Quality control method of particles for eliminating phlegm and stopping cough for children
A quality control method, the technology of reducing phlegm and relieving cough, which is applied in the field of quality control of Xiaoer Huatan Zhike granule, can solve problems such as addiction, affecting product quality, and too simple quality control indicators, so that the quality control method is simple and easy to implement , to ensure clinical efficacy and safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
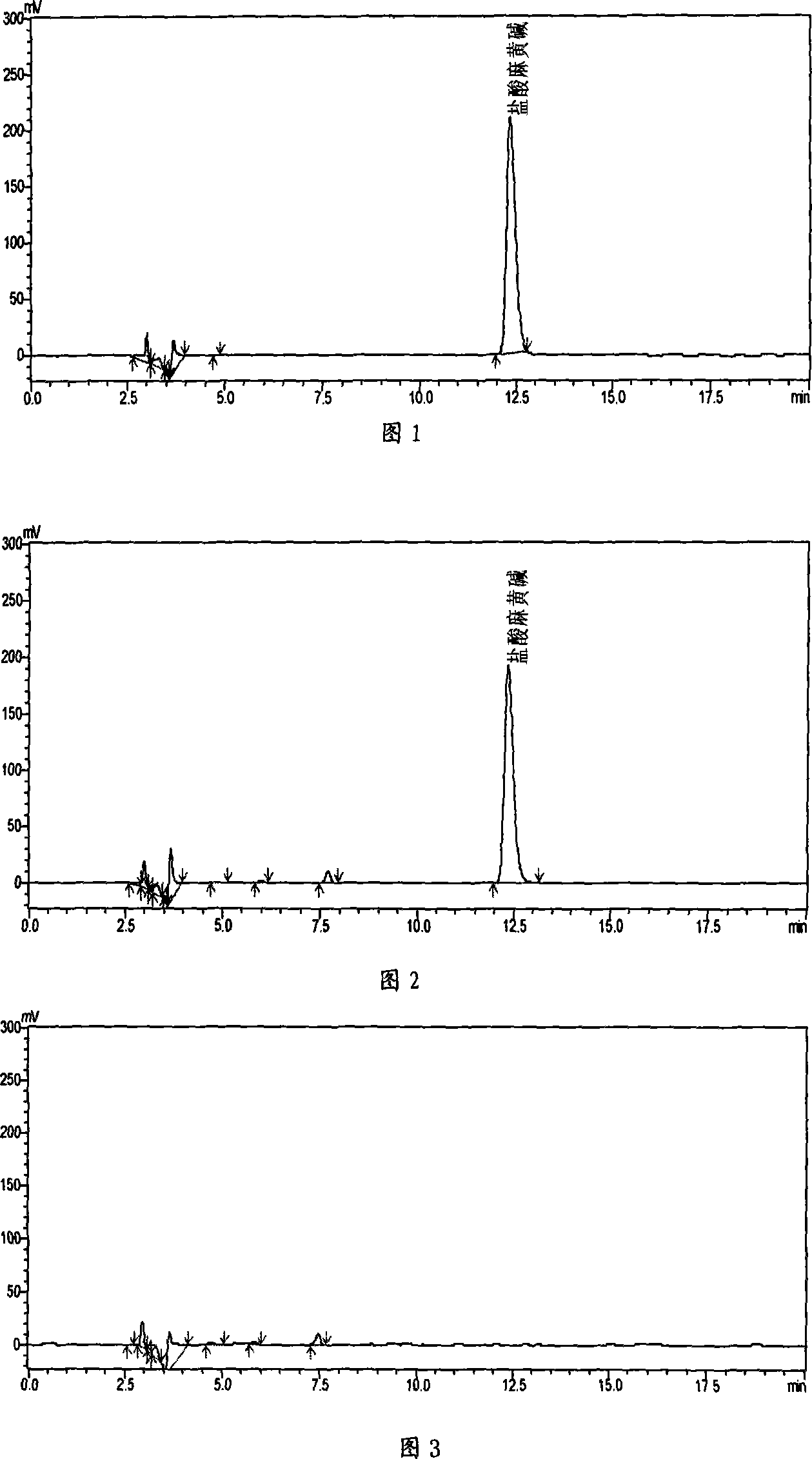
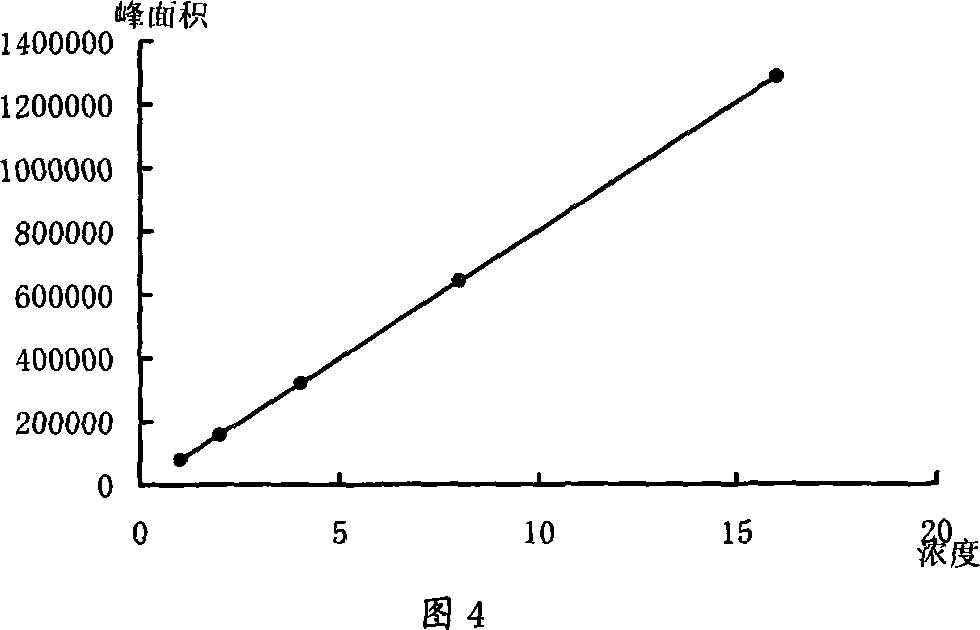
[0018] Embodiment 1: the investigation of ephedrine hydrochloride content determination method
[0019] 1. HPLC chromatographic conditions and preparation and screening of the test solution
[0020] We selected a variety of mobile phase systems to carry out HPLC chromatographic condition screening tests, and the results are shown in the table below.
[0021] Statistical table of chromatographic condition inspection data
[0022] method number
[0023] Through the analysis of the above comparative test results, method 5 has the characteristics of appropriate retention time and good sample separation effect, so it is used as ephedrine hydrochloride-containing mobile phase. Simultaneously according to follow-up test analysis summary, the number of theoretical plates when the separation degree of sample is 1.5 is 2965, therefore the theoretical plate number of regulation ephedrine hydrochloride is not less than 3000.
[0024] Preparation of the test solution Take t...
Embodiment 2
[0055] Embodiment 2: TLC identification of Platycodon grandiflorum medicinal material
[0056] Take 1g of the fine powder of this product, add 10ml of ethanol, heat to boiling, filter, evaporate the filtrate to dryness, add 2ml of dilute hydrochloric acid solution, hydrolyze in a water bath for 1h in a stoppered test tube, adjust the pH to 9-10 with concentrated ammonia water, add 1ml of chloroform, shake fully Shake, centrifuge, and use the chloroform layer as the test solution. Another 0.2 g of bellflower reference drug was taken, and the reference drug solution was prepared in the same way. According to the test of thin-layer chromatography (Appendix VIB, Chinese Pharmacopoeia 2005 Edition), draw 10 μl of each of the above two solutions, respectively spot on the same silica gel G thin-layer plate, and mix with ethyl acetate-butanone-formic acid-water (10: 0.5∶0.5∶0.2) as developing agent, develop, take out, dry in the air, and inspect under ultraviolet light (365nm). In t...
Embodiment 3
[0057] Embodiment 3: TLC identification of tincture of ipecac and ephedrine hydrochloride
[0058] Take about 7.5g of this product, add 15ml of water, heat to dissolve, let it cool, add 2ml of sodium hydroxide test solution, add sodium chloride to make it saturated, shake and extract with 30ml of ether twice, combine the ether solution, evaporate the ether to About 0.5ml, as the test solution. Take another ephedrine hydrochloride reference substance, add methanol to make a solution containing 1mg per 1ml, as the reference substance solution. Then take 2ml of tincture of ipecac reference substance, add 4ml of water, add 2ml of sodium hydroxide test solution, shake and extract twice with 20ml of ether, combine the ether solution, evaporate the ether to about 0.5ml, and use it as the reference substance of tincture of ipecac. According to the test of thin-layer chromatography (Appendix VI B, Chinese Pharmacopoeia, 2005 edition), draw 10-15 μl of each of the above three solutions...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com