Chinese ephedra prescription particle, preparation method and quality control method thereof
A technology of formula granules and ephedra, applied in medical raw materials derived from ephedra, color/spectral characteristic measurement, bulk transportation, etc., can solve problems such as backward large-scale production technology, influence of drug efficacy, limited transfer rate of ephedrine, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Embodiment 1: the preparation method of ephedra formula granule
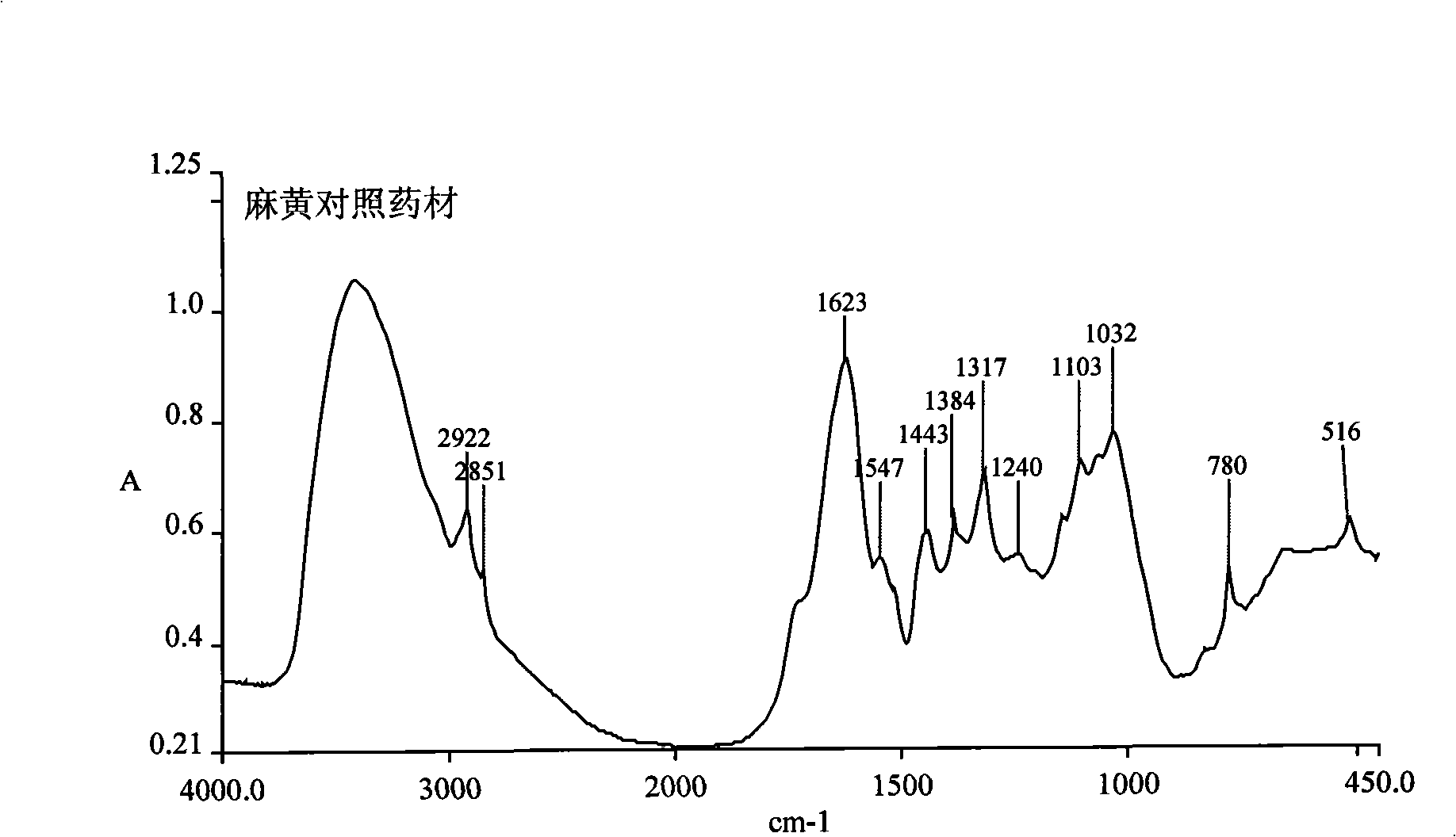
[0064] The quality of the raw material ephedra is identified by infrared fingerprints. The identification method of infrared fingerprints is: use a Fourier transform infrared spectrometer with a measuring range of 4000cm -1 -400cm -1 , DTGS detector, resolution 4cm -1 , the number of scans is 16 times, the interference of water and carbon dioxide is always deducted during the scanning process, the relative humidity of the environment is lower than 60%, and the potassium bromide direct compression method is used for detection: the obtained results are consistent with the standard infrared fingerprint of ephedra; the characteristics of the standard infrared fingerprint of ephedra For: the strongest peak: peak position 1623cm -1 , with 1547cm -1 The peak forms a peak cluster with it; the second strongest peak: the peak position is 1032cm -1 , with 1103cm -1 The peak forms a peak cluster with it; the str...
Embodiment 2
[0066] Embodiment 2: the preparation method of ephedra formula granule
[0067] The quality of the raw material ephedra is identified by infrared fingerprints. The identification method of infrared fingerprints is: use a Fourier transform infrared spectrometer with a measuring range of 4000cm -1 -400cm -1 , DTGS detector, resolution 4cm -1 , the number of scans is 16 times, the interference of water and carbon dioxide is always deducted during the scanning process, and the relative humidity of the environment is lower than 60%. The characteristics of the infrared fingerprint spectrum are: the strongest peak: the peak position is 1623cm -1 , with 1547cm-1 The peak forms a peak cluster with it; the second strongest peak: the peak position is 1032cm -1 , with 1103cm -1 The peak forms a peak cluster with it; the stronger peak: the peak position is 2922cm -1 , at 2851cm -1 There is a peak forming a peak cluster with it; in addition, according to the intensity of the peaks fro...
Embodiment 3
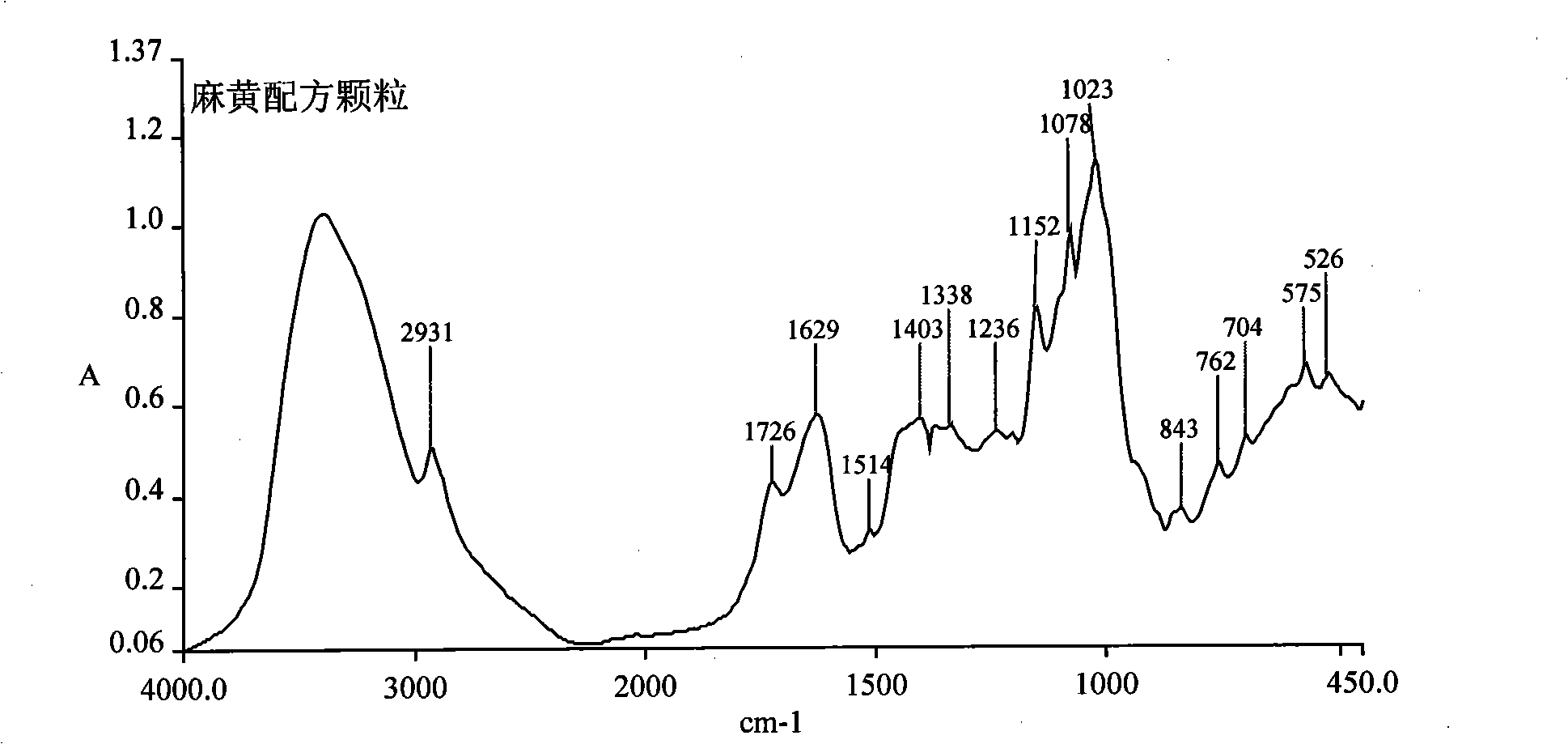
[0069] Embodiment 3: the quality control method of the ephedra formula granule prepared in embodiment 1
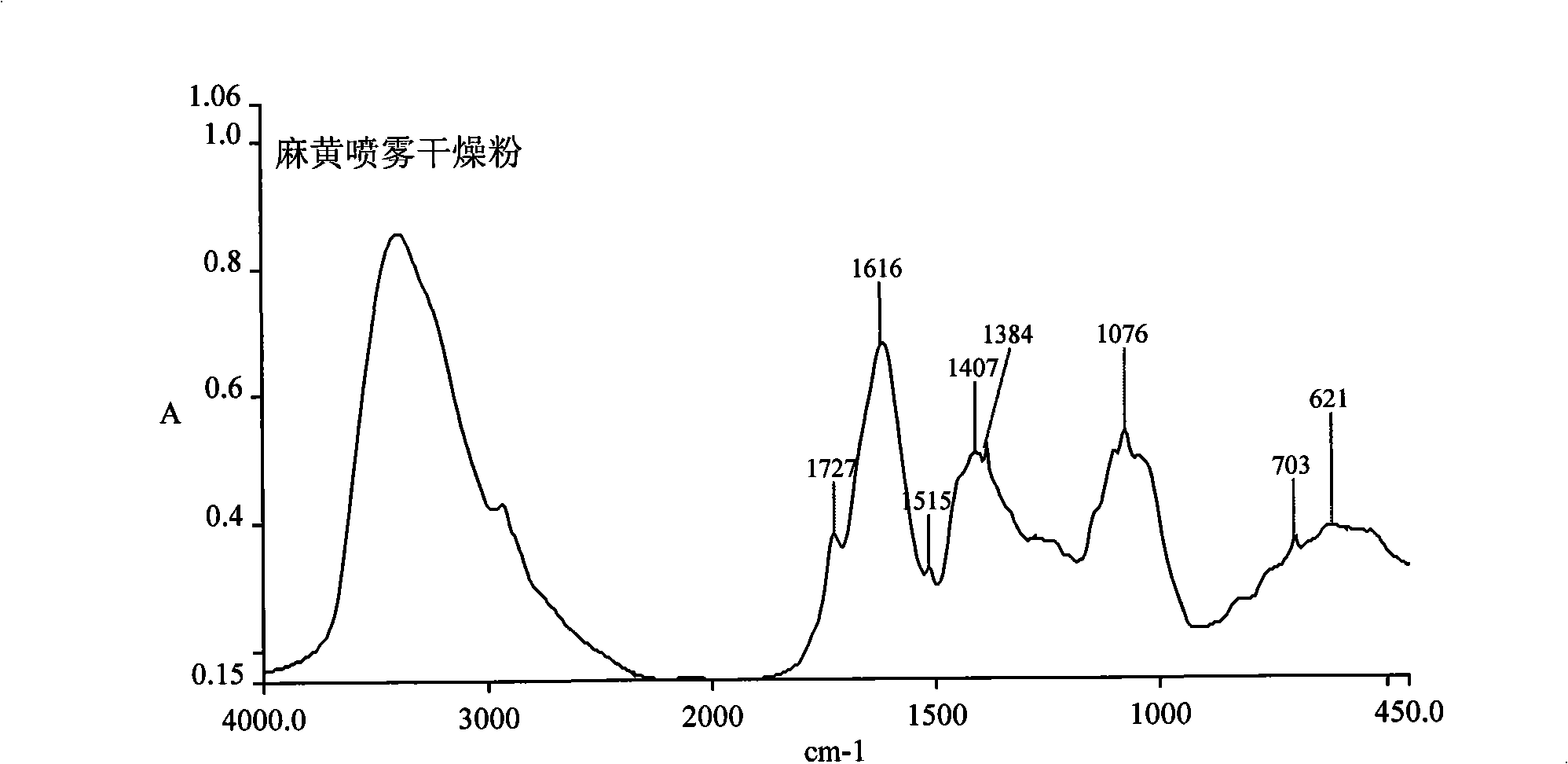
[0070] A. Quality control of ephedra spray-dried powder: ephedra spray-dried powder adopts Fourier transform infrared spectrometer with a measuring range of 4000cm -1 -400cm -1 , DTGS detector, resolution 4cm -1 , the number of scans was 16 times, the interference of water and carbon dioxide was deducted from time to time during the scanning process, the relative humidity of the environment was lower than 60%, and the potassium bromide direct tablet method was used for detection; the obtained results were consistent with the standard infrared fingerprint of ephedra spray-dried powder, ephedra spray-dried The standard infrared fingerprint of the powder ( figure 2 ) is characterized by:
[0071] The strongest peak: peak position 1616cm -1 , with 1727cm -1 、1515cm -1 The two peaks form a peak cluster; the second strongest peak: the peak position is 1076cm -1 ;Stronger...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com