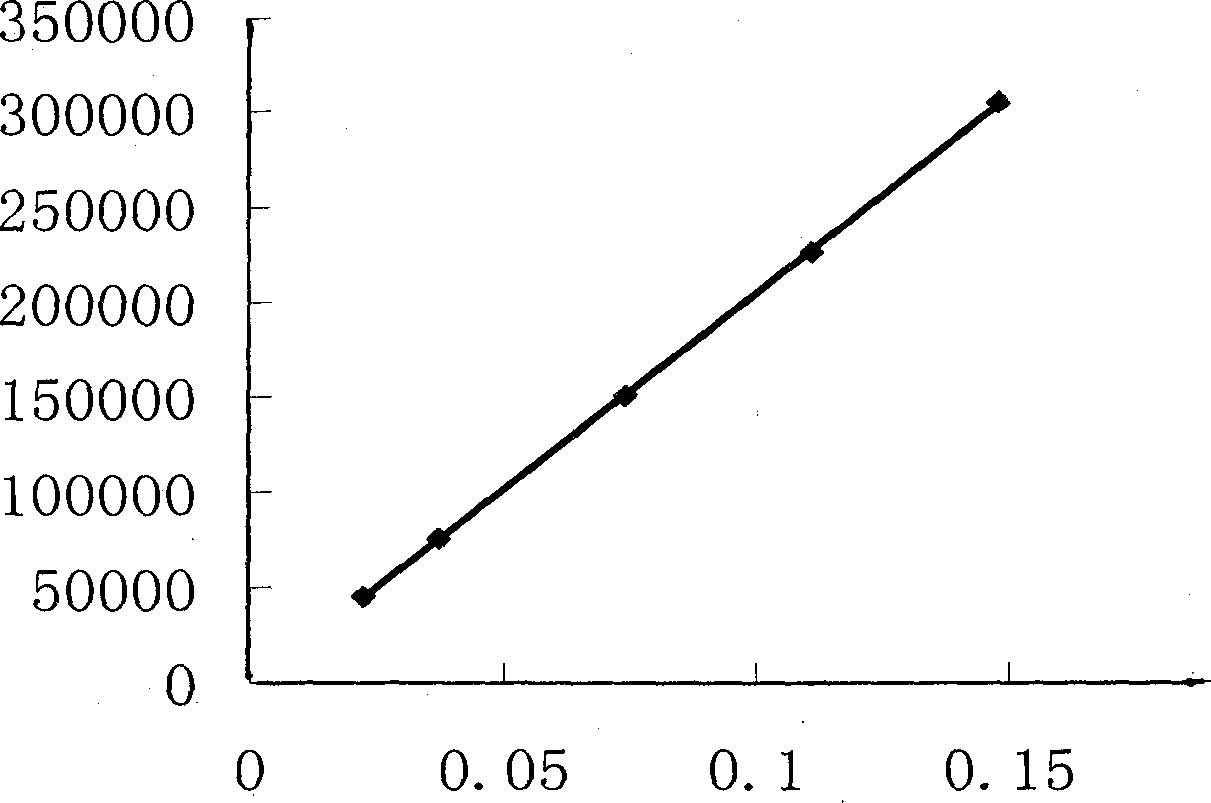
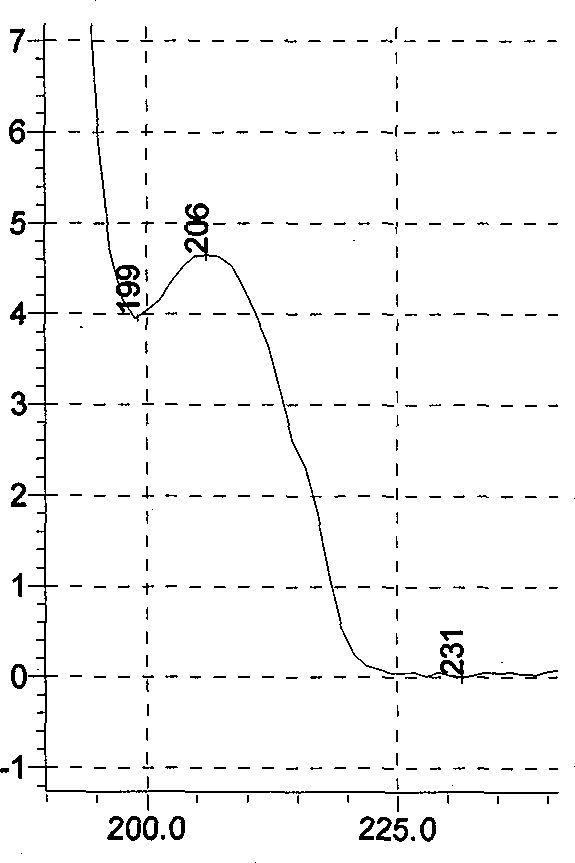
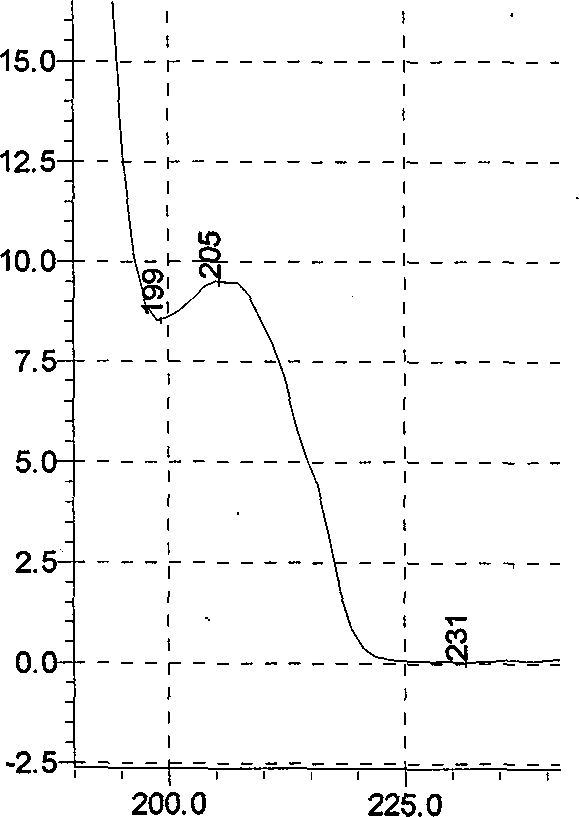
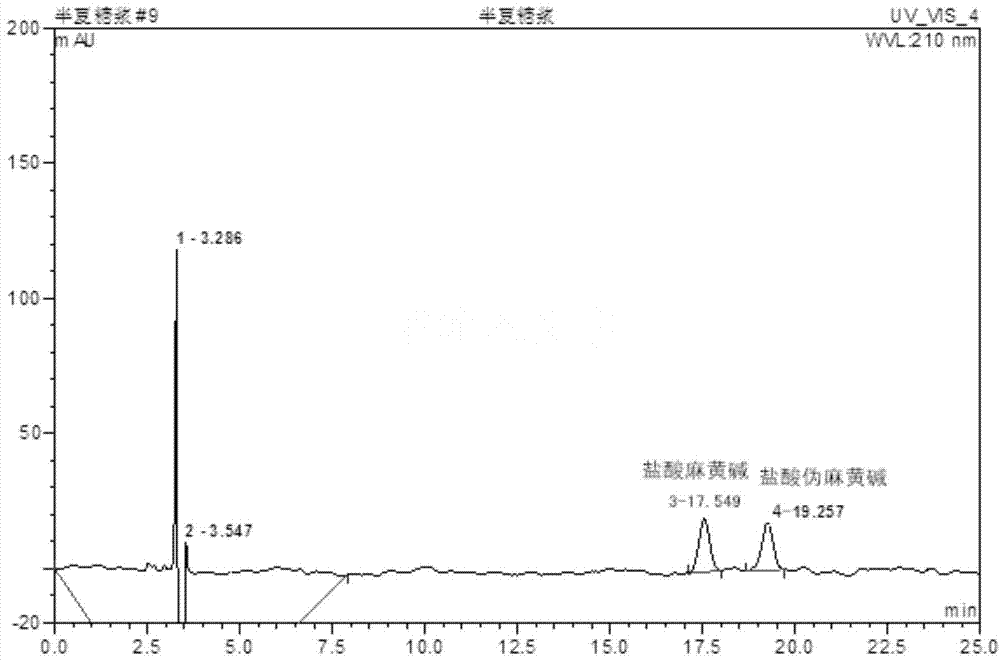
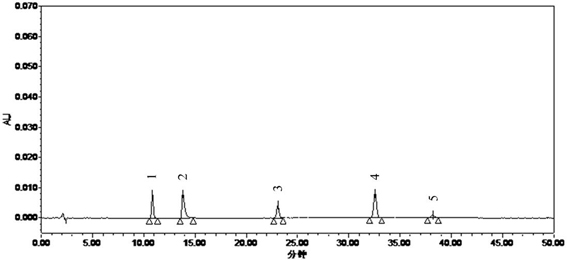
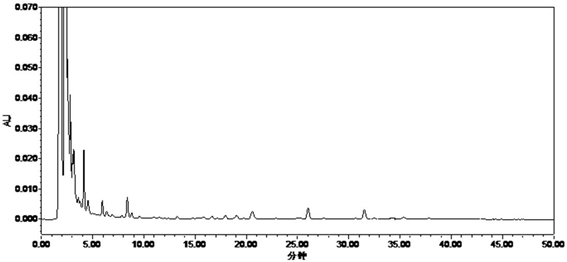
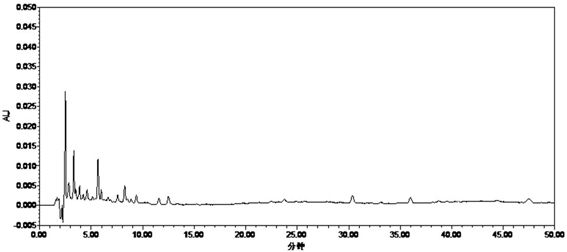
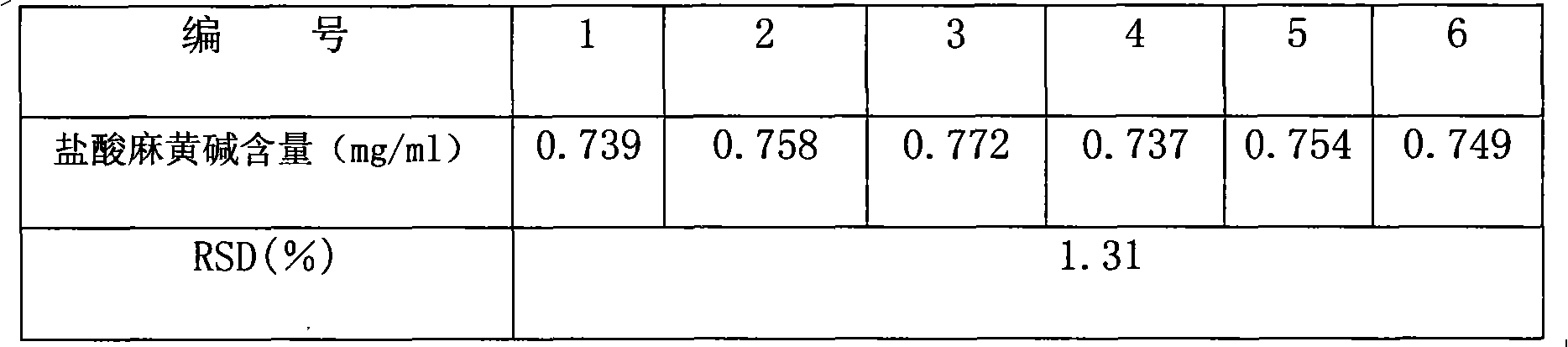
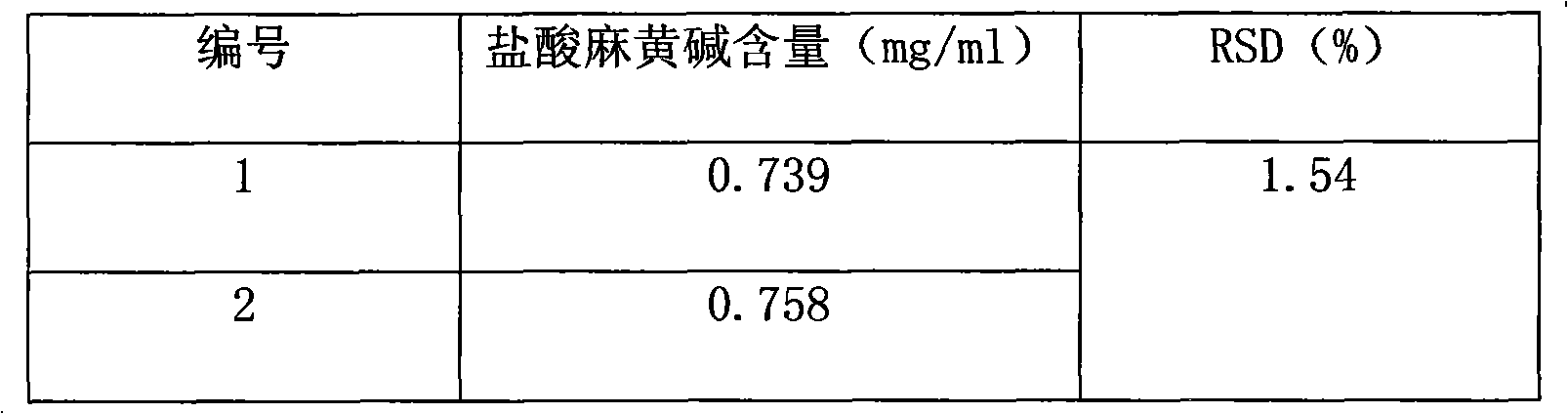
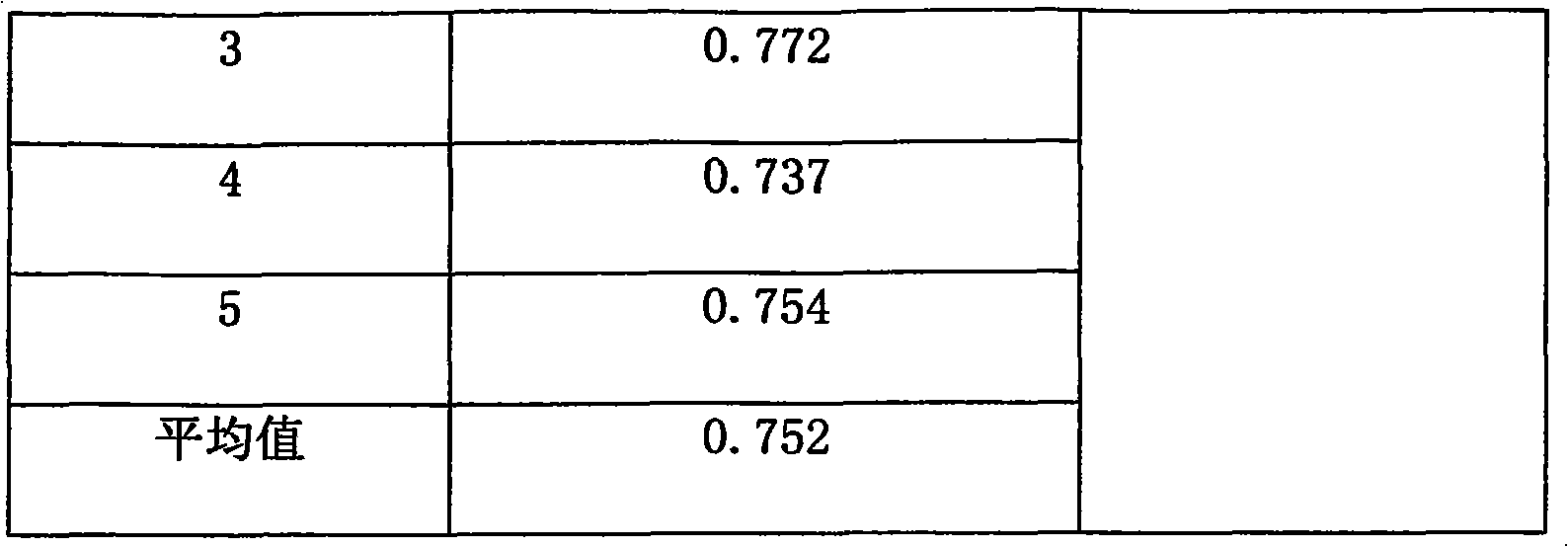
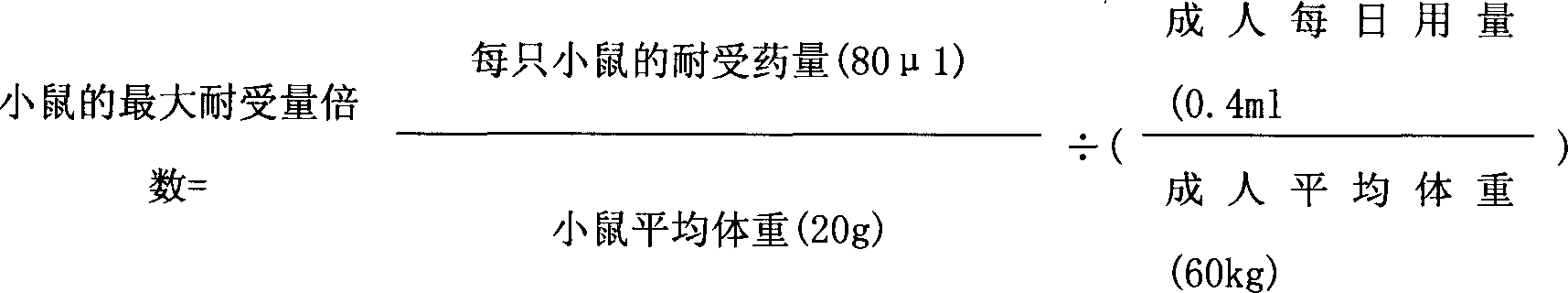
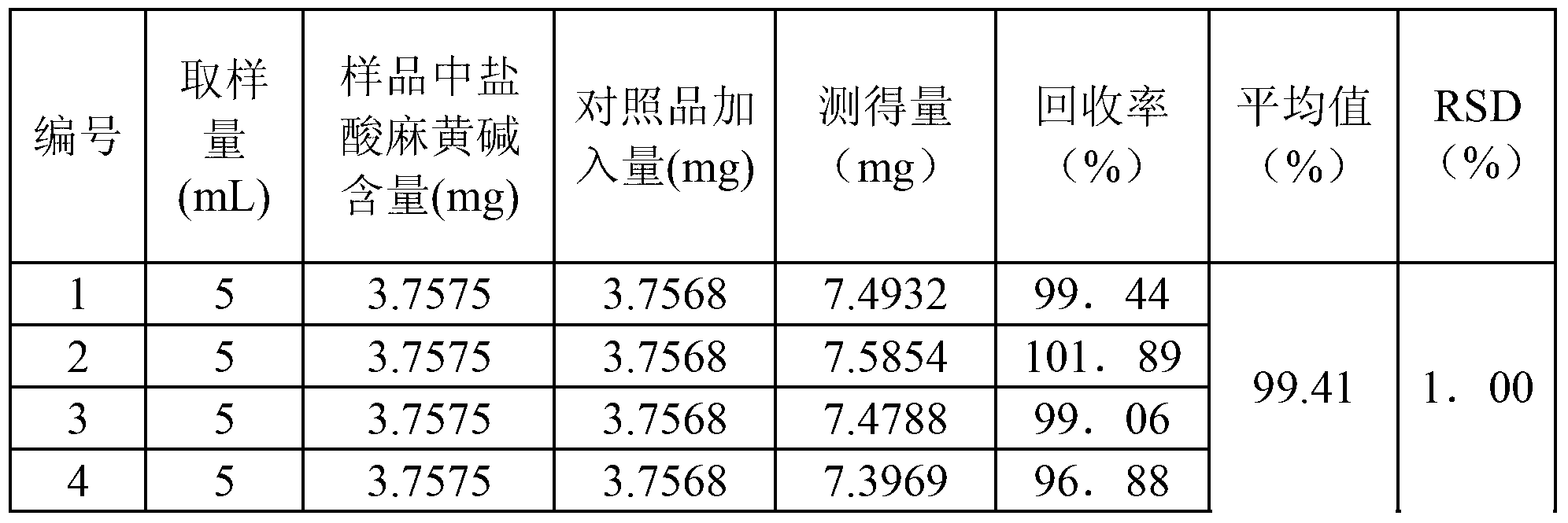
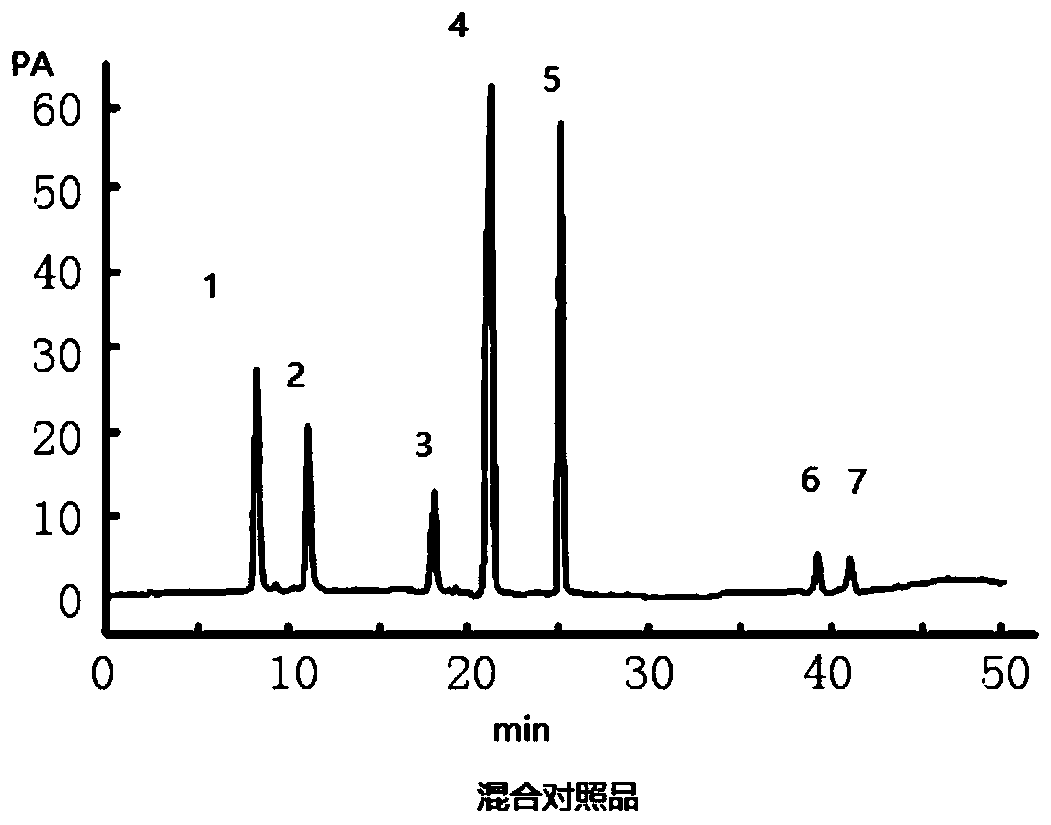
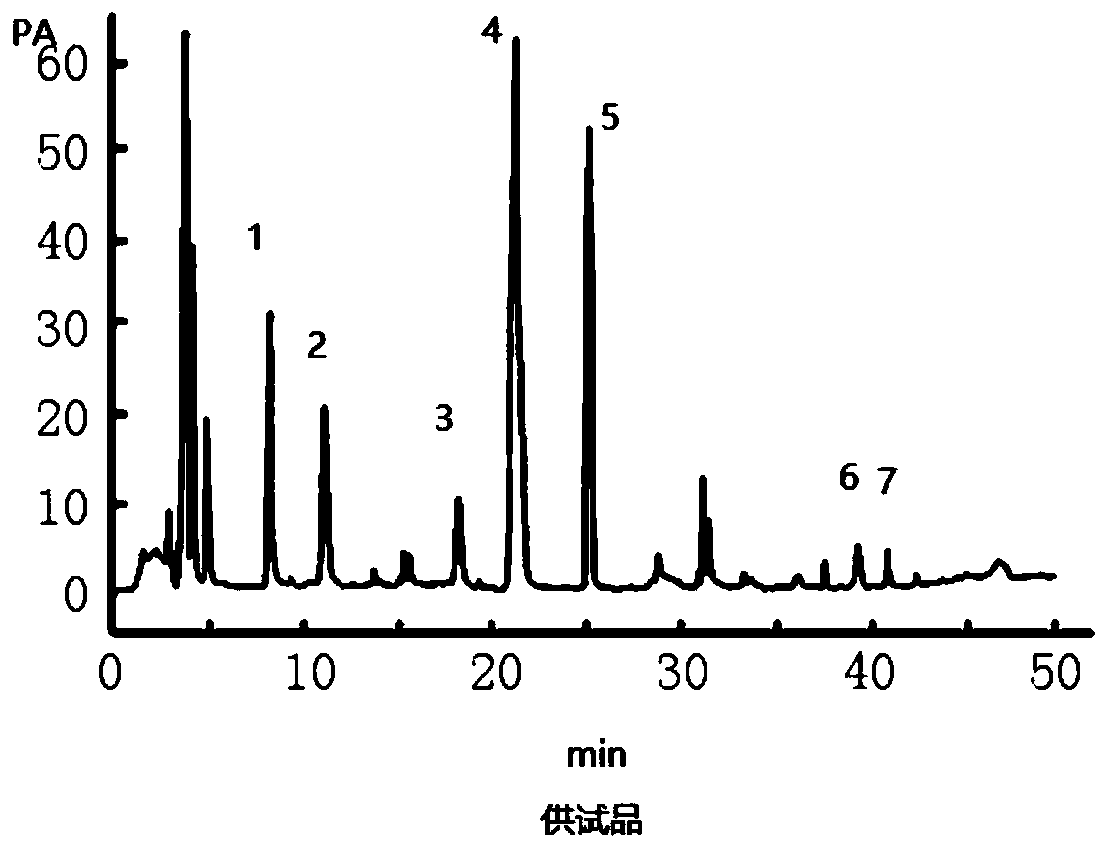
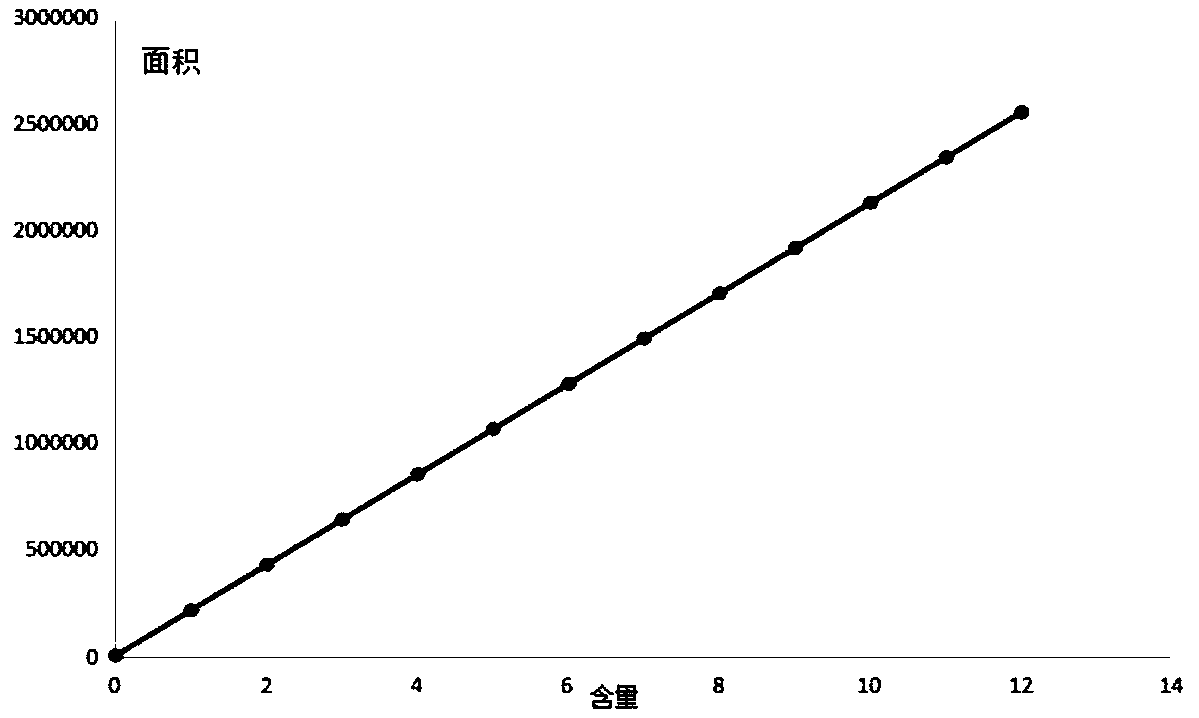
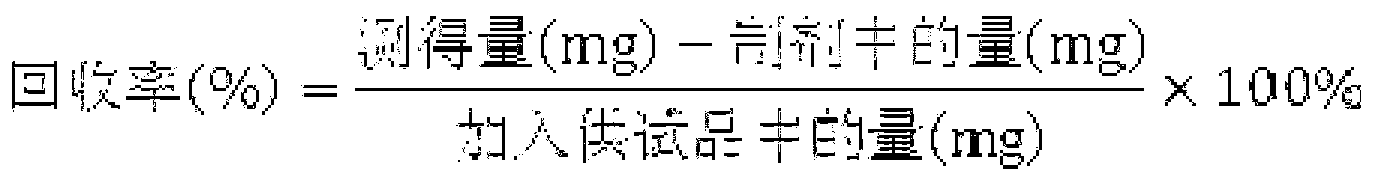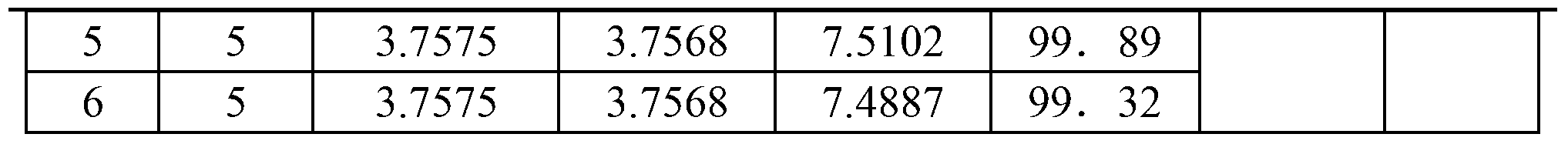Patents
Literature
145 results about "Ephedrine hydrochloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
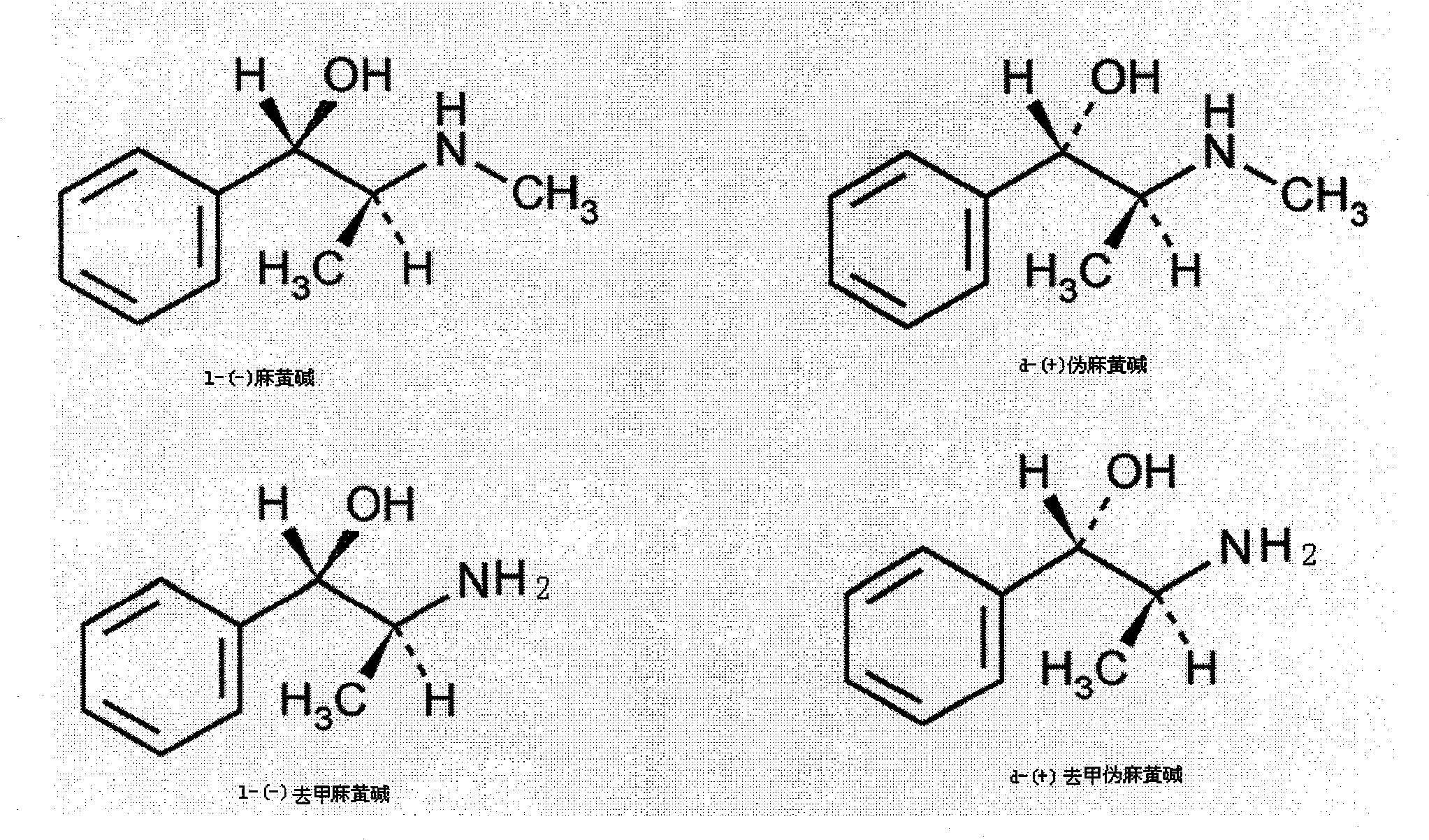
Ephedrine hydrochloride is a phenethylamine found in EPHEDRA SINICA. PSEUDOEPHEDRINE is an isomer. It is an alpha- and beta-adrenergic agonist that may also enhance release of norepinephrine.
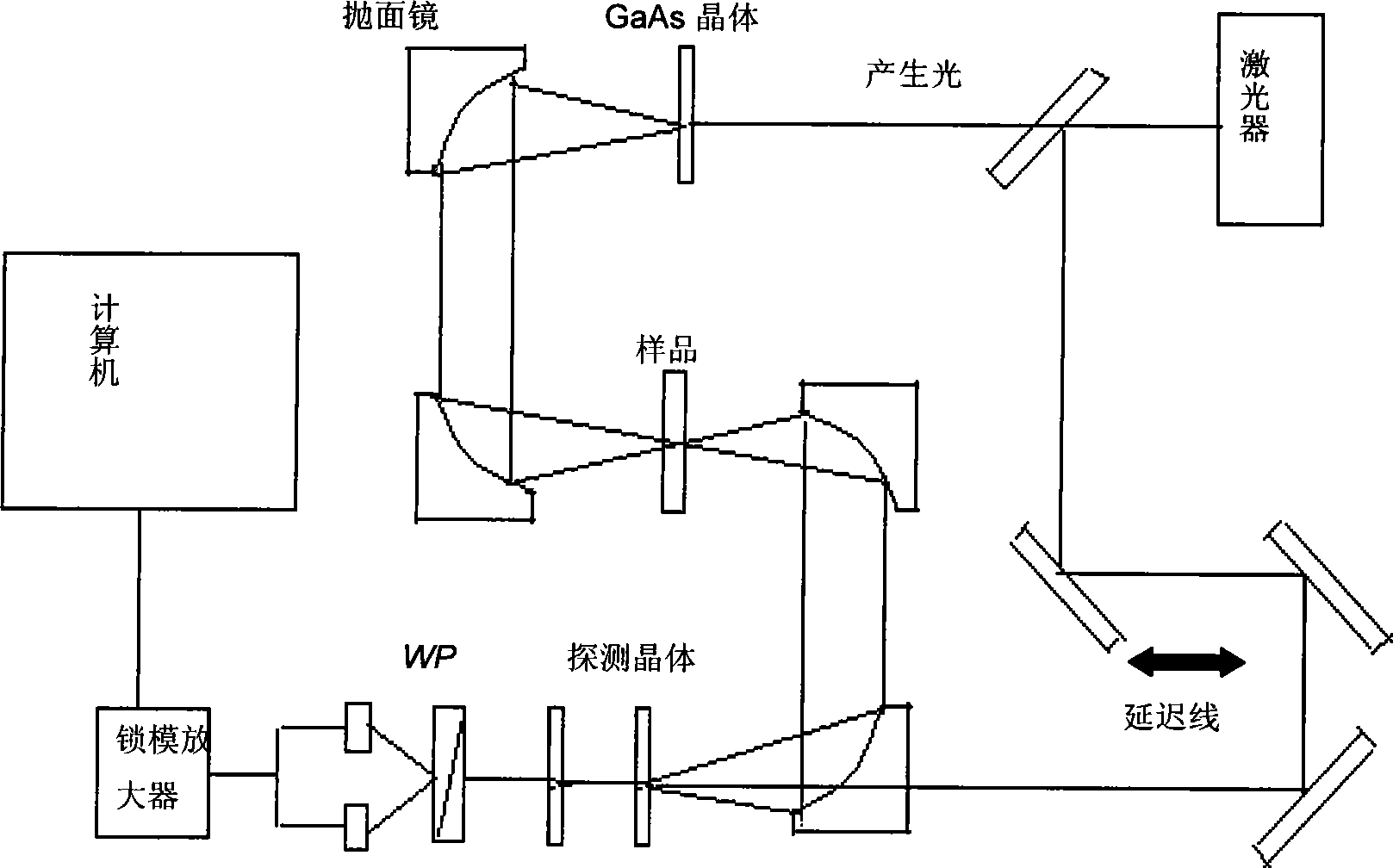
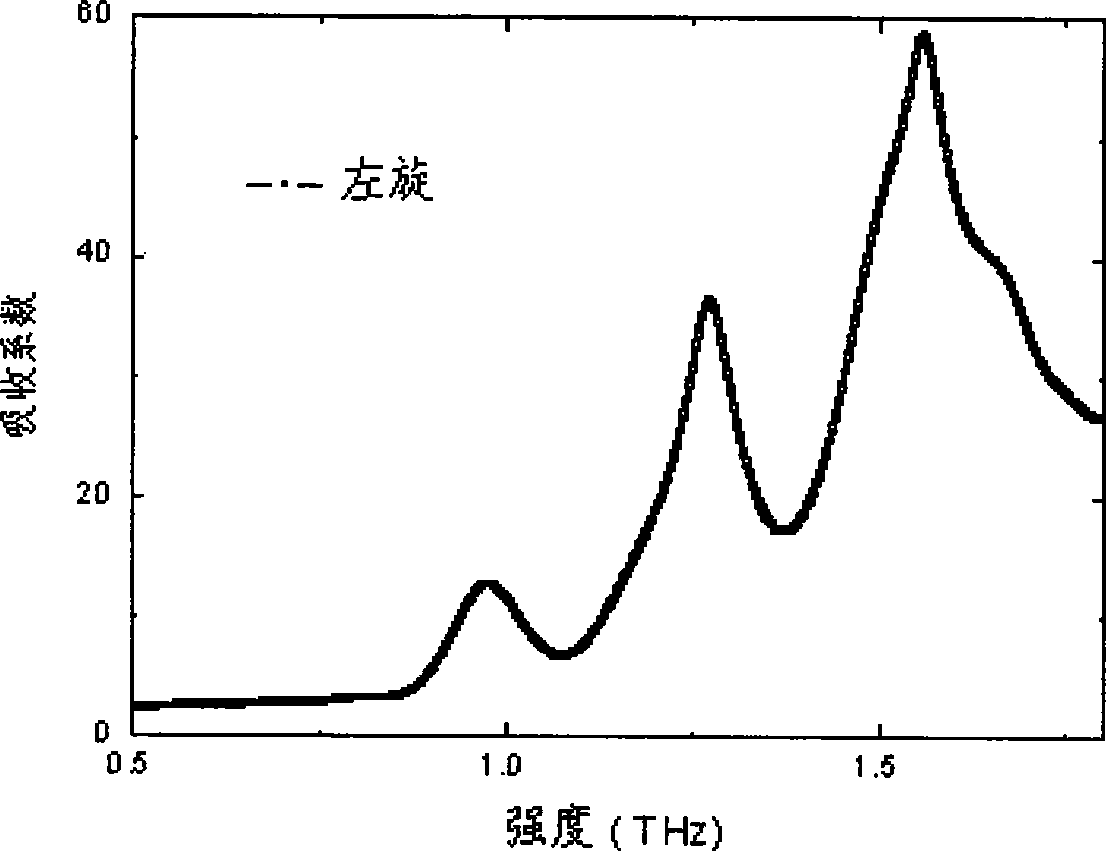
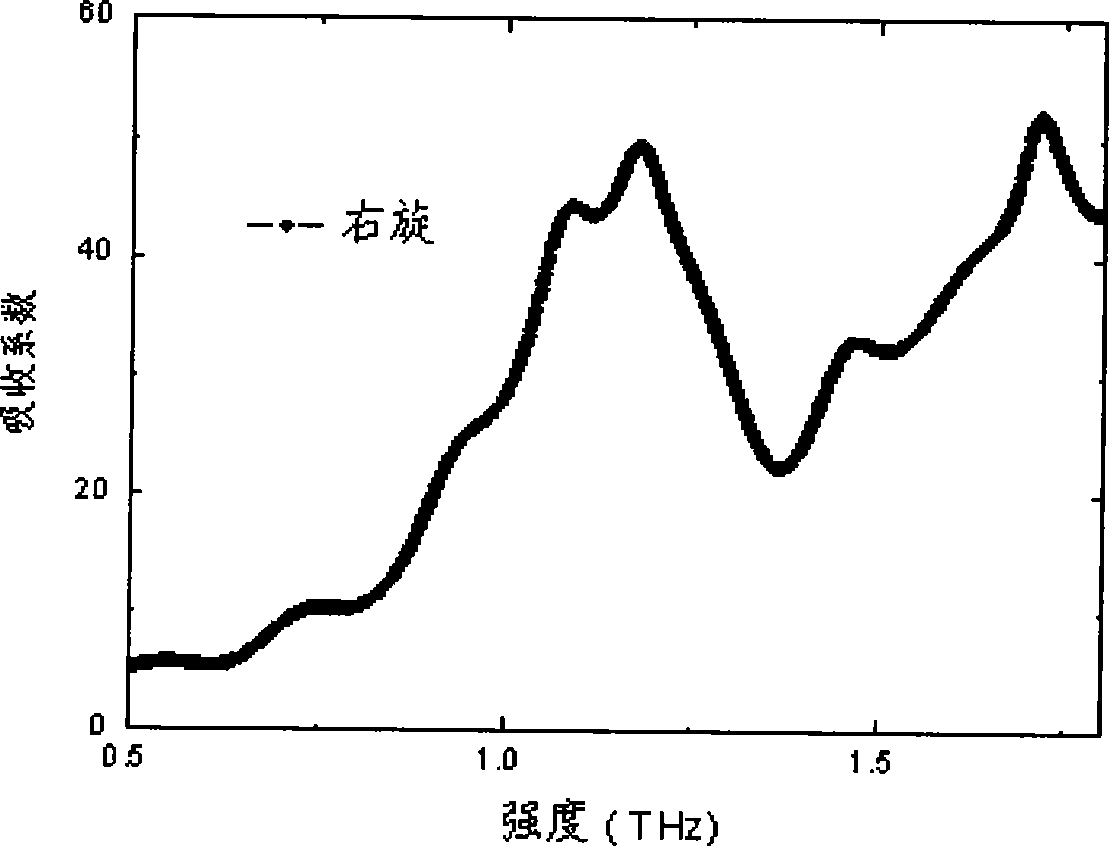
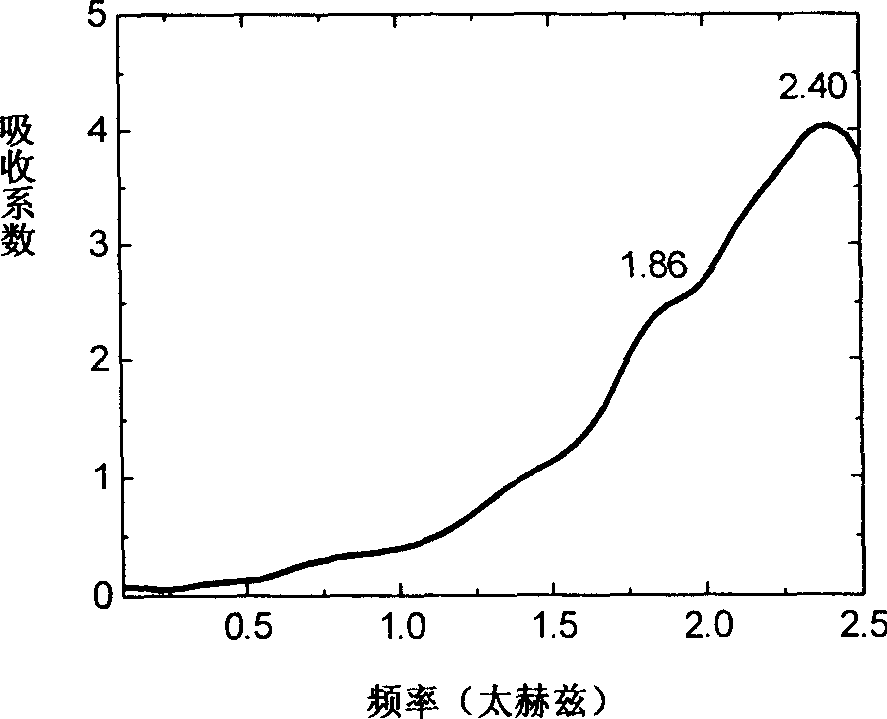
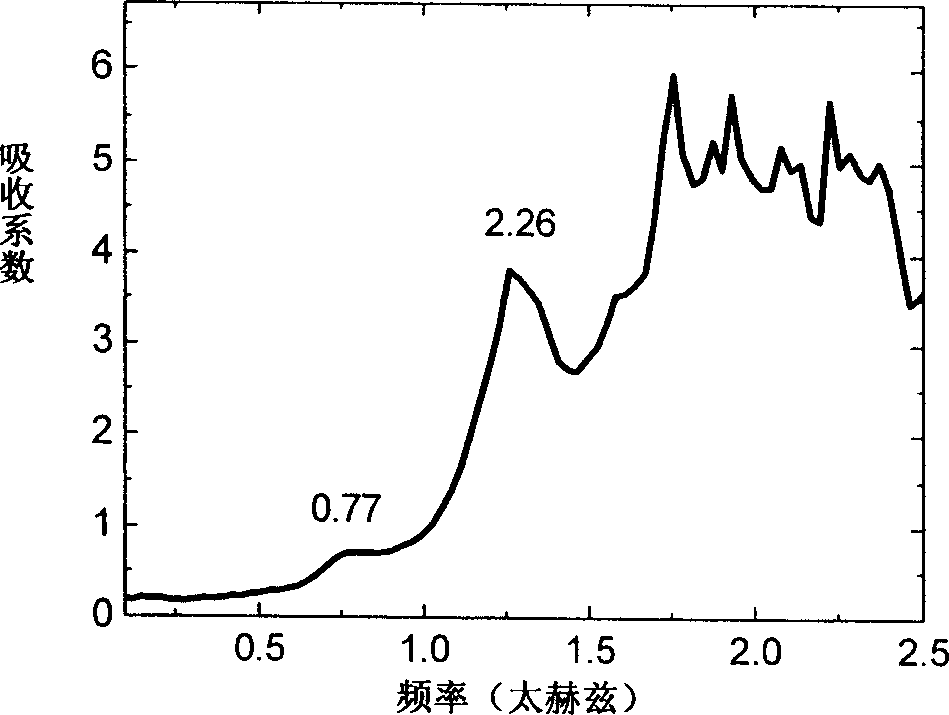
Efedrina for identifying different optical rotation performances by THz-TDS
InactiveCN101435771AWill not harmImprove signal-to-noise ratioPolarisation-affecting propertiesColor/spectral properties measurementsTime domainFrequency spectrum
The invention discloses a method of utilizing THz-TDS to identify ephedrine with different optical activity. The method mainly utilizes a terahertz time-domain spectrum device to detect the THz fingerprint frequency spectra of the ephedrine hydrochloride with different optical activity in a range between 0.5 and 1.8 THz, and the optical activity of an ephedrine hydrochloride sample can be easily identified through the THz fingerprint frequency spectra. The method has the characteristics of rapidity and lossless detection.
Owner:SHANGHAI INST OF APPLIED PHYSICS - CHINESE ACAD OF SCI
THz inspection and fingerprint spectrum for 12 drugs
InactiveCN1818635AAccurate identificationStrong penetrating powerComponent separationColor/spectral properties measurementsPhosphoric acidMorphine
A method for detecting twelve types of narcotic drugs by utilizing TH2 technique includes preparing narcotic drugs standard sample; applying TH2 detection technique to carry out detection on said standard samples and setting up standard fingerprint chart bank; utilizing standard set-up fingerprint chart bank to carry out detection on unknown matter including six types of morphine narcotic drugs, three types of ice narcotic drugs, two types of drugs controlled by the state and one type of new narcotic drugs.
Owner:CAPITAL NORMAL UNIVERSITY
Method for detecting ephedra-perilla leaf cough relieving capsule
InactiveCN104007222ASimple and fast operationEasy to controlComponent separationMedicinal herbsMedicine
The invention relates to a method for detecting an ephedra-perilla leaf cough relieving capsule, which is used for controlling the quality of the ephedra-perilla leaf cough relieving capsule. The method comprises the steps: performing qualitative identification on five Chinese medicinal herbs including ephedra, perilla leaf, lumbricus, burdock and fructus schizandrae in the ephedra-perilla leaf cough relieving capsule by adopting a thin layer chromatography; and determining the content of ephedrine hydrochloride in the ephedra-perilla leaf cough relieving capsule by adopting a high performance liquid chromatography. The method provided by the invention is simple and convenient to operate, scientific, reasonable, good in repeatability, and capable of well controlling the quality of the ephedra-perilla leaf cough relieving capsule, effectively monitoring the production, and increasing the safety of medication of a patient.
Owner:YANGTZE RIVER PHARMA GRP BEIJING HAIYAN PHARMA +1
Quality detection method of five-flavor manna medicine bath preparation
ActiveCN102707007ARaise quality standardsQuality is easy to controlComponent separationSilanesPseudoephedrine Hydrochloride
The invention discloses a quality detection method of a five-flavor manna medicine bath preparation. The five-flavor manna medicine bath preparation is made from raw materials of Juniperus formosana, ephedra, rhododendron anthopogonoide, myricaria and artemisia sieversiana according to a conventional method of pharmaceutics. On the basis of the primary standard, the thin layer chromatography of ephedra and rhododendron anthopogonoide is revised, the thin layer chromatography of Juniperus formosana and artemisia sieversiana is added. Under the simple and convenient condition of mobile phase, octyl silane bonding silica gel or phenyl bonding silica gel is used as filler, and simultaneously, the contents of ephedrine hydrochloride and pseudoephedrine hydrochloride in the ephedra are detected. The invention also provides a method for measuring the content of hyperoside in five-flavor manna preparation rhododendron anthopogonoide, thus ensuring the safety, effectiveness and controllability of product quality. By the quality detection method, the quality standard of the existing five-flavor manna medicine bath preparation is improved correspondingly.
Owner:JINHE TIBETAN MEDICINE
Detection method of Santanning syrup
InactiveCN101897760AEfficient productionProduction controlHydroxy compound active ingredientsMaterial analysis by observing effect on chemical indicatorMENTHOL CRYSTALSThin layer
The invention provides a detection method of Santanning syrup, which comprises the steps of the physicochemical distinguishing of ammonium chloride, thin layer distinguishing of the root bark of white mulberry, the thin layer distinguishing of the menthol crystal, the content determination of ephedrine hydrochloride and the content determination of ammonium chloride. The invention effectively controls the quality of the main components such as the root bark of white mulberry, the ammonium chloride, the ephedrine hydrochloride and the menthol crystal in the prescription, not only can better and more comprehensively reflect the quality of the Santanning syrup, but also can effectively prevent the illegal manufacturer from producing the adulterant Santanning syrup, thereby ensuring the pharmacy safety of the masses.
Owner:SICHUAN FENGCHUN PHARMA
Saccharomycete with stereoselectivity lipase liveness and application in producing S- type betaxolol hydrochloride with biological split method thereof
InactiveCN101220336ALow priceMild reaction conditions for production conversionFungiHydrolasesBacterial strainSalbutamol sulfate
The invention discloses a yeast which has the stereoselective lipase activity and the application of the yeast in the preparation of an S-type betaxolol hydrochloride by using a biological separation method. The method selects one yeast with the stereoselective lipase activity by screening from the soil, utilizes an immobilized cell which is obtained by immobilizing the wet bacteria or sodium alginate-activated carbon-polyethylenimine as an enzyme preparation, carries out an enantiomer separation to a substrate which contains acyl group and prepares the S-type betaxolol hydrochloride. The conversion method is simple, the cost is low and the stereoselectivity is better. The usage of the bacterial strain can carry out the chiral separation of Beta-receptor blocker of betaxolol etc., ephedrine hydrochloride, epinephrine, levodropropizine, salbutamol sulfate, captopril, zofenopril and other compounds which contain hydroxy group or acyl group at the chiral center, thereby having important application value for promoting the development process of the chiral drugs of China.
Owner:ZHENGZHOU UNIV
Detection method for traditional Chinese medicine preparation compounded Sichuan fritillary bulb extract tablets
The invention discloses a detection method for traditional Chinese medicine preparation compounded Sichuan fritillary bulb extract tablets. The detection method comprises the steps of carrying out thin-layer chromatography qualitative identification and carrying out content determination, wherein thin-layer chromatography qualitative identification comprises the steps of identifying ephedrannin and neoephedrine A in the preparation, identifying polygala root and cynanchum atratum and identifying glycyrrhiza uralensis and schisandra fruit; content determination comprises the step of determining the content of ephedrine hydrochloride in the preparation. The detection method disclosed by the invention has the advantages that the mixing of radix ephedrae and cynanchum atratum is effectively controlled, and meanwhile, the composition and content of the compounded Sichuan fritillary bulb extract tablets is effectively controlled, so that the drug can be safer and more effective.
Owner:九寨沟天然药业股份有限公司
Method for simultaneously determining three alkaloids in granules for eliminating phlegm and stopping cough for children
ActiveCN102662024AQuality improvementGood peak separationComponent separationEmetine HydrochloridePhosphoric acid
The invention relates to a method for simultaneously determining three alkaloids in granules for eliminating the phlegm and stopping the cough for children. The method is characterized in that the HPLC (High Performance Liquid Chromatography) method is employed for the first time, an ordinary gradient elution and reversed-phase chromatographic column are adopted, and acetonitrile, methanol and 0.1% phosphoric acid in the volume ratio of (1.5-2.5) : (12.5-11) : (86-86.5) are taken as the mobile phase; the detection wavelength is 205 nm; and the contents of ephedrine hydrochloride, cephaeline hydrochloride and emetine hydrochloride in the granules for eliminating the phlegm and stopping the cough for children are determined simultaneously, so as to end the history of no HPLC determining method for emetine and no quantitative determining indexes for granules for eliminating the phlegm and stopping the cough for children. The method provided by the invention comprises the steps of performing an ultrasonic treatment to a sample with methanol, sucking a certain amount of subsequent filtrate, removing impurities with an alumina column, and determining. The method has the benefits that crest separation of the three alkaloids is excellent, the baseline is stable, and 30 min is required to finish the determining; the quality control aim of being simple, convenient, quick, scientific, standard and multi-component quantitive by one maker is realized; and safety and effectiveness of taking granules for eliminating the phlegm and stopping the cough for children are ensured.
Owner:JING JING PHARMA
Fingerprint spectrum construction method and detection method of Guizhishaoyaozhimu decoction composition
ActiveCN113049724AImprove responseMultiple peaksComponent separationGallic acid esterHplc mass spectrometry
The invention discloses a fingerprint spectrum construction method and a detection method of a Guizhishaoyaozhimu decoction composition, and the fingerprint spectrum construction method comprises the following steps: (1) precisely weighing the Guizhishaoyaozhimu decoction composition, adding water, and carrying out ultrasonic treatment to obtain a test solution; (2) preparing 22 reference substances such as gallic acid, catechin, albiflorin, ephedrine hydrochloride and pseudoephedrine hydrochloride into a reference substance solution; and (3) precisely sucking the test solution and the reference solutions, injecting the test solution and the reference solutions into an ultra-high performance liquid chromatograph for chromatographic analysis at the detection wavelength of 210 nm to obtain a test fingerprint spectrum and a reference chromatographic spectrum, and formulating a standard fingerprint spectrum of the Guizhishaoyaozhimu decoction composition. The quality of a product to be detected can be comprehensively reflected through the standard fingerprint spectrum, and the product quality of the Guizhishaoyaozhimu decoction preparation can be effectively controlled.
Owner:SINOPHARM GUANGDONG GLOBAL PHARMA CO LTD +1
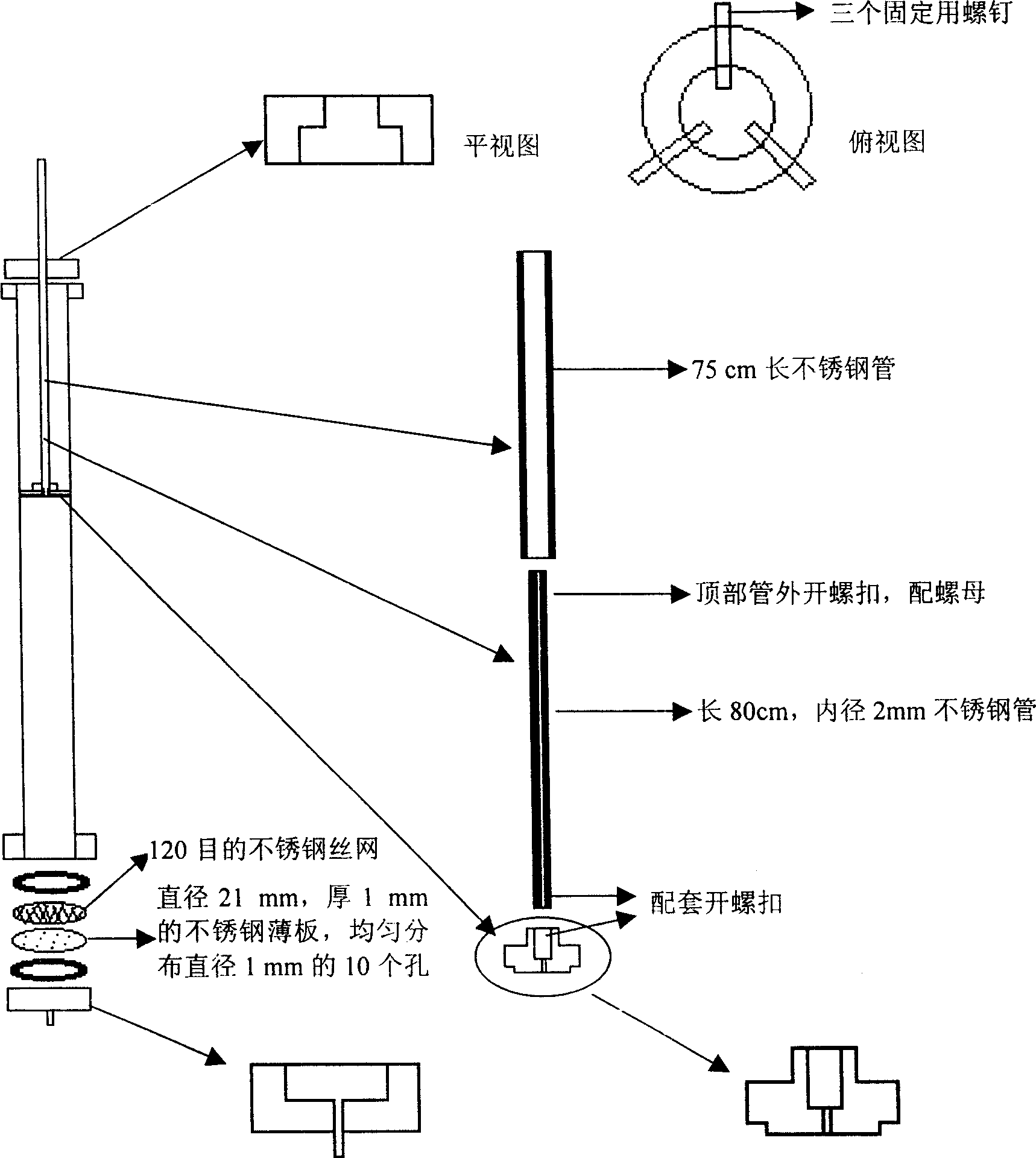
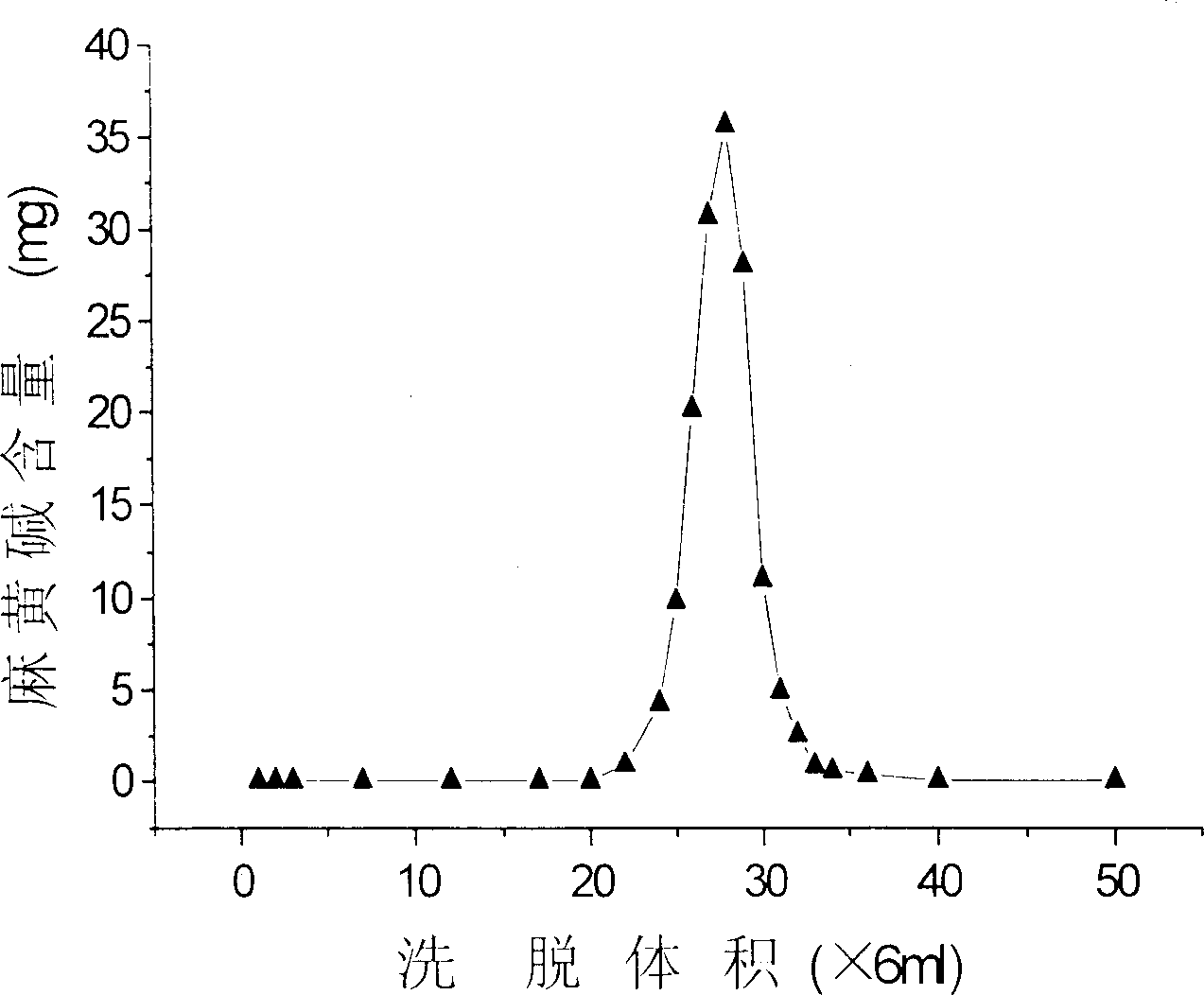
Method for one-step purification and separation of ephedrine by cation exchange resin and expanded bed integrated technology
InactiveCN1704398AImprove adsorption capacityEfficient removalOrganic compound preparationAmino-hyroxy compound preparationPurification methodsOrganic solvent
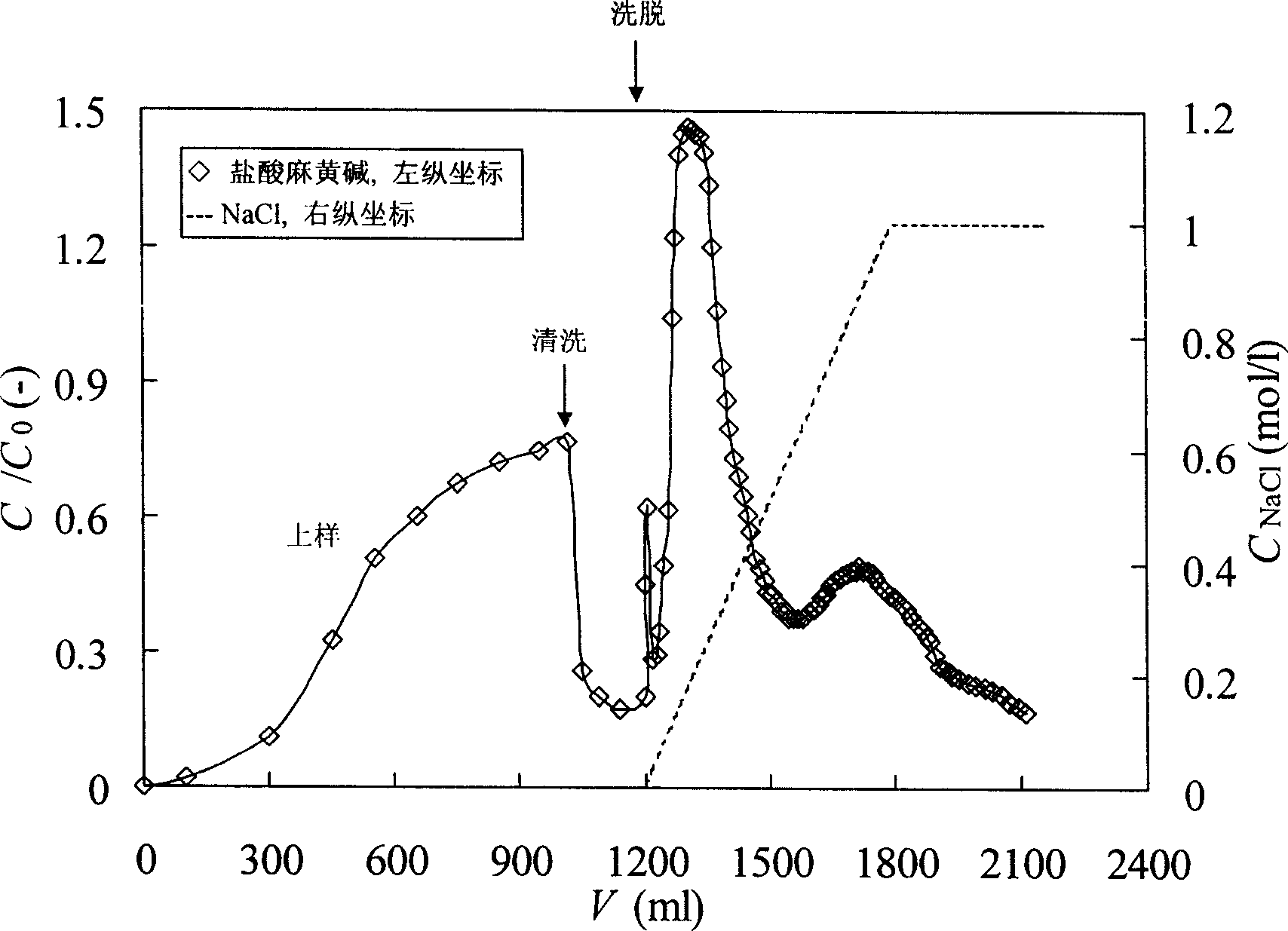
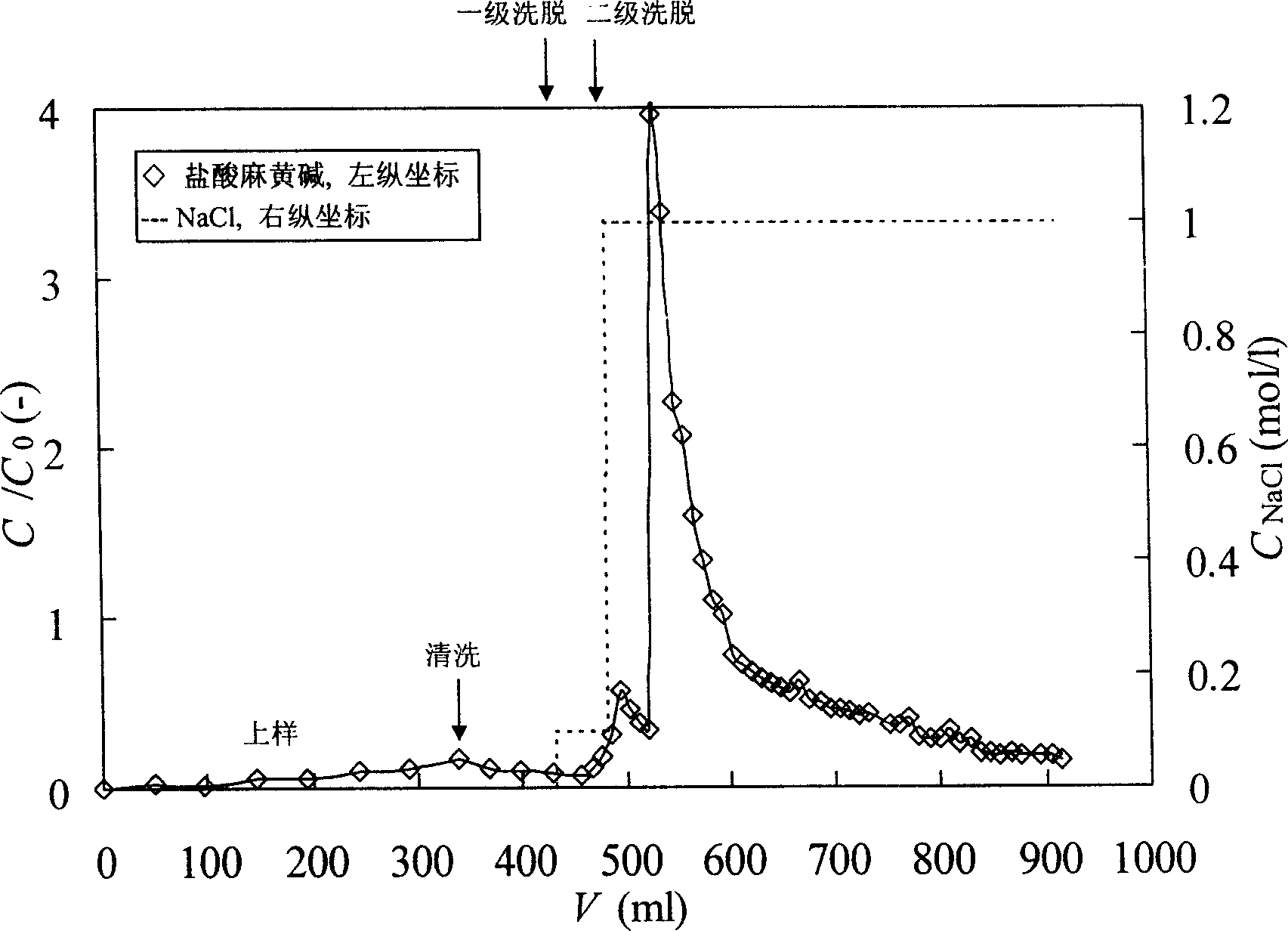
The invention provides a process for one-step purification and separation of ephedrine which comprises the following steps, using 2-3 times of cushioning liquid A to dilute raw feed liquid containing ephedrine hydrochloride pretreated by 0.05M diluted hydrochloric acid and obtained through the conventional Chinese ephedra plant extraction, synthesis, biofermentation method or cell culture method, adsorbing hydrochloric ephedra sinica stapf in one step by employing a liquid distributor filled by phenylethene group cationic resin 001X7 Styrene DVB, carrying out two phase step elution by employing 0.01M phosphates cushioning liquid containing 0.1M NaCl and 1.0M NaCl, finally collecting the eluent of the second elution peak.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Chinese ephedra medicinal material, and content determination method of three alkaloids in preparation thereof
ActiveCN103512998AExtend your lifeSimplify flushing proceduresComponent separationMethylephedrine hydrochloridePseudoephedrine Hydrochloride
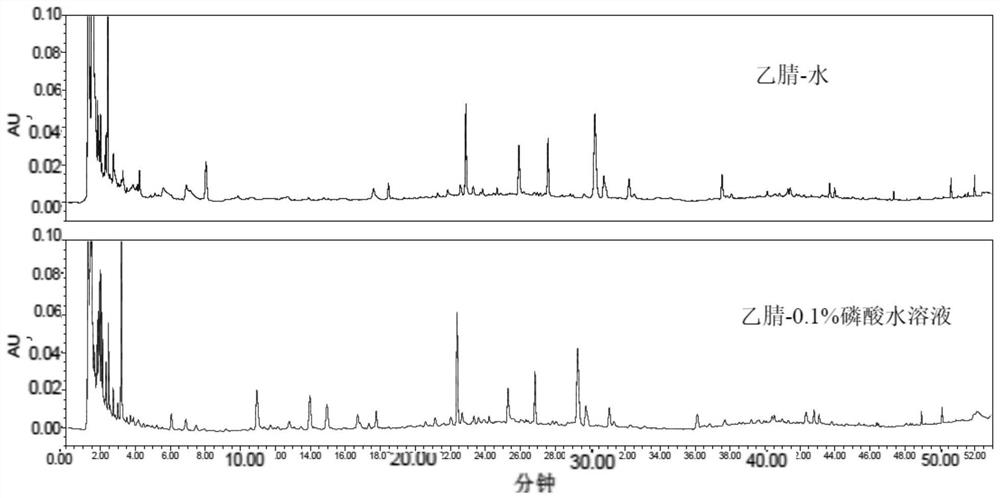
The invention relates to a Chinese ephedra medicinal material, and a content determination method of three alkaloids in a preparation thereof. The method has the characteristics that: a common reversed-phase column and a non-buffer salt which is a mobile phase of acetonitrile-methanol-0.1% phosphoric acid with a volume ratio of 1-1.5:4-4.2:94.5-95.0 are adopted; at 207+ / -2nm, contents of ephedrine hydrochloride, pseudoephedrine hydrochloride, and ephedrine methyl hydrochloride in the Chinese ephedra medicinal material and the preparation thereof are simultaneously determined. A sample is only processed through water-containing methanol ultrasonic extraction and alumina impurity removing, such that a near-colorless transparent solution is obtained. The method is simple, fast, reproducible, and accurate. According to the quantitative chromatogram of the three alkaloids, a baseline is stable, peak separation is good, and the peak appearing is completed within 20min. Compared with a traditional method with a buffer-salt solution, a specific chromatographic column, and unconventional organic phase ratio and flow rate, with the method provided by the invention, instrument and chromatographic column service lives are prolonged, cost is reduced, and detection conditions are communized. The method is suitable for popularization and application of basic units.
Owner:丰宁满族自治县七环旅游开发有限公司
Method for determining content of active ingredients such as ephedrine hydrochloride and pseudoephedrine hydrochloride in pinellia ternata syrup
InactiveCN104764820AImprove stabilityImprove accuracyComponent separationPseudoephedrine HydrochlorideBULK ACTIVE INGREDIENT
The invention discloses a method for determining the content of active ingredients such as ephedrine hydrochloride and pseudoephedrine hydrochloride in pinellia ternata syrup. The method comprises the following steps of: preparation of reference solution, preparation of to-be-tested sample solution and high-performance liquid chromatographic determination; and if every 1mL of product contains more than or equal to 30 micrograms of ephedrine hydrochloride and pseudoephedrine hydrochloride in total, the product is qualified. The method adopts the high-performance liquid chromatography to determine the content of the ephedrine hydrochloride and the pseudoephedrine hydrochloride which are used for investigating the quality of the pinellia ternata syrup product; and compared with the existing standard in absence of a content determining item, the method can control the internal quality of medicines directly by detecting the content of ephedrine hydrochloride and pseudoephedrine hydrochloride; in the method, the separating effect for ephedrine hydrochloride and pseudoephedrine hydrochloride is good, the stability and the accuracy are high, and the repeatability is good, so that the quality of the pinellia ternata syrup product can be well controlled.
Owner:HUBEI DUANZHENG PHARMA CO LTD
Detection method of Wuweiganlu preparation
ActiveCN102645493AQuality improvementStable quality detection methodComponent separationPreparing sample for investigationPseudoephedrine HydrochlorideJuniperus formosana
The invention provides a detection method of a Wuweiganlu preparation. The method comprises the following steps of: performing microscopic identification of the microscopic characteristics of juniperus formosana and myricaria in the Wuweiganlu preparation; performing thin-layer chromatography identification of rhododendron anthopogonoide, juniperus formosana and artemisia sieversiana in the Wuweiganlu preparation; and measuring the content of ephedrine hydrochloride and pseudoephedrine hydrochloride in ephedra and the content of artemisetin in artemisia sieversiana in the preparation by a high performance liquid chromatography. The detection method provided by the invention has the advantages of good reproducibility and stability, high precision, strong specificity, clear spot color, high separation degree, accurate content and the like, and is simple to operate; and by creating a reliable quality detection method with strong specificity, the quality of the Wuweiganlu preparation can be effectively controlled so that the quality of the Wuweiganlu preparation is stable, safe and controllable.
Owner:TIBET QIZHENG TIBETAN MEDICINE
Quality control method of dampness-resolving toxin-vanquishing composition
ActiveCN111983106AGuaranteed stabilityQuality improvementComponent separationRadix Astragali seu HedysariPinellia
The invention discloses a quality control method of a dampness-resolving toxin-vanquishing composition. The dampness-resolving toxin-vanquishing composition is mainly prepared from the following components: herba ephedrae, fried semen armeniacae amarae, gypsum, liquorice root, herba pogostemonis, cortex magnoliae officinalis, rhizoma atractylodis fried with bran, fried semen tsaoko, rhizoma pinellinae praeparata, poria cocos, radix et rhizoma rhei, radix astragali, semen lepidii and radix paeoniae rubra. The quality control method of the dampness-resolving toxin-vanquishing composition comprises the following steps: (1) determining the content of total anthraquinone, the content of free anthraquinone, the content of ephedrine hydrochloride, the content of pseudoephedrine hydrochloride andthe content of paeoniflorin in the dampness-resolving toxin-vanquishing composition by adopting high performance liquid chromatography, and calculating the content of combined anthraquinone, wherein the combined anthraquinone content is equal to the sum of the total anthraquinone content and the free anthraquinone content; and (2) identifying ephedra, liquorice and mangnolia officinalis by adopting thin-layer chromatography. By implementing the method, each link in the production process of the dampness-resolving toxin-vanquishing composition can be well controlled, and the quality stability and controllability of the product are effectively ensured.
Owner:GUANGDONG YIFANG PHARMA
Detection method for lung-soothing syrup
ActiveCN101961472AImprove quality control standardsProduction controlOrganic active ingredientsComponent separationClinical efficacyQuality control
The invention provides a quality detection method for lung-soothing syrup. The method comprises part or all of properties, inspection, identification and content measurement items, wherein the properties need to satisfy relevant regulations under Section Syrup; in the inspection, the syrup needs to conform to the relevant regulations in Section Syrup, Appendix IH, Part 1, Chinese Pharmacopedia (2005 Edition); in the component identification, ammonium chloride and licorice are identified; and in the content measurement, the content of ephedrine hydrochloride and the content of ammonium chloride are measured. The quality detection method has the advantages of high precision, high stability, favorable reproducibility, high recovery rate and accurate measurement result, enhances the quality control standard of lung-soothing syrup, and can effectively control illegal manufacturers from producing fake and poor-quality lung-soothing syrup, thereby ensuring the clinical curative effect of the preparation.
Owner:九寨沟天然药业股份有限公司
Compound preparation for treating bronchitis, its preparation method and quality control method
The invention discloses a process for preparing compound granule and the quality control method, wherein the compound granule is prepared from flitilarry bulb fluid extract, liquid extract of liquorice, platycodon glaucus, roor of sessile stemona, peucedanum root, pinellia tuber, dried orange peel, ammonium chloride, ephedrine hydrochloride and menthanol. The quality control method is characterized by the thin layer chromatogram authentication to dried orange peel, peucedanum root and licorice root, as well as the content measuring method for ephedrine hydrochloride.
Owner:张友生
Chinese ephedra prescription particle, preparation method and quality control method thereof
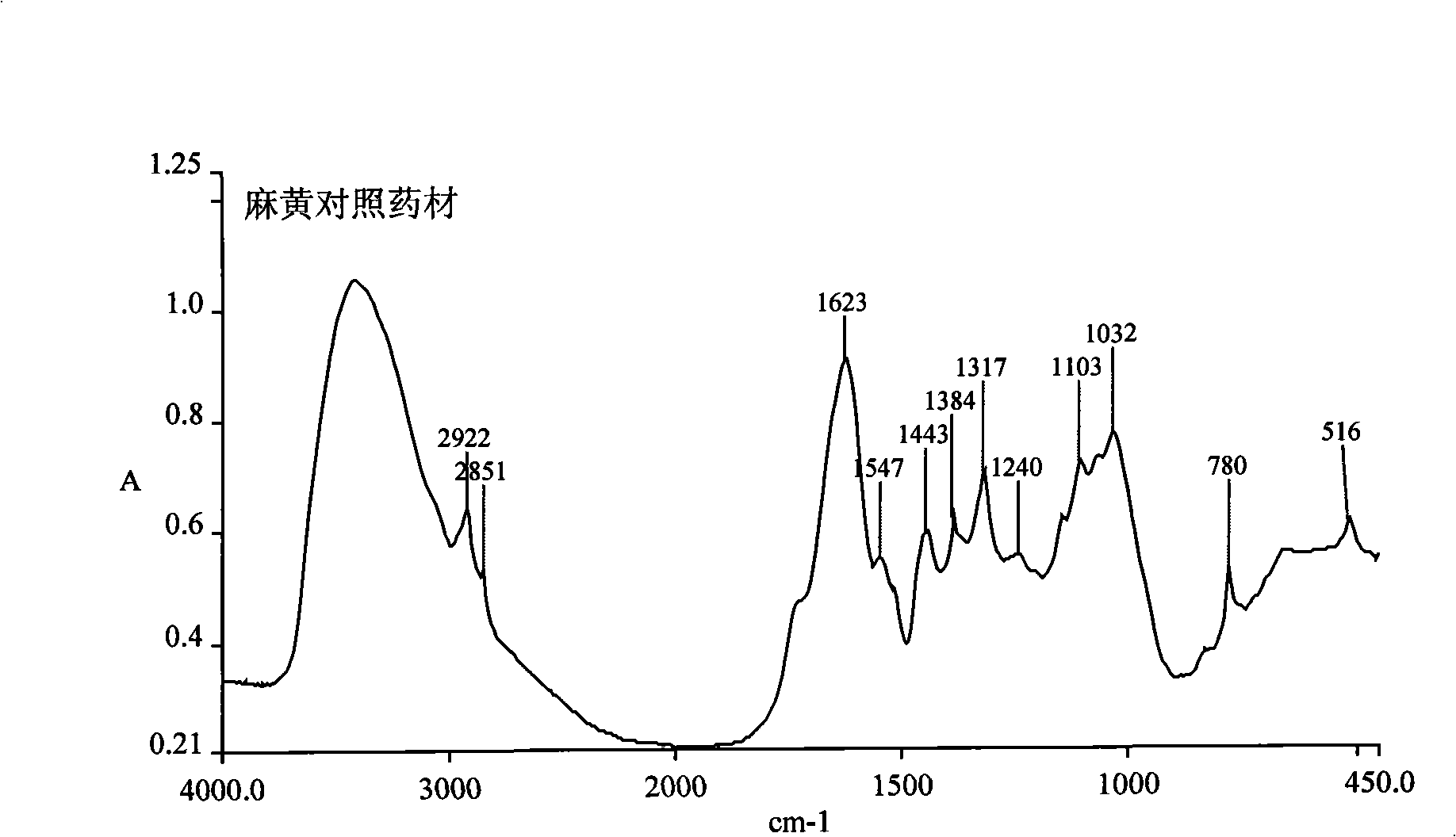
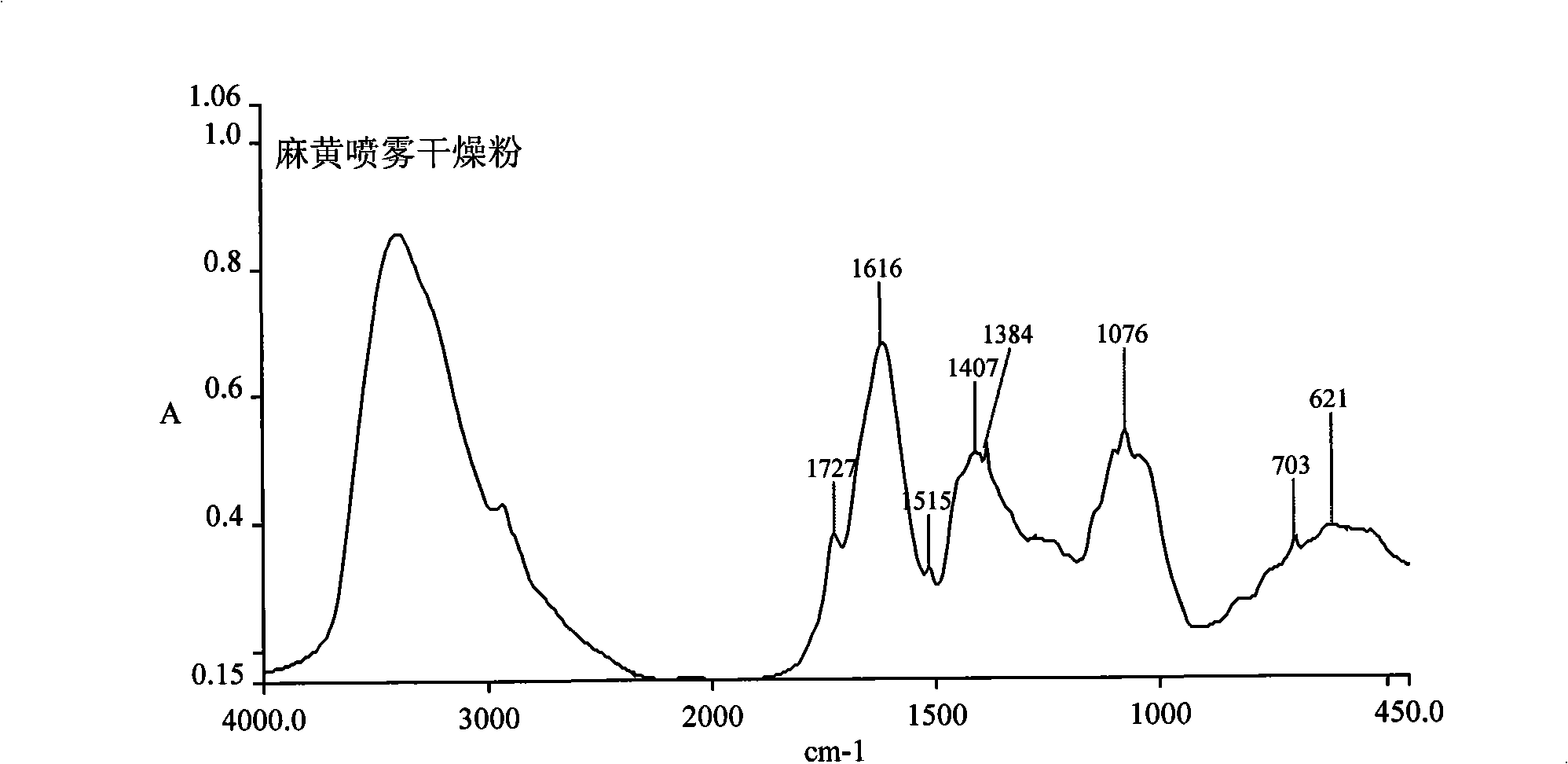
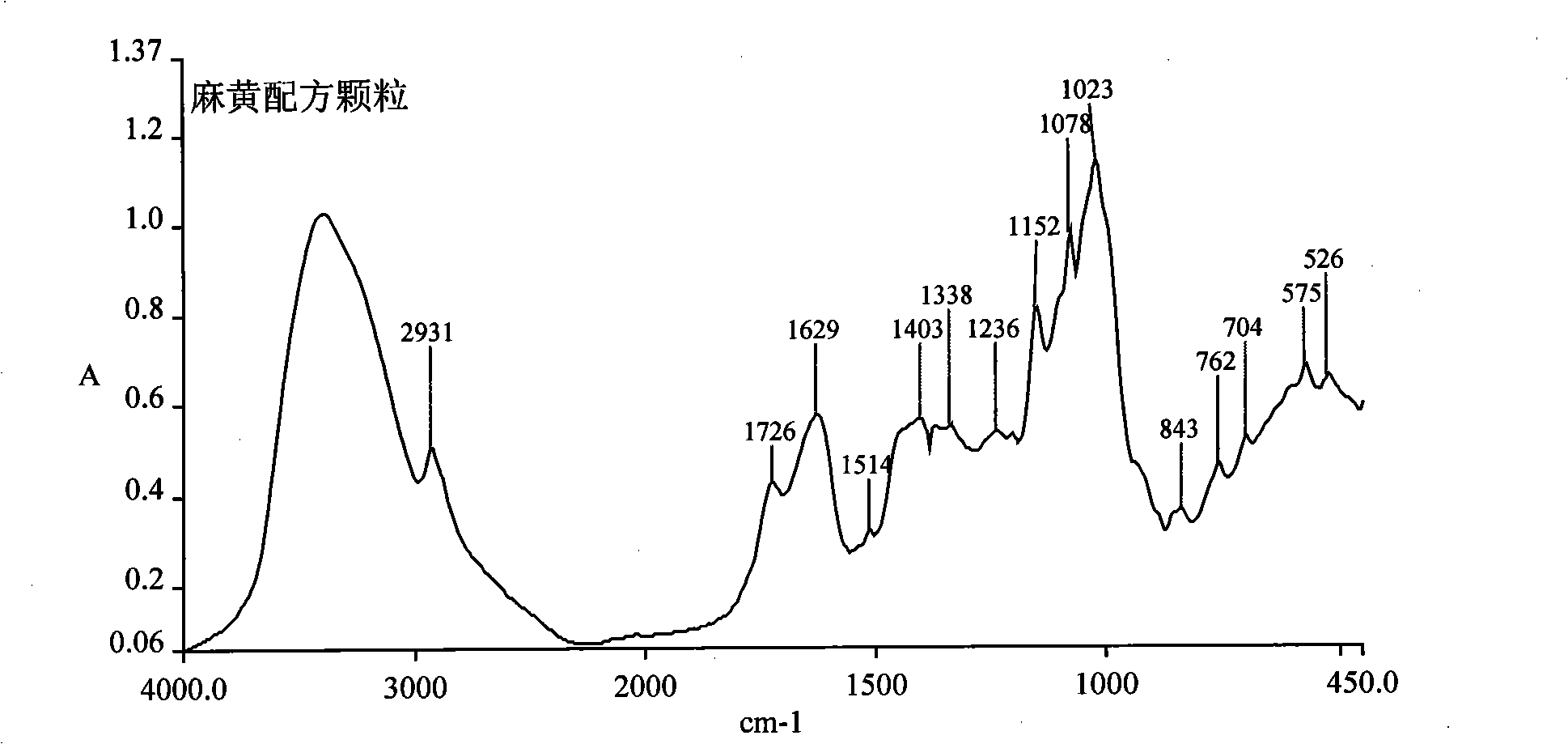
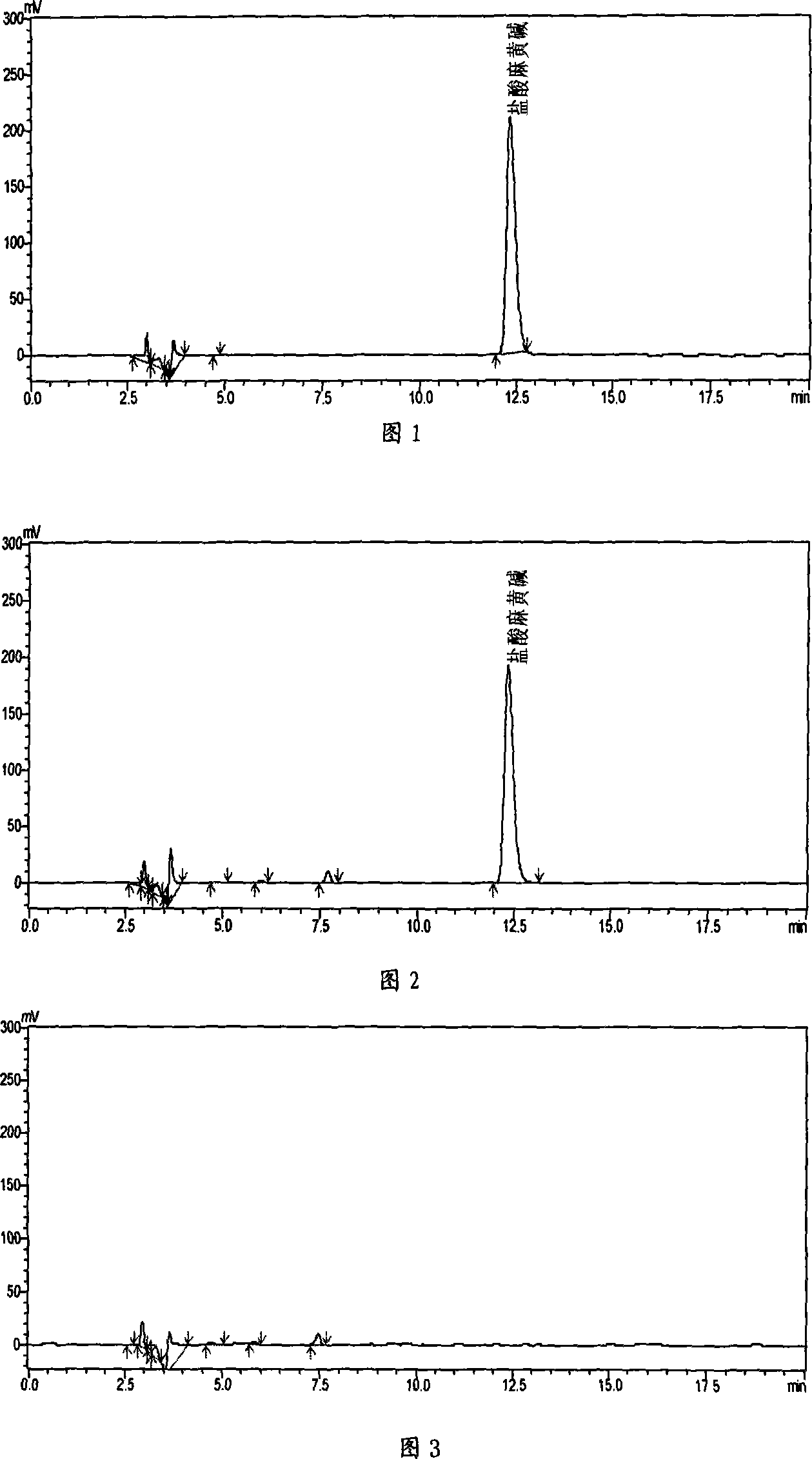
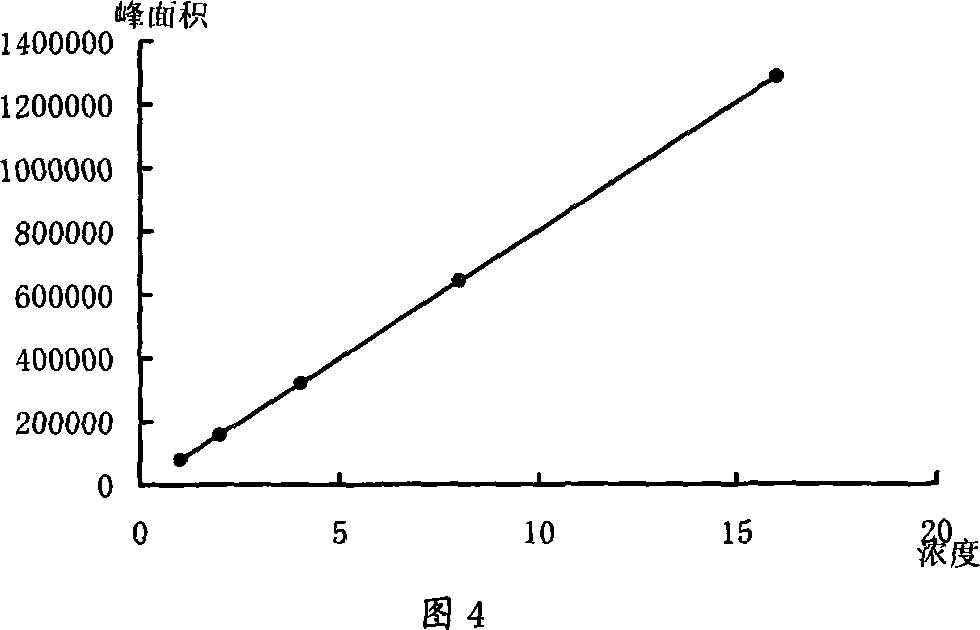
The invention discloses a herba ephedra prescription particle and a preparation method and a quality control method thereof. The prescription particle adopts the herba ephedrae as raw materials. Spraying dried herba ephedra powder is made by extracting the herba ephedrae with acidified water, concentrating the herba ephedrae under low pressure and low temperature and spray-drying the herba ephedrae. The herba ephedra particle is made by the spraying dried herba ephedra powder directly or by mixing the praying dried herba ephedra powder with proper quantities of auxiliary materials. Each herba ephedra prescription particle is equivalent to 10 grams of crude drug and the content of the ephedrine hydrochloride in the particle is not less than 3.0 percent. The herba ephedra prescription particle of the invention is provided with an IR fingerprint spectra as indicated in figure3.
Owner:BEIJING KANGRENTANG PHARMA
Method for separating and purifying ephedrine by using weak acid cation exchange resin and macroporous resin
InactiveCN1443749AEasy to eluteImprove adsorption capacityOrganic compound preparationAmino-hyroxy compound preparationOrganic solventFiltration
The present invention relates to a method for separating and purifying ephedrine by using weak acid cationc exchange resin and macroporous resin. Said method includes the following steps: pulverizingephedrae herba, mixing it with solvent to make ultrasonic extraction, filtering leaching liquor, using NaOH solution to regulate pH to 8-14, after filtration, making the filtrate pass through the weak acid cation exchange resin column or macroporous resin column to make exchange and adsorption, then using hydrochloric acid or mixed liquor of hydrochloric acid and organic solvent to make elution, making the eluant undergo the process of reduced pressure concentration to obtain ephedrine hydrochloride crystal, and the resin after elution can be washed with organic solvent and can be reused. Itsextraction efficiency can be up to above 95%.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Quality control method of particles for eliminating phlegm and stopping cough for children
InactiveCN101112422AQuality improvementSimple and easy quality control methodOrganic active ingredientsComponent separationClinical efficacyCurative effect
The present invention discloses a quality control method of pediatric phlegm-resolving and cough-relieving granules, which pertains to the quality control field of the traditional Chinese medicine preparations. The present invention firstly establishes the content determination of the ephedrine hydrochloride in the pediatric phlegm-resolving and cough-relieving granules and the TLC identification of the radix platycodi medicinal material. The present invention includes the following steps: (1) determination of the content of ephedrine hydrochloride by HPLC, the ephedrine hydrochloride (C10H15NO HCl) in each bag (5g) of the product is 1.7 to 2.1mg; (2) the TLC identification of the radix platycodi in the pediatric phlegm-resolving and cough-relieving granules. The quality control method of the present invention has simple and easy operation, accuracy, reliability and high precision. The present invention can better control the quality of the traditional Chinese medicine granules, so as to ensure the safety of drugs, further to ensure the clinical efficacy of the drugs.
Owner:贵州省科晖制药有限公司
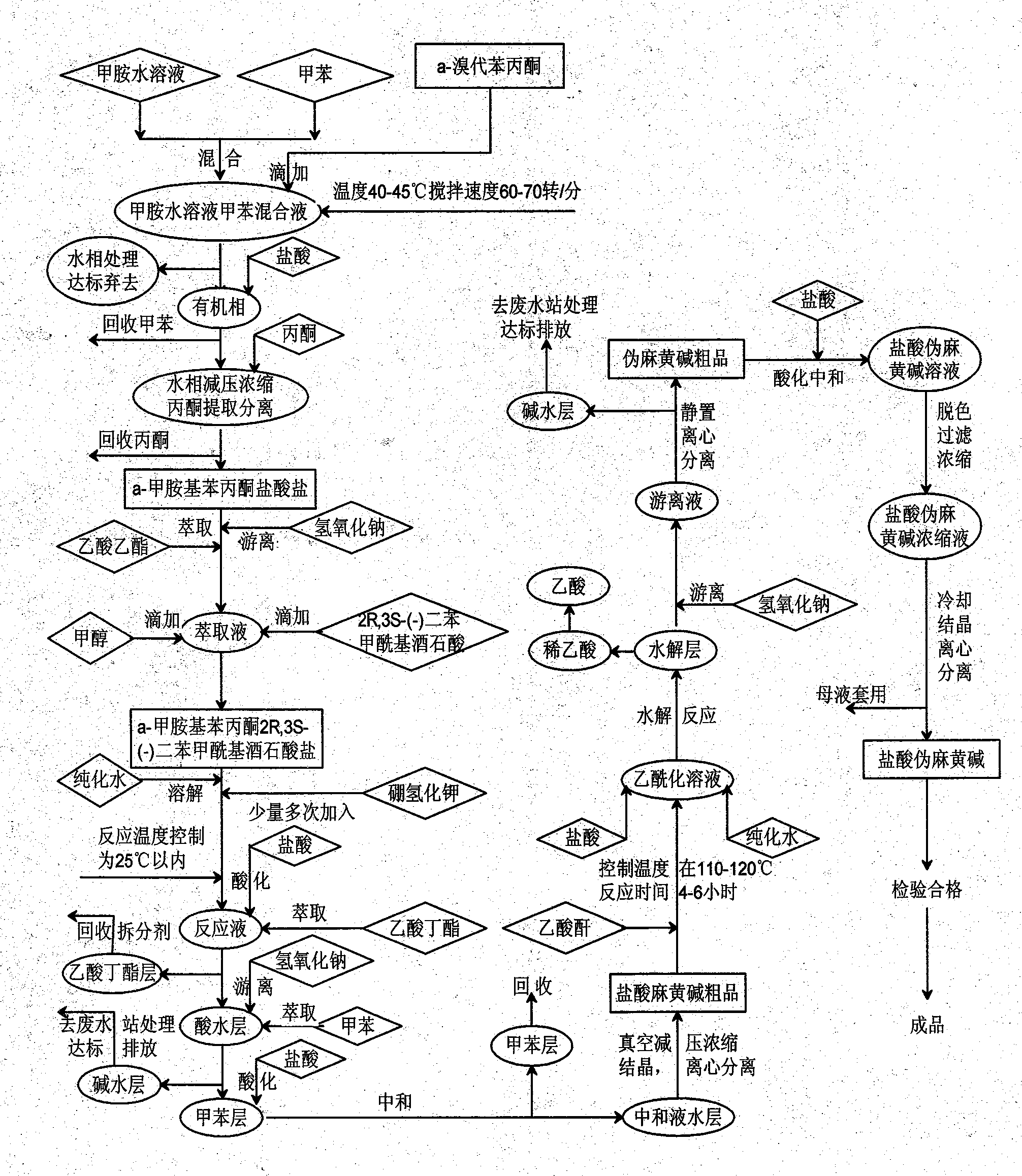
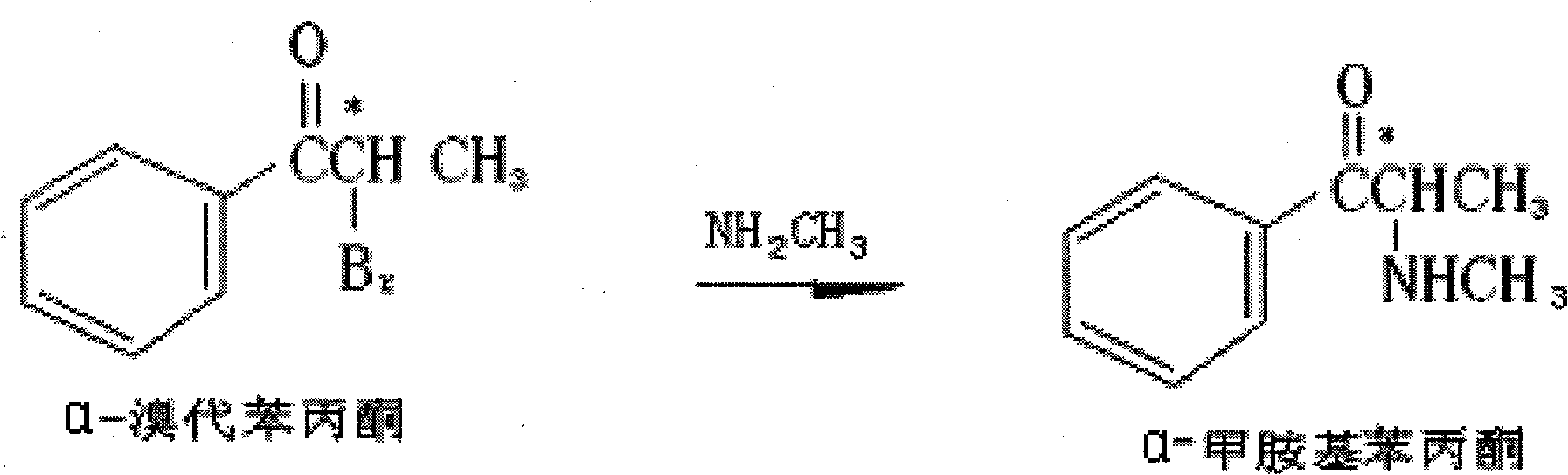
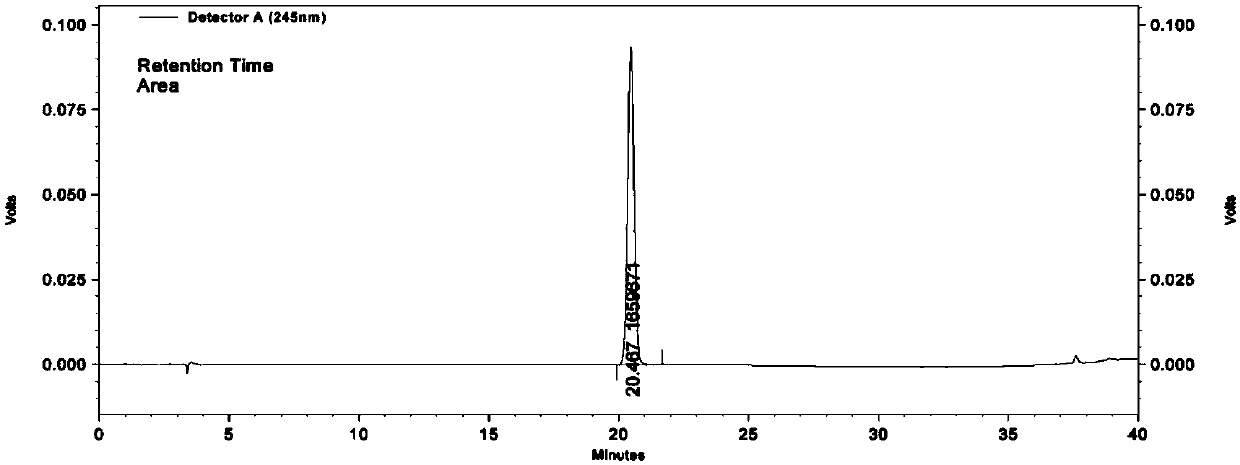
Preparation method of L-(-)-ephedrine chloride and d-(+)-pseudoephedrine hydrochloride
InactiveCN101870660AMild reaction conditionsRaw materials are easy to getOrganic compound preparationOptically-active compound separationPseudoephedrine HydrochlorideKetone
The invention relates to a method for preparing L-(-)-ephedrine chloride and d-(+)-pseudoephedrine hydrochloride by taking alpha-bromophenyl ethyl ketone as a raw material through steps of methylamination, resolution, reduction, acylation, acidolysis, hydrolysis and the like. The method has the advantages of mild reaction conditions, available raw material, small equipment investment, simple three wastes treatment, high yield and the like. The ephedrine chloride and the pseudoephedrine hydrochloride which are prepared by the invention belong to beta2 adrenoceptor agonists, and the preparations (tablets and capsules, such as new contac capsules produced by Tianjin Smith Kline & French laboratories Ltd.) of the ephedrine chloride and the pseudoephedrine hydrochloride release norepinephrine by mainly stimulating sympathetic nerve ending after being orally taken to take the sympathomimetic nerve effect in an indirect way. The L-(-)-ephedrine chloride and the d-(+)-pseudoephedrine hydrochloride are used for the adjuvant therapy of cold clinically and can soothe nasal mucosa congestion caused by the cold, allergic rhinitis, rhinitis and nasosinusitis.
Owner:QINGHAI LAKE PHARMA COMPANY
Quality control method of concentrated-type oral liquid for treating cough and asthma of children
InactiveCN107894488AStrong specificityEasy to separateComponent separationPediatric coughPseudoephedrine Hydrochloride
The present invention relates to a quality control method of traditional Chinese medicine oral liquid, in particular to a quality control method of children's Kechuanling oral liquid. Alkaline and pseudoephedrine hydrochloride content detection. The quality control method has high accuracy, good stability, and good reproducibility; it improves the current situation that there is no accurate quality control method for Xiaoer Kechuanling Oral Liquid, and ensures the accuracy and comprehensiveness of the quality control of Xiaoer Kechuanling Oral Liquid ; Ensure the quality of Kechuanling oral liquid for children, thereby ensuring the safety and effectiveness of medication for patients.
Owner:HARBIN KANGLONG PHARM CO LTD
Method for detecting quality of lung clearing and phlegm eliminating pill
ActiveCN101732552AImprove quality control standardsHigh level of quality standardsComponent separationPill deliveryPublic healthMedicine
The invention relates to a method for detecting quality of a lung clearing and phlegm eliminating pill in Volume 12 of Drug Standard of Ministry of Public Health of Peoples Republic of China (traditional medicine prescribed preparation). The method for detecting quality of a lung clearing and phlegm eliminating pill comprises microscopic identification, a thin layer chromatography identification method for orange peel and bitter orange, a thin layer chromatography identification method for ephedrine hydrochloride in herba ephedrae, a thin layer chromatography qualitative identification method for balloon flower, and scutelloside content measurement. The invention enhances the quality control standard of the lung clearing and phlegm eliminating pill, establishes content measuring and detecting indexes in main medicines of preparation and a detecting method thereof, increases the thin layer chromatography qualitative identification methods for orange peel, bitter orange, ephedrine hydrochloride and balloon flower, and ensures the higher quality standard levels of the compound preparation.
Owner:KUNMING CHINESE MEDICINE FACTORY
Quality detection method of dampness-resolving toxin-vanquishing composition
ActiveCN111735889AGuaranteed stabilityEnsure controllabilityComponent separationPaeoniae RadixOfficinalis
The invention discloses a quality detection method of a dampness-resolving toxin-vanquishing composition. The dampness-resolving toxin-vanquishing composition is mainly prepared from the following components: herba ephedrae, fried semen armeniacae amarae, gypsum, liquorice root, herba pogostemonis, cortex magnoliae officinalis, rhizoma atractylodis fried with bran, fried semen tsaoko, rhizoma pinellinae praeparata, poria cocos, rheum officinale, astragalus membranaceus, semen lepidii, and radix paeoniae rubra. The quality detection method of the dampness-resolving toxin-vanquishing compositioncomprises the following steps: measuring the content of total anthraquinone, the content of free anthraquinone, the content of ephedrine hydrochloride, the content of pseudoephedrine hydrochloride and the content of paeoniflorin in the dampness-resolving toxin-vanquishing composition by adopting high performance liquid chromatography, and calculating the content of combined anthraquinone; whereinthe combined anthraquinone content = the total anthraquinone content - the free anthraquinone content. The method is high in specificity and stability, high in durability and capable of effectively guaranteeing the stability and controllability of the product quality of the dampness-resolving toxin-vanquishing composition in the large-scale production process.
Owner:GUANGDONG YIFANG PHARMA
Pinellia ternate medicinal material detection method
InactiveCN104267111AThe method is accurate and simpleSimple methodComponent separationColor/spectral properties measurementsPinelliaPesticide residue
The invention relates to a medicinal material detection method and concretely relates to a method for detecting alkaloid content and pesticide residue content of a pinellia ternate medicinal material. The method comprises alkaloid content determination: by chloroform, extracting a sample to be detected so that the extract is obtained and is used as a sample solution, preparing a contrast solution from ephedrine hydrochloride, determining a light absorption value by ultraviolet analysis and carrying out comparison calculation, and pesticide residue content determination: preparing a contrast solution based on acetonitrile protection, preparing a sample solution by acetone ultrasonic treatment, GPC gel penetration chromatogram purification and cyclohexane-ethyl acetate elution, determining content of a plurality of pesticide residues in the pinellia ternate medicinal material by a gas chromatography-mass spectrometry combined method and carrying out concentration calculation. The method provided by the invention solves the technical problem of operation complexity and limitation of the traditional physicochemical property discriminating method, has high accuracy, can be operated simply, has a low cost and has a good application prospect and economic benefits.
Owner:LONGXI ZHONGTIAN PHARM CO LTD
Pharmaceutical composition for treating nose disease and its preparation method
ActiveCN1806815AEffective treatmentHydroxy compound active ingredientsPharmaceutical delivery mechanismDiseaseClinical efficacy
The invention discloses a pharmaceutical composition for treating nasal diseases, which is prepared from the following raw material medicaments: ephedrine hydrochloride, glycoside of baikal skullcap root, lily magnolia oil, boneol and honeysuckle flower. The pharmaceutical composition can be made into any clinically acceptable preparations through conventional methods in the prior art, preferably nasal drops, aerosols and sprays.
Owner:SINOPHARM GRP DEZHONG (FOSHAN) PHARM CO LTD
Method for detecting wind-cold cold granules
The invention discloses a method for detecting wind-cold cold granules. The method comprises thin layer identification of Chinese ephedra and platycodon root and content determination of ephedrine hydrochloride, wherein identification of the Chinese ephedra is performed by taking ephedrine hydrochloride as a reference substance and a mixed solution including ethyl acetate, methanol and water as a developing agent and using a thin layer chromatography; and the identification of the platycodon root is performed by taking a platycodon root contrast medicinal material as a reference substance and a mixed solution including n-hexane, ethyl acetate and glacial acetic acid as a developing agent and using the thin layer chromatography. By adopting the method, the quality of the main components including the Chinese ephedra and the platycodon root, which cause problems easily, in a prescription is effectively controlled, so that the quality of the wind-cold cold granules is better and more comprehensively reflected, fake and shoddy wind-cold cold granules produced by illegal manufacturers can be effectively detected and identified, and then the safety and effectiveness of mass medication are ensured.
Owner:SICHUAN FENGCHUN PHARMA
Method for simultaneously measuring seven index components in formula granules of Huagai powder for treatment of wind-cold-caused common cold and asthma by using high performance liquid chromatography
InactiveCN109856270ASimple processing methodFully extractedComponent separationPretreatment methodPseudoephedrine
The invention discloses a method for simultaneously measuring seven index components in formula granules of Huagai powder for treatment of wind-cold-caused common cold and asthma by using high performance liquid chromatography. The seven index components include ephedrine hydrochloride, pseudoephedrine, amygdalin, glycyrrhizic acid, liquiritin, sanggenon C, and sanggenon D. The method comprises the following steps: step one, preparing a standard solution; step two, preparing a test sample solution; step three, carrying out liquid chromatogram separation; step four, performing a content calculation method. According to the invention, the pretreatment method of sample extraction is simple and the index components can be extracted fully. The method is performed accurately and rapidly with high sensitivity and low cost; and various methodological indexes can satisfy the actual detection demands. Seven measured compounds have high linearity in a standard curve linear range, wherein R2 is larger than 0.9990; the within-day precision relative standard offsets of all components are less than 1.0%; and the day-to-day precision relative standard offsets are less than 3.0%. The recovery ratesof the seven index components are in a range of 90.0% to 110.9%.
Owner:ZHEJIANG PHARMA COLLEGE +1
Chemical method for synthesizing ephedrine
ActiveCN101570492ASimple production processEasy to useOrganic compound preparationAmino-hyroxy compound preparationChemical synthesisOrganic acid
The invention relates to a chemical method for synthesizing ephedrine. In the technology, (+ / -)alpha-methylaminophenylpropanone hydrochloride is taken as a raw material and reduced to the mixtures of (+ / -)ephedrine and (+ / -)pseudoephedrine by a proper reducing agent; the (+ / -)ephedrine is separated, and the l-ephedrine or l-ephedrine hydrochloride is separated by using chiral organic acid as a resolving agent. The method enjoys simple technology, less equipment investment, less environment pollution, less hazardous and poisonous chemical reagents which are used and the like.
Owner:QINGHAI LAKE PHARMA COMPANY
Quality inspection method of cough pills
ActiveCN101732553AImprove quality control standardsHigh level of quality standardsInorganic boron active ingredientsComponent separationChemical reactionMagnolol
The invention relates to a quality inspection method of cough pills prescribed in Volume 11 of Drug Standard of Ministry of Public Health of the People's Republic of China (Chinese Traditional Patent Formulation Preparations). The quality inspection method comprises microscopic identification of cough pills, a thin-layer chromatography identification method of ephedrine hydrochloride, magnolol and dried orange peel, a thin-layer chromatography qualitative identification method of bitter orange and a content determination method of ephedrine hydrochloride in the cough pills. The quality inspection method raises the quality control standards of cough pills and establishes content determination inspection indexes of main drugs in a preparation and a inspection method thereof; and meanwhile, the quality inspection method deletes physical and chemical reaction identification of poor specialization and adds the thin-layer chromatography qualitative identification method of ephedrine hydrochloride, magnolol, dried orange peel and bitter orange, thereby ensuring a higher quality standard level of compound preparations of cough pills.
Owner:KUNMING CHINESE MEDICINE FACTORY
Quality testing method for perilla-ephedra cough-relieving capsule
ActiveCN108490083AMonitor qualityFormulation stabilizer is goodComponent separationContent determinationEphedrine hydrochloride
The invention relates to a quality testing method for a perilla-ephedra cough-relieving capsule. The method specifically comprises determination methods for the contents of ephedrine, pseudoephedrineand arctiin. The method brings forward simultaneous quality detection of ephedrine hydrochloride and pseudoephedrine in the perilla-ephedra cough-relieving capsule for the first time; and the method is accurate, has good resolution and repeatability and high specificity, and is especially applicable to content determination of the perilla-ephedra cough-relieving capsule.
Owner:北京东方运嘉科技发展有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com