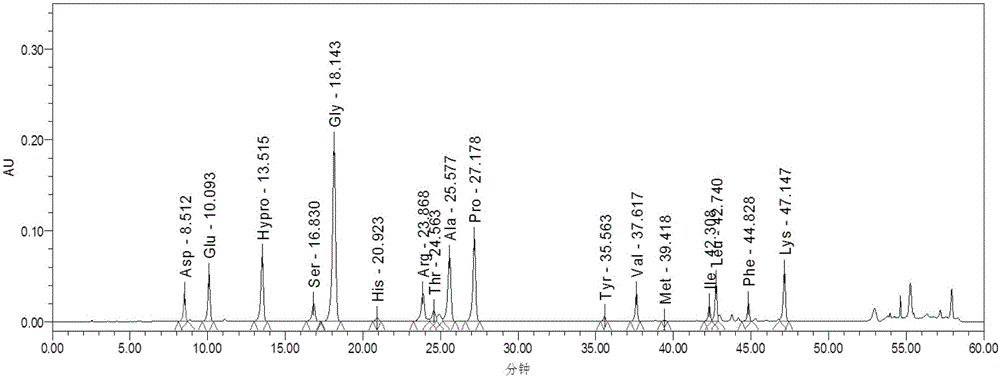
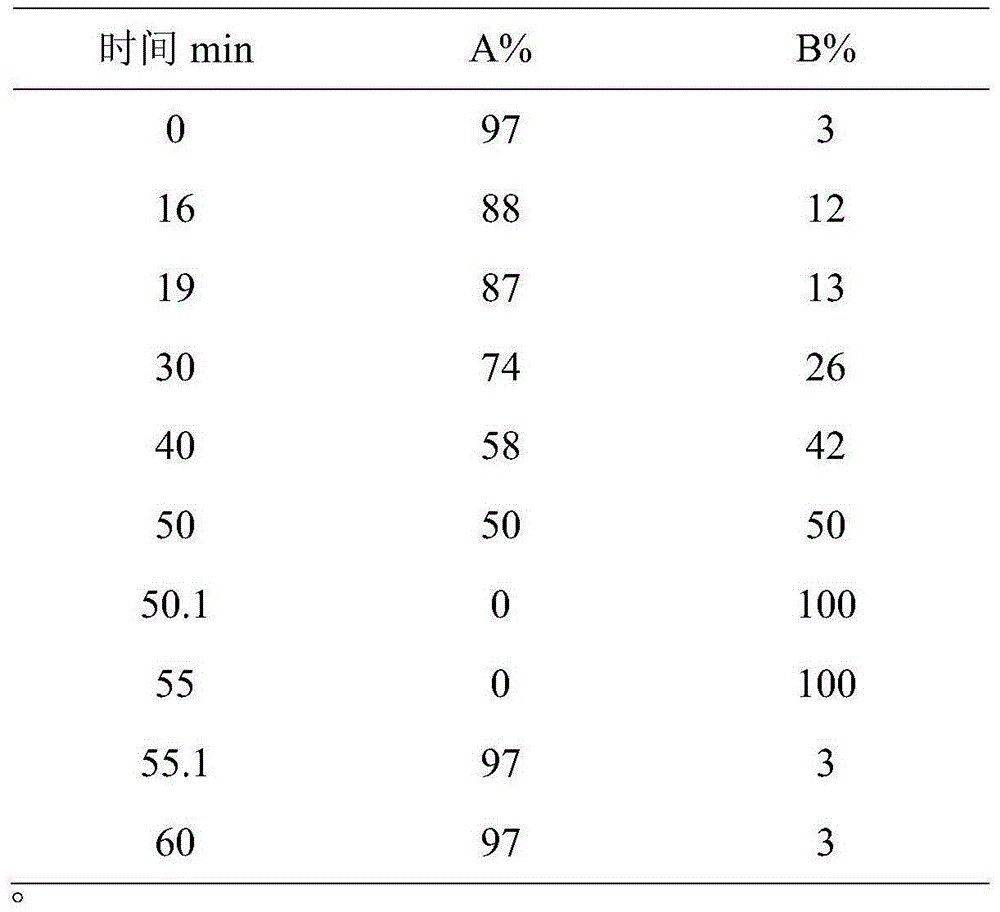
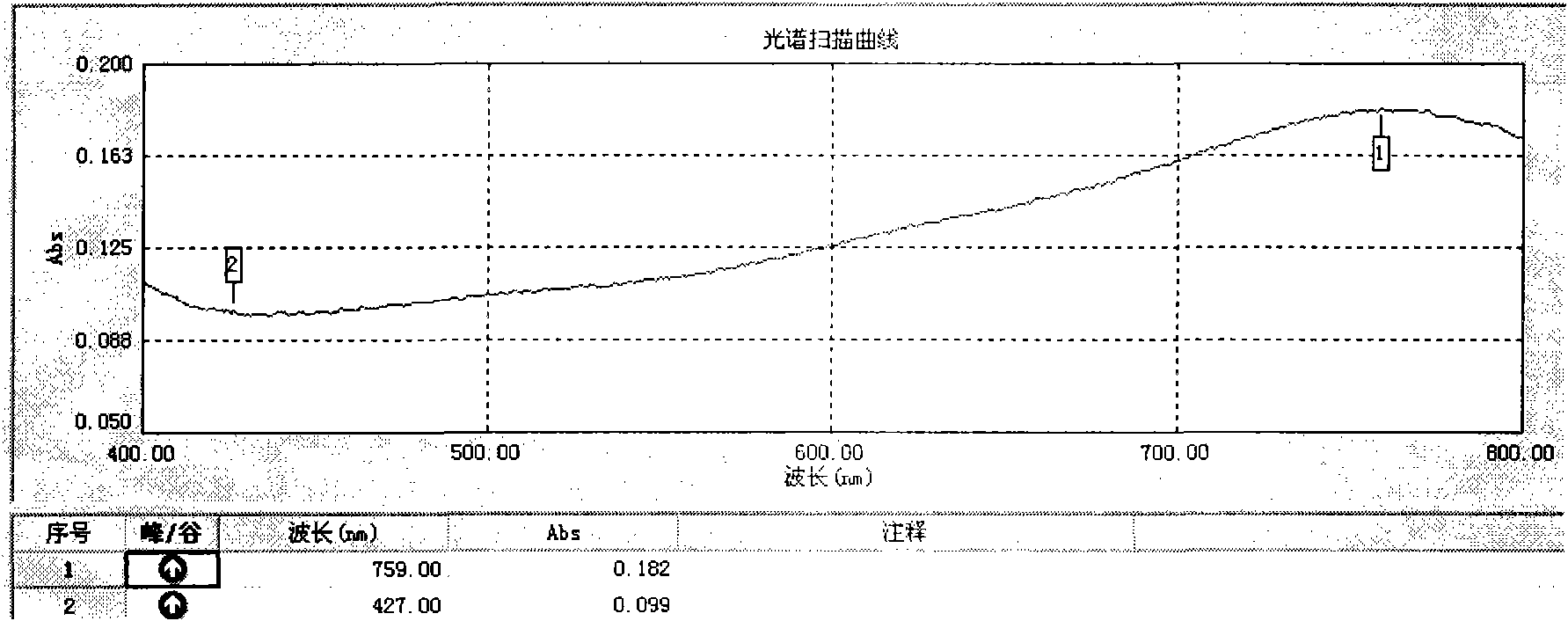
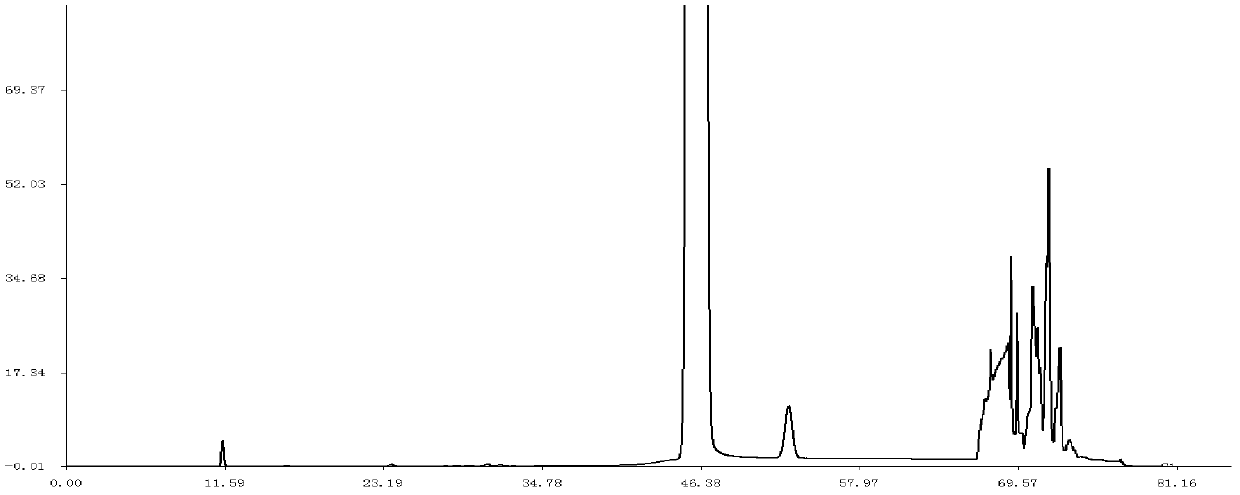
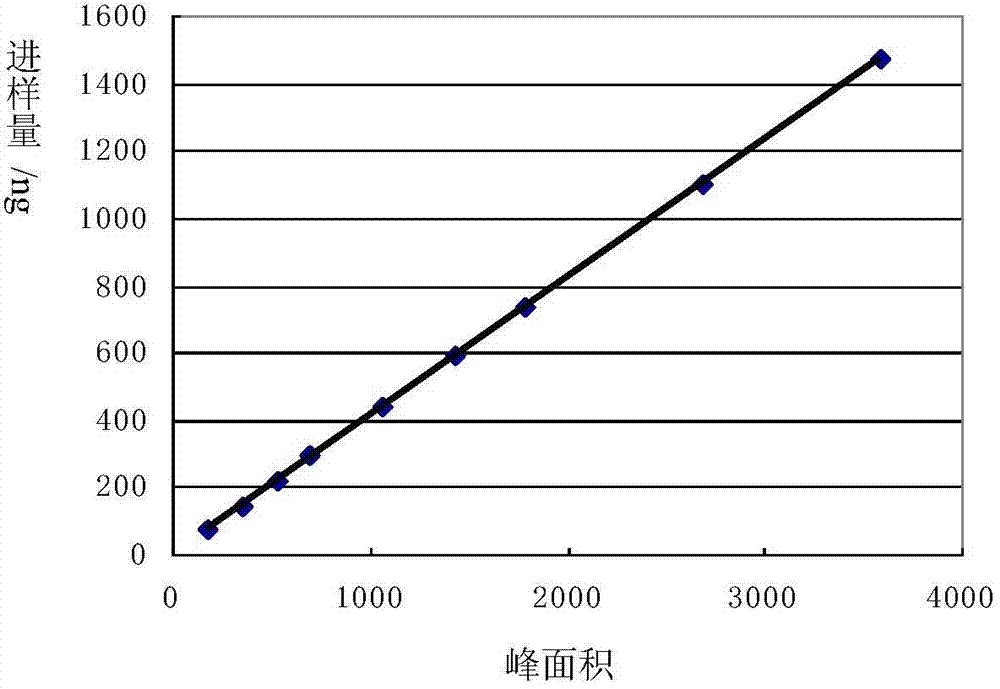
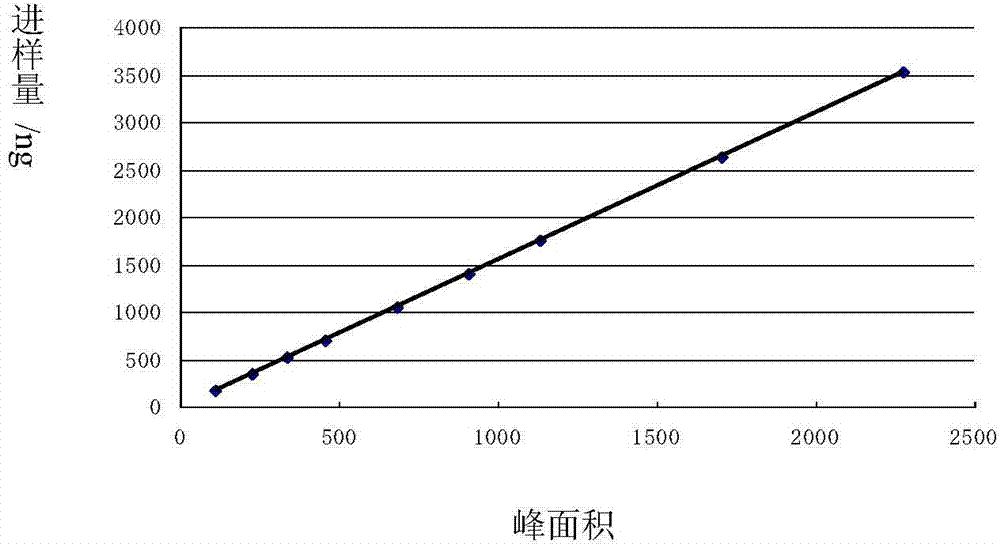
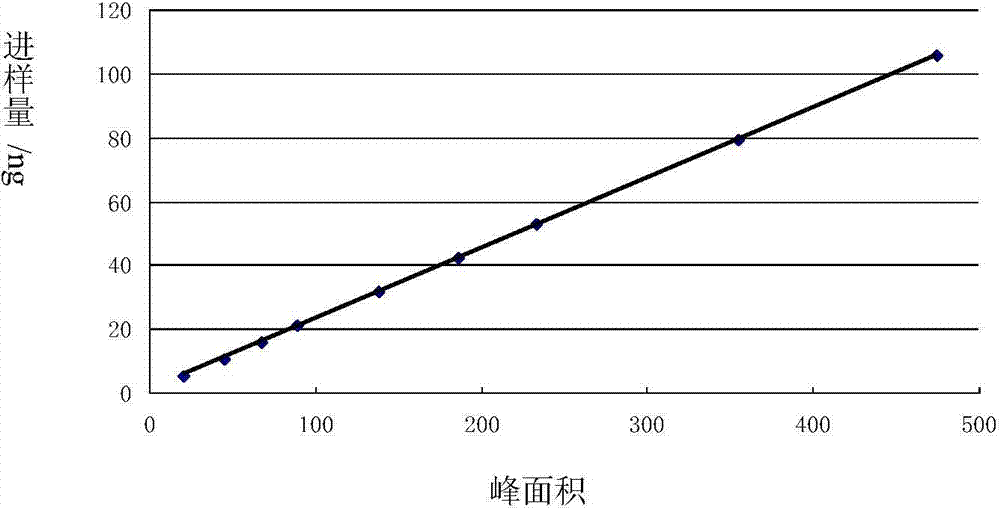
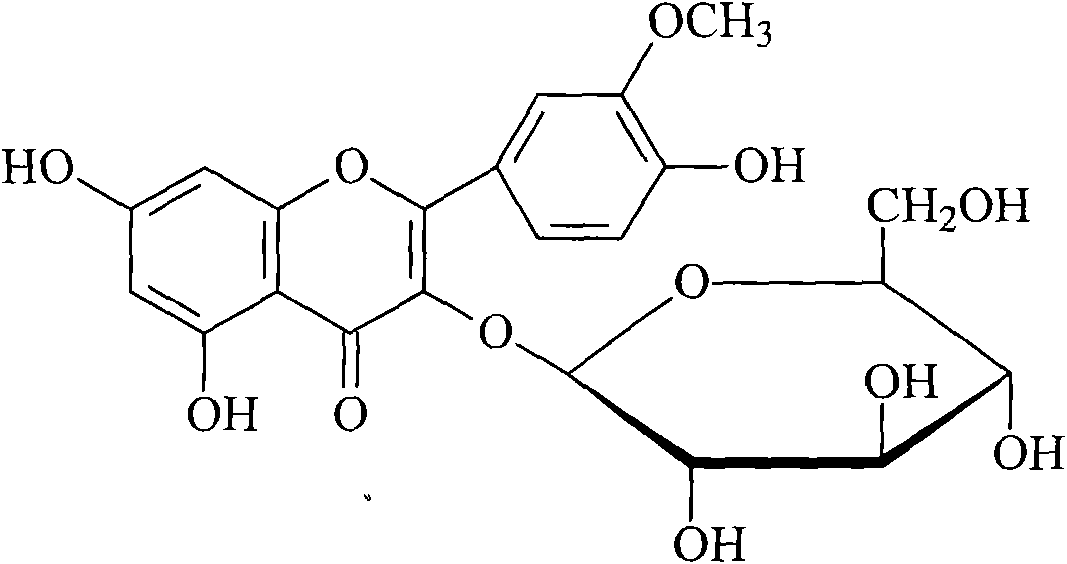
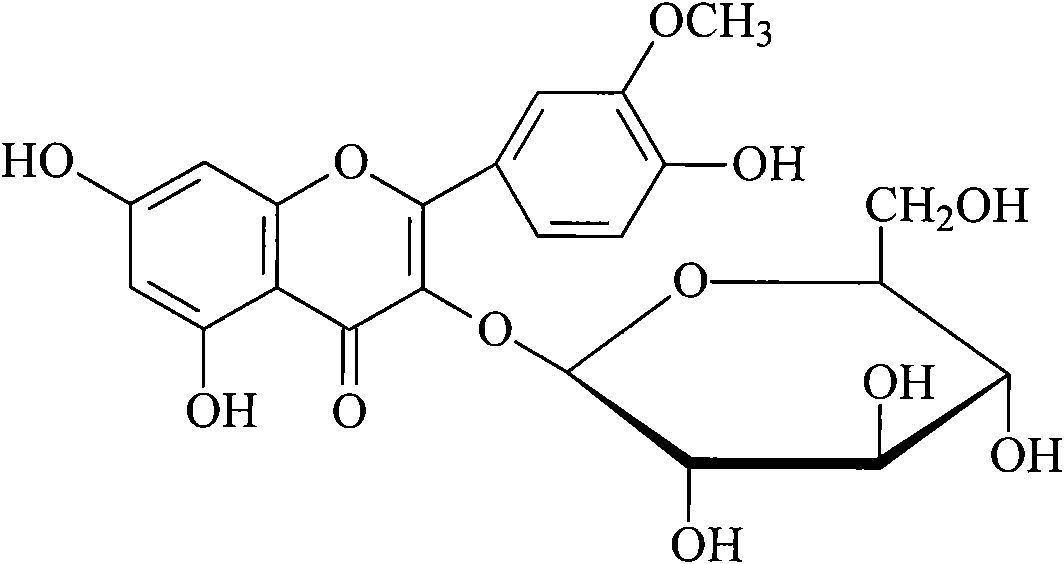
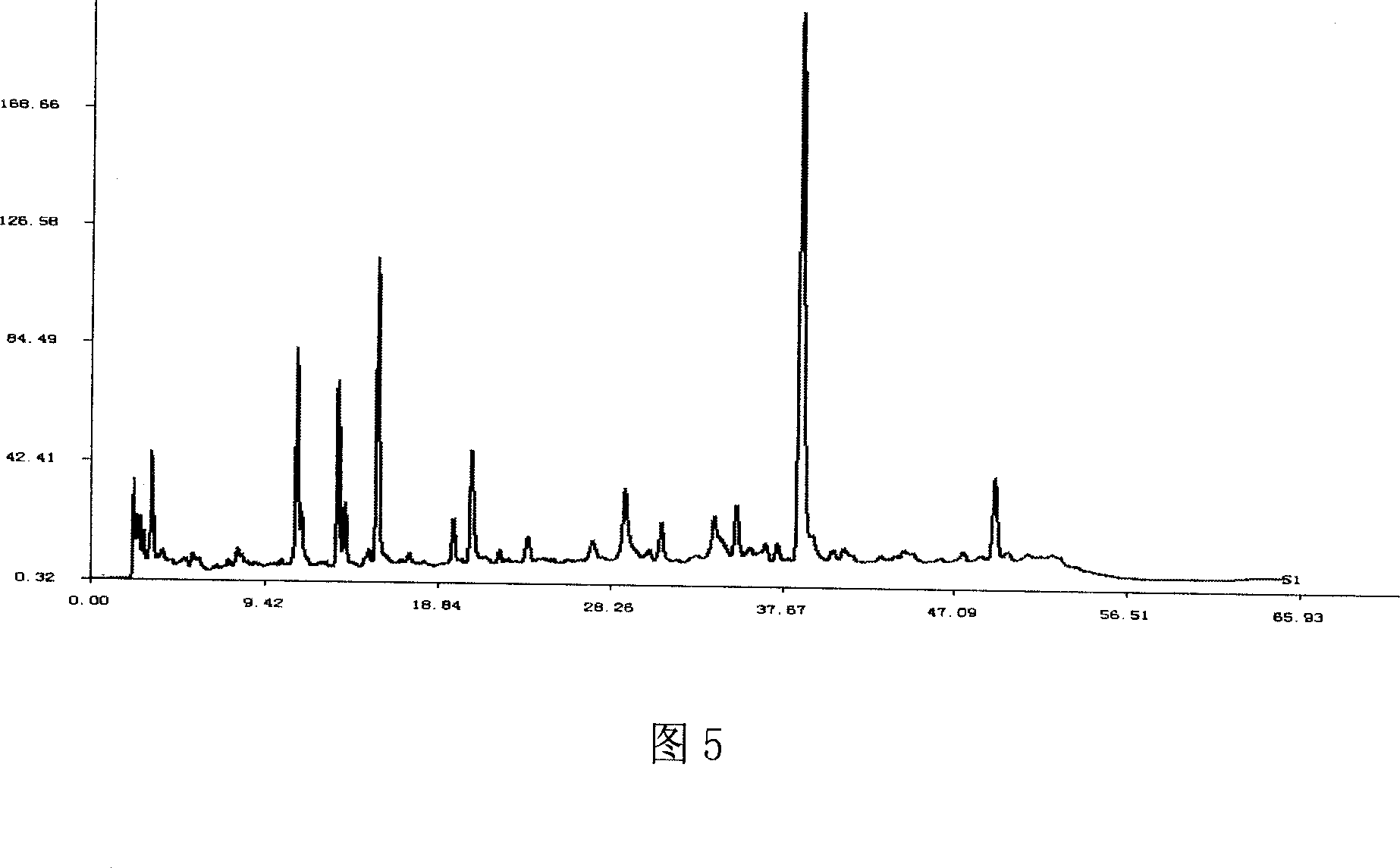
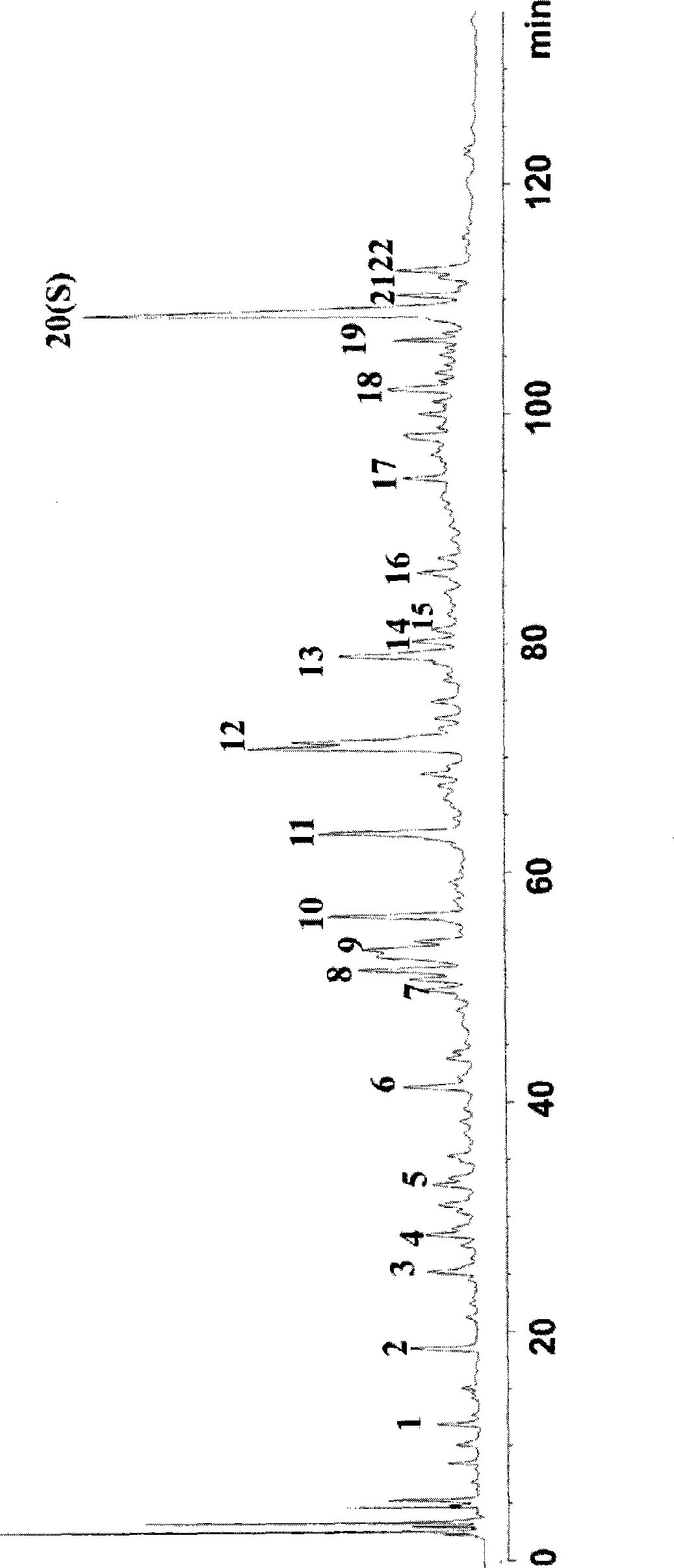
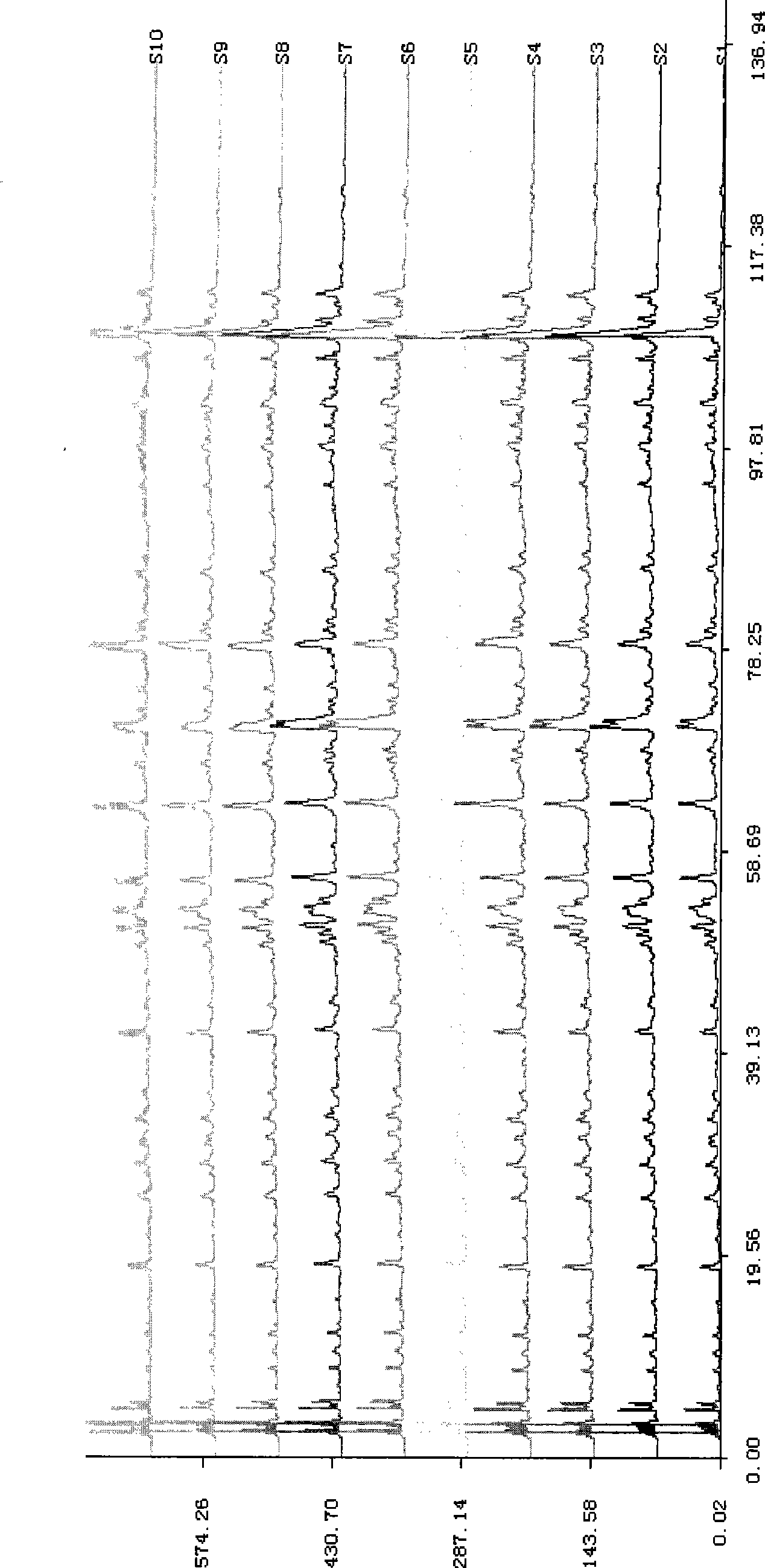
Patents
Literature
107results about How to "Improve quality control standards" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Quality control method of pulse-invigorating and heart-nourishing pill
ActiveCN101396519AImprove quality control standardsGrasp the qualityComponent separationUnknown materialsActive componentMedicine
The invention discloses a quality control method of a pulse-invigorating and heart-nourishing pill. The method comprises the following steps: the contents prepared tuber fleece flower and Chinese magnolia vine fruit are identified, and the contents are tested according to the method described in the pharmacopoeia, and liquoric root in the contents is assayed. The quality of the related drugs can be guaranteed more accurately by the assay of the active component liquoric root in the pulse-invigorating and heart-nourishing pill to effectively ensure safety, uniformity, stability, effectiveness and controllability of the pulse-invigorating and heart-nourishing pill. The new quality control method is simple and convenient, gives accurate result and better reproducibility, and can achieve the purpose of quality control.
Owner:津药达仁堂集团股份有限公司乐仁堂制药厂
Method for detecting quality of mulberry and chrysanthemum lonicera and forsythia powder
ActiveCN101732608AImprove quality control standardsHigh level of quality standardsPowder deliveryAnthropod material medical ingredientsThin-layer chromatographyQuality standard
The invention relates to a method for detecting quality of mulberry and chrysanthemum lonicera and forsythia powder according to standards described in volume XI of Medicament Standard of Ministry of Health of the People's Republic of China (Chinese medicinal set prescription preparation), which comprises a microscopic identification method of chrysanthemum, lonicera confuse and great burdock achene, a thin-layer chromatography identification method of forsythia, a thin-layer chromatography identification method of liquoric root and a content measuring method of arctigenin in medicaments. The quality detection method of the mulberry and chrysanthemum lonicera and forsythia powder improves the quality control standard of the mulberry and chrysanthemum lonicera and forsythia powder, establishes content measuring indexes in the preparation and a detection method thereof, improves the thin-layer chromatography identification method of the forsythia and the liquoric root by making the thin-layer chromatography identification method of the forsythia and the liquoric root more specific and accurate, increases microscopic characteristics of the chrysanthemum and the great burdock achene, revises the microscopic characteristic of the lonicera confuse to make the description of the lonicera confuse more standard and accurate, and ensures the high quality standard level of the compound preparation.
Owner:KUNMING CHINESE MEDICINE FACTORY
Bone-strengthening drug quality detection method
ActiveCN105021729AImprove quality control standardsHigh level of quality standardsComponent separationColor/spectral properties measurementsMicrowaveMedicine
The invention discloses a bone-strengthening drug quality detection method, and belongs to the drug quality detection technical field. The adopted technical scheme comprises that the bone-strengthening drug is made into capsules by an artificial tiger bone powder and auxiliary materials, and the quality detection method includes the following determining steps: 1) after the bone-strengthening drug is hydrolyzed, determining the content of total amino acids in the samples, wherein each capsule contains not less than 65 mg of the total amino acids; and 2) determining the content of total calcium in the bone-strengthening drug by adopting atomic absorption spectrophotometry, wherein each capsule contains not less than 55 mg of calcium. The determination method for determining the content of the total amino acids in the preparation by adopting microwave hydrolysis and automatic online derivation, using a full-automatic amino acid analyzer or adopting traditional hydrolysis and PITC derivation method and using high performance liquid chromatography is established, the determination method for determining the content of calcium in the samples by adopting the atomic absorption spectrophotometry is increased, the accuracy and advancement of the quality detection method is ensured, and the product quality of Jintiange capsules can be effectively controlled.
Owner:XIAN DAQING PHARMA FACTORY JINHUA ENTERPRISE GROUP CORP
Chinese medicinal composition for treating gastropathy as well as preparation and quality control method thereof
ActiveCN101485872ASignificant effectGood pain reliefChemical analysis using combustionChemical analysis using titrationGinger RhizomeDisease
The invention discloses a traditional Chinese medicine composition for treating gastrosis. The traditional Chinese medicine composition is prepared from starfish, alum, dried orange peels, blood clam shells, oysters, Atractylodes macrocephaia, Astragalus membranaceus, dried ginger, peppers and other medicines according to weight ratio, and has good effect of treating diseases such as weakness of the spleen and the stomach, stomach cold and pain, acid stomach and gastroduodenal ulcer. Moreover, the invention discloses a method for preparing the traditional Chinese medicine composition and a quality control method for the traditional Chinese medicine composition.
Owner:大连美罗中药厂有限公司
Quality detection method for Jinhuaxiaocuo pills
ActiveCN101732463AImprove quality control standardsHigh level of quality standardsComponent separationDigestive systemChemical reactionAdditive ingredient
The invention relates to a quality detection method for Jinhuaxiaocuo pills (WS3-B-2169-96) in the eleventh volume of Drug Standard of Ministry of Public Health of the Peoples Republic of China (Chinese patent medicaments). Through the quality detection method for the Jinhuaxiaocuo pills, the quality control standard for the Jinhuaxiaocuo pills is improved, a method for detecting the content of a main ingredient in a preparation is established, physical and chemical reaction identification with poor specificity is eliminated, and thin layer chromatography identification methods for geniposide and berberine hydrochloride are improved to have the advantages of simplness and clear characteristic spots, thin layer chromatography identification methods for rhubarb, golden thread, amur corktree bark and baical skullcap root in the Jinhuaxiaocuo pills are increased, and the high quality standard level of the compound preparation is guaranteed.
Owner:KUNMING CHINESE MEDICINE FACTORY
Method for measuring content of tannide in nakedflower beautyberry
ActiveCN101625313AImprove quality control standardsColor/spectral properties measurementsBlood disorderTanninComputer science
The invention relates to a method for measuring the content of tannide in nakedflower beautyberry. The invention adopts a light splitting photometric method to measure the content of the tannide in the nakedflower beautyberry, further enhances the controllability of nakedflower beautyberry medicinal materials and preparations and ensures the quality of nakedflower beautyberry extracts.
Owner:HAINAN JIUZHITANG PHARMACY
Building method and mass detection method for fingerprint of total alkaloid components of cortex mori radicis medicinal material or cortex mori radicis extract
ActiveCN102890125AImprove quality control standardsGood stabilityComponent separationHigh-performance liquid chromatographyMedicine
The invention provides a building method and a mass detection method for a fingerprint of the total alkaloid components of a cortex mori radicis medicinal material or a cortex mori radicis extract. The building method comprises the following steps of: (1) preparing a cortex mori radicis medicinal material solution or a cortex mori radicis extract solution; and (b) using a high performance liquid chromatography to detect either the fingerprint of the total alkaloid components of the cortex mori radicis medicinal material or the fingerprint of the total alkaloid components of the cortex mori radicis extract. Either the fingerprint of the total alkaloid components of the cortex mori radicis medicinal material or the fingerprint of the total alkaloid components of the cortex mori radicis extract is obtained by the building process, thereby comprehensively reflecting the total alkaloid active components in the cortex mori radicis medicinal material. Therefore, the mass control standards of the cortex mori radicis medicinal material and the cortex mori radicis extract are improved, and the building method of the fingerprint provided by the invention has the advantages of good stability and good reproducibility. The mass problem of the existing cortex mori radicis medicinal material can be accurately adjusted in the market by comparing with the fingerprints.
Owner:BEIJING WBL PEKING UNIV BIOTECH
Method for detecting contents of baicalin, forsythin, indirubin and glycyrrhizic acid in Qingreling granules
ActiveCN103698422AImprove quality control standardsComponent separationLicorice acidGLYCYRRHIZA EXTRACT
The invention discloses a method for detecting contents of baicalin, forsythin, indirubin and glycyrrhizic acid in Qingreling granules. The method comprises the following steps: pretreating the Qingreling granules to prepare a sample to be detected, and injecting the sample to be detected to a high performance liquid chromatograph for detecting to obtain a high performance liquid chromatogram of the sample to be detected; and calculating the contents of the baicalin, the forsythin, the indirubin and the glycyrrhizic acid in the sample to be detected according to the peak areas of corresponding absorption peaks in the high performance liquid chromatogram of the sample to be detected and a standard curve of each standard sample. The method disclosed by the invention can be used for measuring the components of the baicalin under the content determination items of the Qingreling granules in the first part of Pharmacopoeia of the People's Republic of China published in 2010, and also can be used for additionally measuring the content of the forsythin in fructus forsythiae, the content of the indirubin in folium isatidis and the content of the glycyrrhizic acid in liquorice and greatly improving the quality control standard of the Qingreling granules. Proved through content measurement methodology, the method completely conforms to the specification and is an advanced quality control method. The method can be used for shortening the detection time and reducing the detection cost.
Owner:CHIATAI QINGCHUNBAO PHARMA
Mass detection method for motherwort grains
InactiveCN103245753AImprove quality control standardsHigh level of quality standardsComponent separationAngelica Sinensis RootThin layer chromatographic
The invention provides a mass detection method for motherwort grains. The mass detection method comprises the steps of identifying, and measuring the content of motherwort, namely (1) carrying out a chromatograph condition and system suitability test, (2) preparing a reference solution, (3) preparing a test solution, and (4) measuring to ensure that the motherwort content per bag is not less than 10.0mg based on stachydrine hydrochloride (C7H13NO2.HCl). With the adoption of the mass detection method for the motherwort grains provided by the invention, the mass control standard of the motherwort grains is improved, a content determination detection index and a detection method of a main drug, namely motherwort, in a preparation are established, and a thin-layer chromatography qualitative identification method for angelica sinensis and ligusticum wallichii is added, so that the high mass standard level of the compound preparation is guaranteed.
Owner:KUNMING CHINESE MEDICINE FACTORY
Parametric modeling method and system for steel structure bridge model based on BIM technology
InactiveCN108804770AAchieve standardizationImprove pictorial thinkingGeometric CADSpecial data processing applicationsModel parametersData format
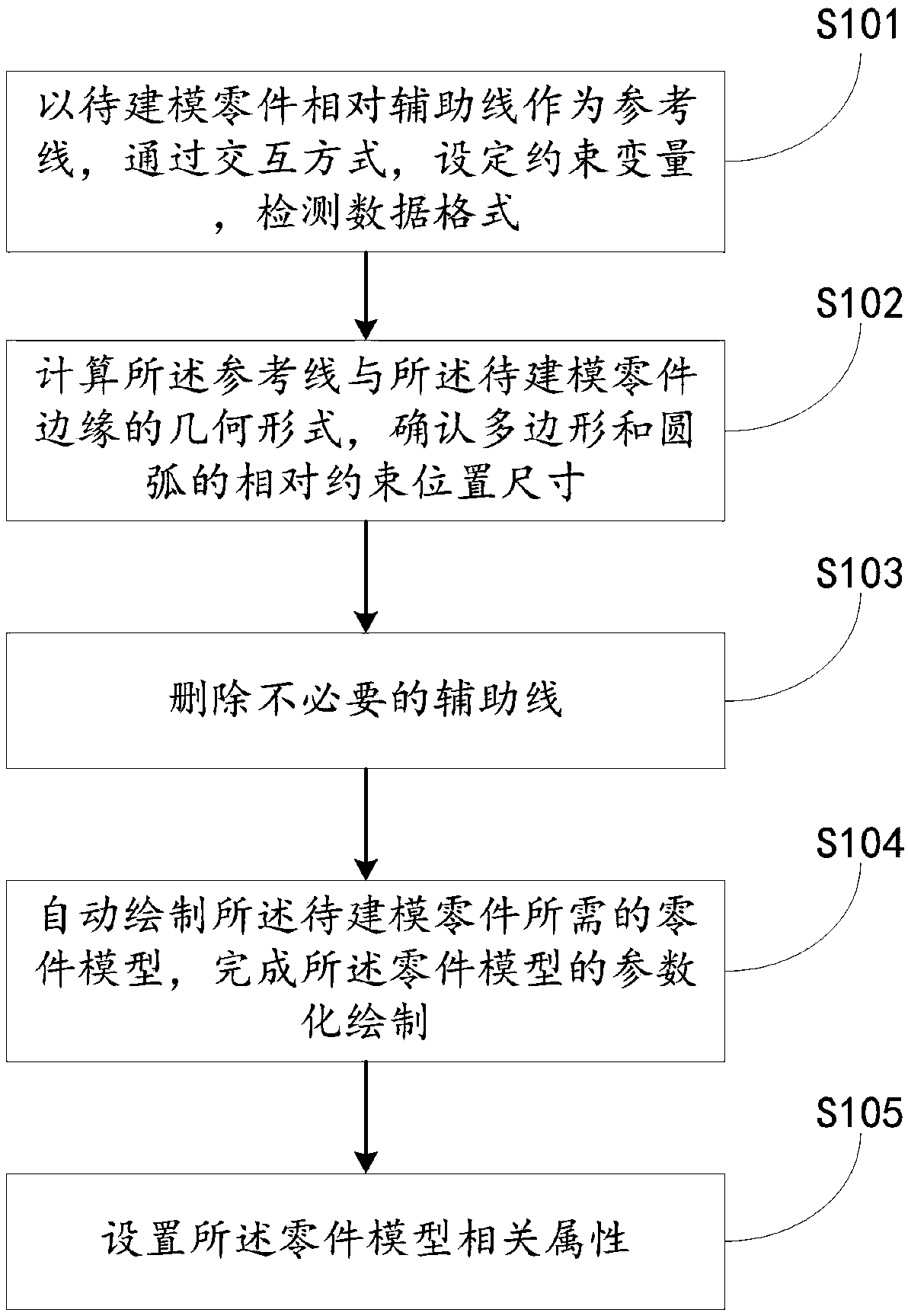
The invention discloses a parametric modeling method and system for a steel structure bridge model based on BIM technology. The parametric modeling method for the steel structure bridge model based onthe BIM technology comprises the following steps: setting a constraint variable and checking the data format through an interaction mode with the relative auxiliary line of a part to be modeled as the reference line; calculating the geometric form of the reference line and the edge of the part to be modeled, and confirming the relative constrained position size of a polygon and an arc; deleting unnecessary auxiliary lines; automatically drawing the part model required for the part to be modeled, and completing the parametric drawing of the part model; and setting the relevant properties of the part model. The parametric modeling method for the steel structure bridge model based on the BIM technology provided by the application can be used to develop a parametric design software for the steel bridge model in the field of deepening detailed drawings of steel structure bridges; and the refined parametric drawings method is adopted, the original drawing thinking is improved, the efficientdesign algorithm and interactive management and control mode are used, therefore, and the project design and manufacturing cycle is shortened.
Owner:CHINA RAILWAY SHANQIAO GRP CO LTD
Method for testing quality of Nguyen supernatant pills
ActiveCN101773560AImprove quality control standardsHigh level of quality standardsInorganic boron active ingredientsHydroxy compound active ingredientsMatrineChemical reaction
The invention relates to a method for testing the quality of Nguyen supernatant pills, which are described in the 20th volume of 'Drug Standard of Ministry of Public Health of the People's Republic of China' (Chinese medicament formulation preparations). The method comprises microscopic identification method of catechu and caper, physical and chemical reactions, thin-layer chromatography identification method of matrine, thin-layer chromatography identification method of borneol, thin-layer chromatography identification method of the catechu and content measurement method of the catechu in the Nguyen supernatant pills. The method for testing the quality of the Nguyen supernatant pills improves the quality control standard of the Nguyen supernatant pills, creates the content measurement and testing index and a testing method of the catechu which is a main medicament of the preparation, deletes physical and chemical reaction identification without specificity, increases the microscopical identification method of the catechu and the capers and the thin-layer chromatography identification method of the borneol, the catechu and the matrine, and ensures high quality control level of the compound preparation.
Owner:KUNMING CHINESE MEDICINE FACTORY
Detection method for lung-soothing syrup
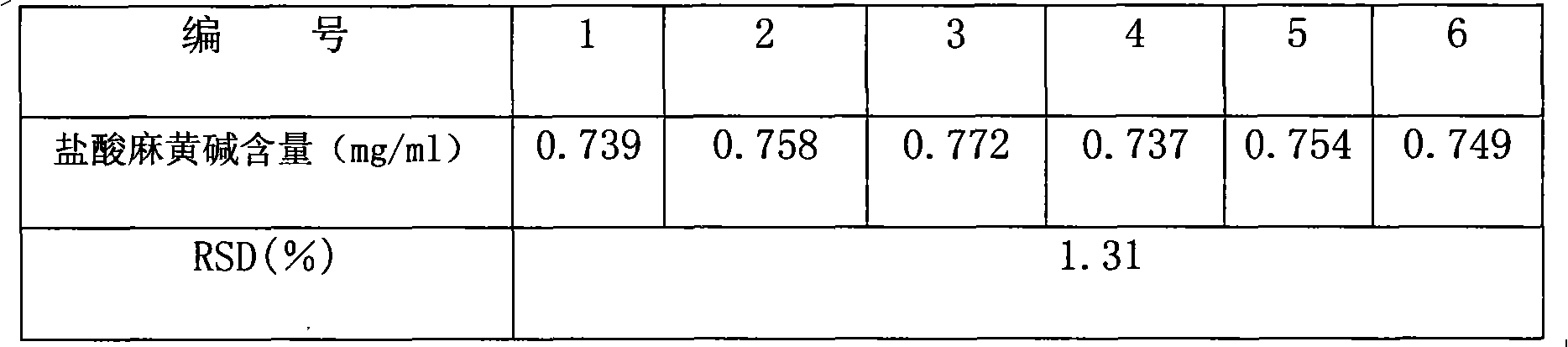
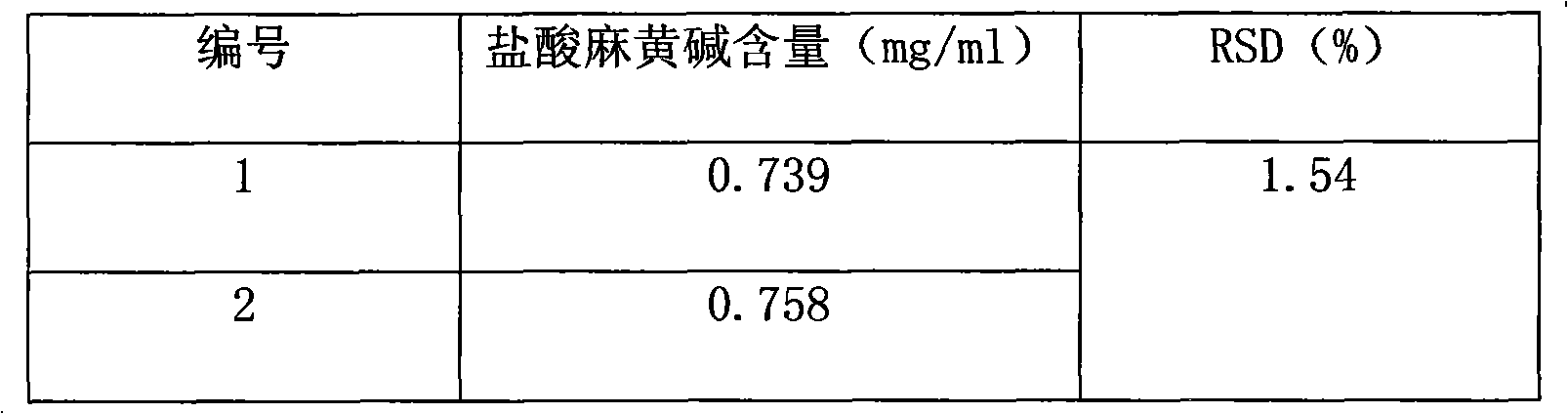
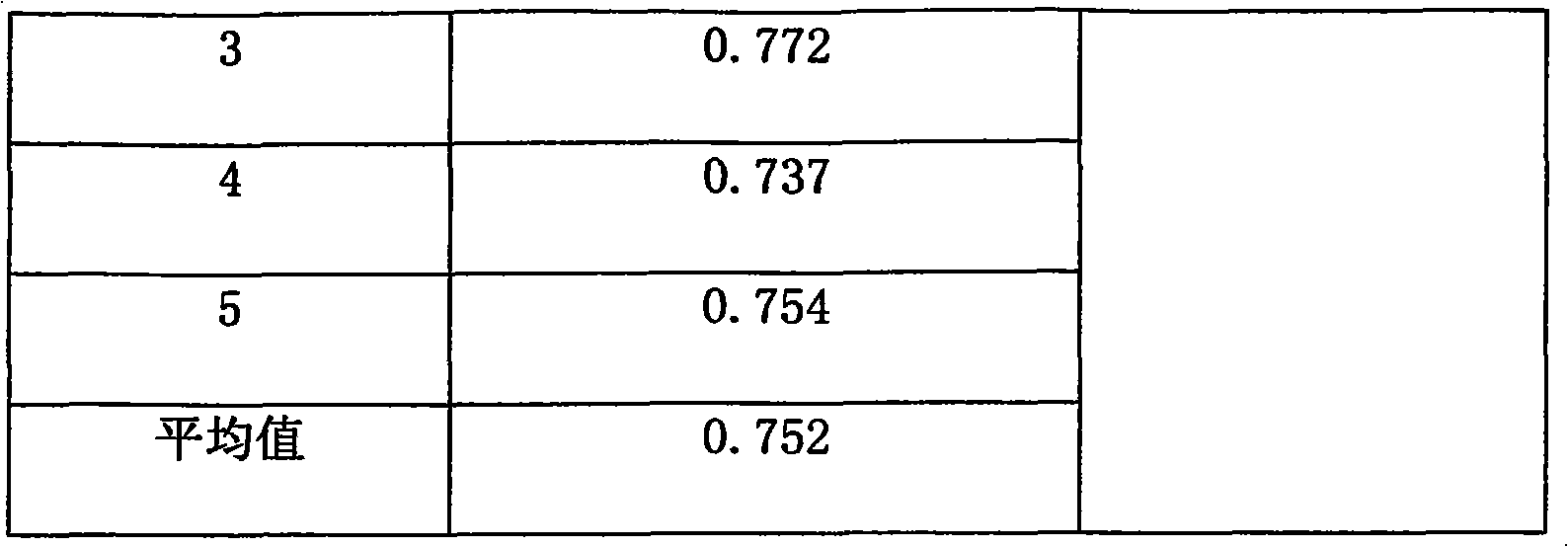
ActiveCN101961472AImprove quality control standardsProduction controlOrganic active ingredientsComponent separationClinical efficacyQuality control
The invention provides a quality detection method for lung-soothing syrup. The method comprises part or all of properties, inspection, identification and content measurement items, wherein the properties need to satisfy relevant regulations under Section Syrup; in the inspection, the syrup needs to conform to the relevant regulations in Section Syrup, Appendix IH, Part 1, Chinese Pharmacopedia (2005 Edition); in the component identification, ammonium chloride and licorice are identified; and in the content measurement, the content of ephedrine hydrochloride and the content of ammonium chloride are measured. The quality detection method has the advantages of high precision, high stability, favorable reproducibility, high recovery rate and accurate measurement result, enhances the quality control standard of lung-soothing syrup, and can effectively control illegal manufacturers from producing fake and poor-quality lung-soothing syrup, thereby ensuring the clinical curative effect of the preparation.
Owner:九寨沟天然药业股份有限公司
Granule contg. Radix astragalic, donkey-hide gelatin, rattletop and others for increasing leucocyte and its prepn. method
InactiveCN1537575AImprove quality control standardsQuantitative increaseUnknown materialsGranular deliveryAlcoholMedicine
An improved Chinese medicine 'Qijiao Shengbai granule' for treating dizziness, night sweat, leukocytopenia, etc is prepared through extracting in alcohol and decocting for part of raw materials, direct decocting for part of raw materials, collecting volatile oil and decocting for part of raw materials, pulverizing the rest, granulating, adding additive and volatile oil, and granulating again. Its advantages are high curative effect and high safety.
Owner:毛友昌
Method for detecting salvia heart-soothing capsules
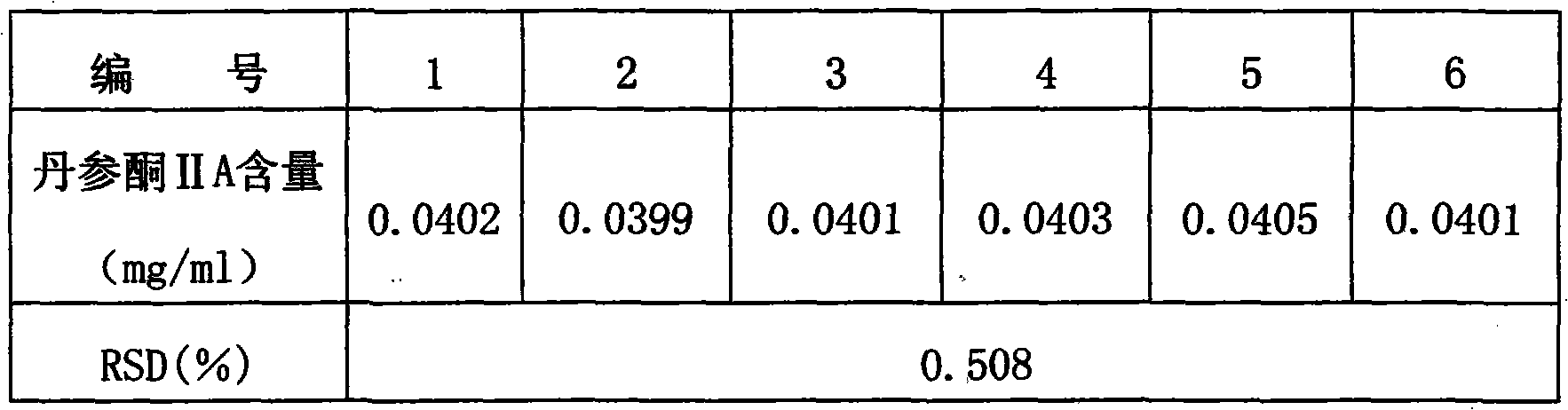
InactiveCN101982189AImprove quality control standardsGuaranteed clinical efficacyComponent separationCapsule deliveryClinical efficacySalvianolic acid B
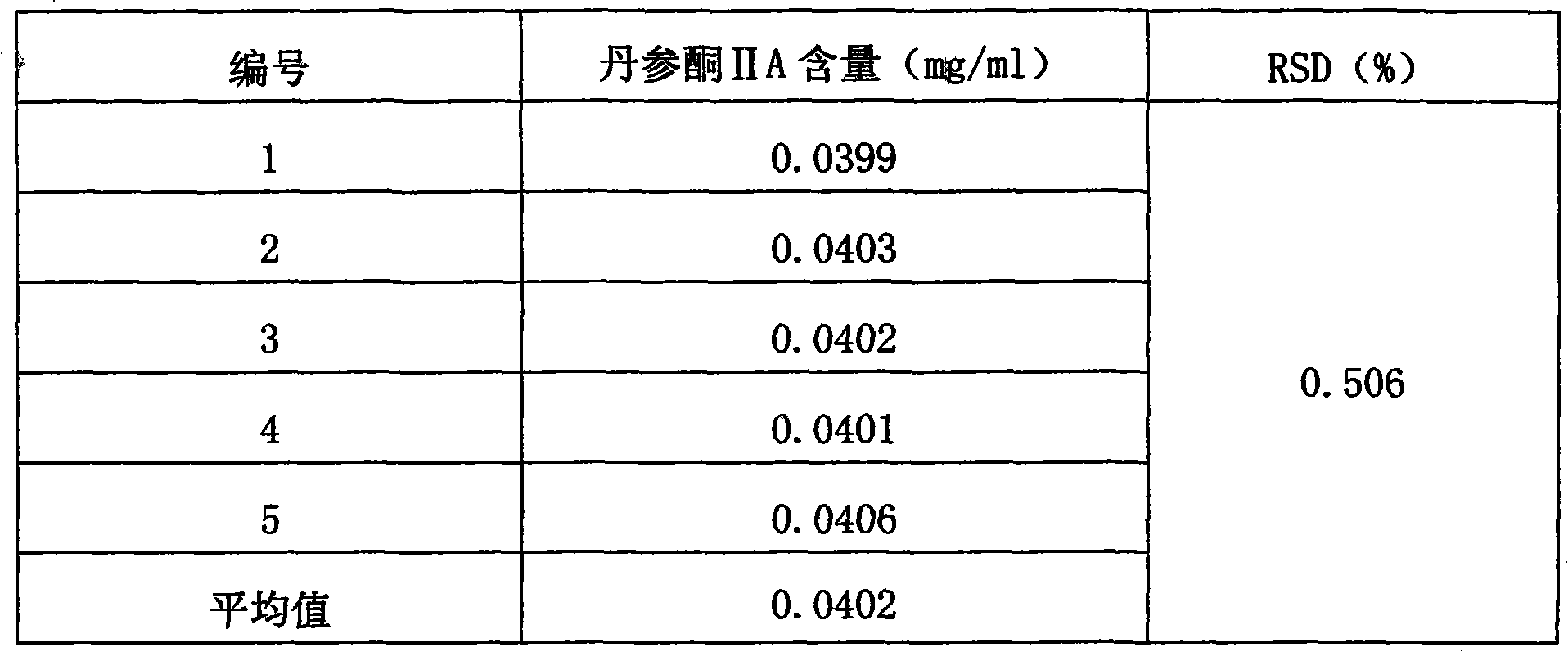
The invention provides a method for detecting the quality of salvia heart-soothing capsules. The method comprises all or partial items of character detection, identification, inspection and content measurement, wherein the character is capsules which are filled with dark brown powder, and have mild and subastringent taste; the identification means to use salvia contrast medicament as contrast, and a thin-layer chromatography method is adopted for salvia qualitative identification; the inspection needs to accord with regulations related to capsule item in the appendix IL of Chinese pharmacopoeia 2010 (vol.1); and the content measurement is to detect the content of main components tanshinone IIA and salvianolic acid B of the capsules by adopting high-efficiency liquid chromatography method. The quality detecting method has the advantages of strong specificity, high accuracy, good stability, good reproducibility, high reclamation rate, accurate measurement and simpleness and convenience, measures the contents of water-soluble ingredients and ethanol soluble ingredients of the capsules, improves the quality control standard of the salvia heart-soothing capsules, and can effectively control fake salvia heart-soothing capsule production of illegal manufacturers or manufacturers with low level of process control technology so as to ensure the clinical curative effect of the preparation.
Owner:SICHUAN FENGCHUN PHARMA
Method for controlling quality of Xingsu cough syrup
InactiveCN103175937AImprove quality control standardsAvoid product qualityComponent separationClinical efficacyBULK ACTIVE INGREDIENT
The invention provides a method for controlling the quality of Xingsu cough syrup. The method mainly comprises part or all of identification and content determination items, wherein the identification comprises thin-layer chromatography identification of perilla leaves, balloon flower, ephedra, thunberg fritillary bulb and baikal skullcap root; and the content determination means to determine the content of the main active ingredient amygdalin of bitter almonds in a preparation by adopting a high-performance liquid chromatography, and method validation is carried out, so that a reliable and accurate quality control method with strong specificity is established. By adopting the method, the quality control standard of the Xingsu cough syrup is improved, the quality of a product can be effectively controlled, and then the clinical curative effect of the product is definitely ensured.
Owner:蚌埠市第三人民医院
Method for detecting Jinze coronary disease capsules
InactiveCN101979027AImprove quality control standardsEffectively control productionComponent separationMetabolism disorderClinical efficacyAdditive ingredient
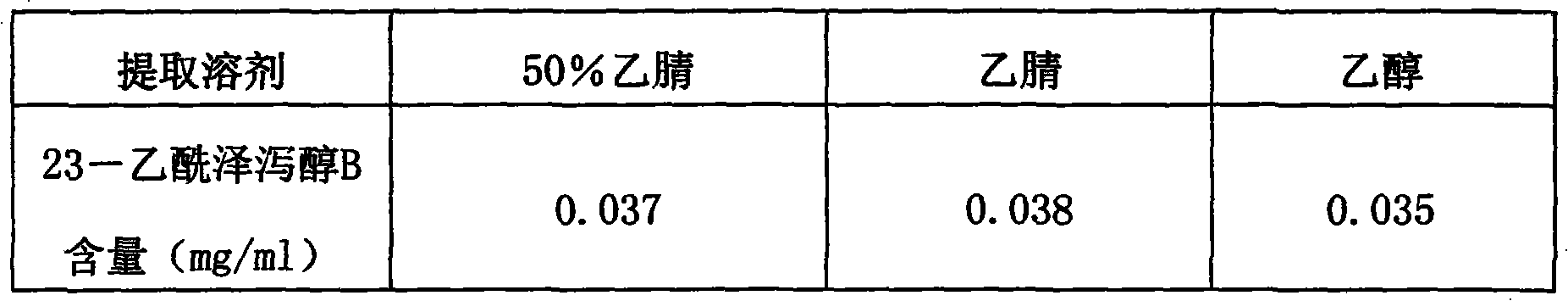
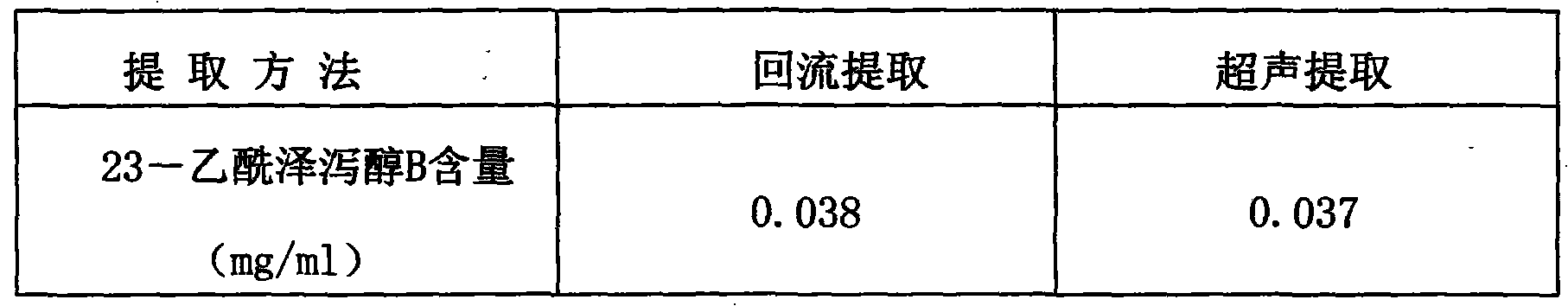
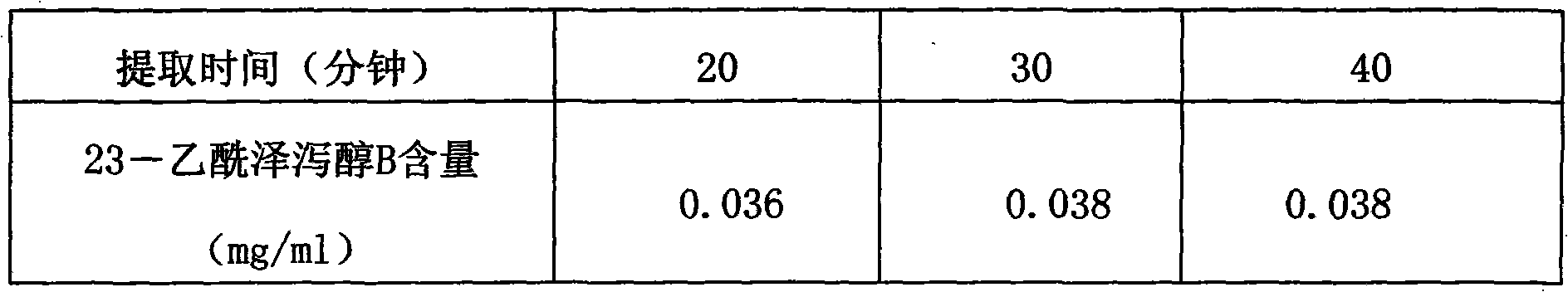
The invention provides a method for detecting the quality of Jinze coronary disease capsules. The method comprises part or all items including character, identification, inspection and content determination, wherein the character is required to comply with related regulations on capsules; the ingredient identification is to identify rhizoma alismatis and / or hemsleya amabilis; the inspection is required to comply with all regulations on capsule of A appendix interpretative language (IL) of 'Chinese Pharmacopoeia' of the 2010 version; and the content determination is to determine the alisol B 23-acetate content of the rhizoma alismatis. The quality detection method in the invention has high specificity, high precision, high stability, high repeatability, a high recovery rate and an accurate measuring result, is simple and convenient, improves the quality control standards of the Jinze coronary disease capsules, and can effectively prevent illegal manufacturers from producing the fake Jinze coronary disease capsules and therefore guarantee the clinic curing effects of the preparation.
Owner:SICHUAN FENGCHUN PHARMA
Bacteriostatic component extracted from ageratum conyzoides and extraction method thereof
InactiveCN101906121AImprove quality control standardsAntibacterial agentsOrganic active ingredientsAdditive ingredientAgeratum conyzoides
The invention discloses a bacteriostatic effective component isorhamcomin-3-O-glucoside of ageratum conyzoides and an extraction method thereof. The isorhamcomin-3-O-glucoside belongs to flavonoid glycoside compounds, has the bacteriostatic function and can be used as a contrast substance for measuring the content of the ageratum conyzoides. The invention lays the foundation for developing a novel product of an ageratum conyzoides preparation aiming at improving the quality control standard of the medicinal material of the ageratum conyzoides.
Owner:福建省医学科学研究所
Traditional Chinese medicine preparation-Liuwei Anxiao Pills and preparing method thereof
InactiveCN102526574AEasy to carryEasy to takeDigestive systemAluminium/calcium/magnesium active ingredientsEngineeringDrug efficiency
The invention relates to a traditional Chinese medicine preparation-Liuwei Anxiao Pills and a preparing method of the traditional Chinese medicine preparation, belonging to the field of traditional Chinese medicines. The traditional Chinese medicine preparation is characterized by comprising the following components in weight percent: 40-55 parts of Tibet inula, 160-200 parts of rhubarb, 80-100 parts of Kaempferia galangal, 200-250 parts of Gypsum Gypsum Rubrum, 110-150 parts of myrobalan and 250-300 parts of Tronae. In the preparing method, on the basis of the traditional process, the original single crushing pattern is changed, a sterilization process is changed, i.e. the original moist heat sterilization process for medicine powder produced after medicinal slices are crushed is changed into a cobalt 60 irradiation process, and the pattern of collecting and coating volatile oil is changed. The preparing method sufficiently ensures that volatile components in the Tibet inula are not lost and oily components in the Tibet inula are not lost. In addition, the drug effect is more effectively ensured, the product name quality is ensured, and benefits of patients are guaranteed.
Owner:江西青春康源制药有限公司
Method for detecting quality of lung clearing and phlegm eliminating pill
ActiveCN101732552AImprove quality control standardsHigh level of quality standardsComponent separationPill deliveryPublic healthMedicine
The invention relates to a method for detecting quality of a lung clearing and phlegm eliminating pill in Volume 12 of Drug Standard of Ministry of Public Health of Peoples Republic of China (traditional medicine prescribed preparation). The method for detecting quality of a lung clearing and phlegm eliminating pill comprises microscopic identification, a thin layer chromatography identification method for orange peel and bitter orange, a thin layer chromatography identification method for ephedrine hydrochloride in herba ephedrae, a thin layer chromatography qualitative identification method for balloon flower, and scutelloside content measurement. The invention enhances the quality control standard of the lung clearing and phlegm eliminating pill, establishes content measuring and detecting indexes in main medicines of preparation and a detecting method thereof, increases the thin layer chromatography qualitative identification methods for orange peel, bitter orange, ephedrine hydrochloride and balloon flower, and ensures the higher quality standard levels of the compound preparation.
Owner:KUNMING CHINESE MEDICINE FACTORY
Method for detecting quality of blood fat recovery capsule
ActiveCN1987449AImprove quality control standardsGuaranteed efficacyComponent separationPhosphoric acidGradient elution
The method includes following steps: using octadecylsilane chemically bonded silica as filler; using acetonitrile as mobile phase A, and 0.05% phosphoric acid as mobile phase B to carry out gradient elution; under condition of detection wavelength as 256nm, column temperature as room temperature, velocity of flow as 1.0ml / min, weighing up reference substance 1mg of rovastatin in 5ml volumetric flask, and adding constant volume of acetonitrile to scale so as to obtain solution of reference substance; taking content substance 0.3g of Xuezhikang capsule, adding acetonitrile 2ml, carrying out supersound extraction for 10-20min, using 0.45 micros microporous membrane to carry out filtering operation so as to obtain sample solution; mensuration according to high performance liquid chromatography; fingerprint of sample solution should possess good comparability to the referenced fingerprint, degree of similarity larger than 0.90. Features are: simple operation and good reproducibility.
Owner:BEIJING WBL PEKING UNIV BIOTECH
Honghua Qili tablet contg. safflower carthamus, and its prepn. method
InactiveCN1537580AOvercome the inaccurate dosage and the inconvenience of takingRaise quality standardsAntipyreticAnalgesicsInjury causeMedicinal herbs
An improved Chinese medicine 'Honghua Qili tablets' for treating traumatic injury, ecchymoma and traumatic hemorrhage is prepared through superfine pulverizing of cinnabar, proportionally mixing it with others, and tabletting. Its advantages are high curative effect and high safety.
Owner:毛友昌
Fingerprint spectrum determination method of ligusticum wallichii tea-blending granular preparation
ActiveCN102590423AImprove quality control standardsQuality control appliesComponent separationSolventFingerprint
The invention relates to a fingerprint spectrum determination method of a ligusticum wallichii tea-blending granular preparation, which comprises the following steps: (a) preparing ligusticum wallichii tea-blending preparation test sample: precisely weighing a proper quantity of ligusticum wallichii tea-blending preparation, adding a solvent for reflux extraction, centrifuging the extracting solution, evaporating and drying supernate, dissolving with water, extracting with n-butyl alcohol, collecting n-butyl alcohol extracting solution, evaporating and drying, dissolving with 70% methyl alcohol, and filtering by using a microfiltration membrane to obtain the test sample; (b) chromatograph condition: with octadecylsilane bonding silica gel as the chromatograph column of filler, carrying out gradient elution, wherein a flowing phase A is acetonitrile, the flowing phase B is 0.01-0.2% formic acid aqueous solution, and the detection wavelength is 230-280nm; and (c) determination: precisely adsorbing test sample solution, introducing in a liquid chromatograph, recording the chromatogram to finally obtain the characteristic fingerprint spectrum of the ligusticum wallichii tea-blending preparation. The HPLC (High Performance Liquid Chromatography) characteristic fingerprint spectrum analysis method for ligusticum wallichii tea-blending preparations, such as the granular preparation, is simple to operate, has the advantages of high precision and repeatability, and is suitable for controlling the quality of the ligusticum wallichii tea-blending preparations, such as the granular preparation.
Owner:GUIZHOU WARMEN PHARMA
Method for measuring content of ginsenoside in Aidi preparation
InactiveCN101614708AImprove quality control standardsHigh precisionComponent separationClinical efficacyCurative effect
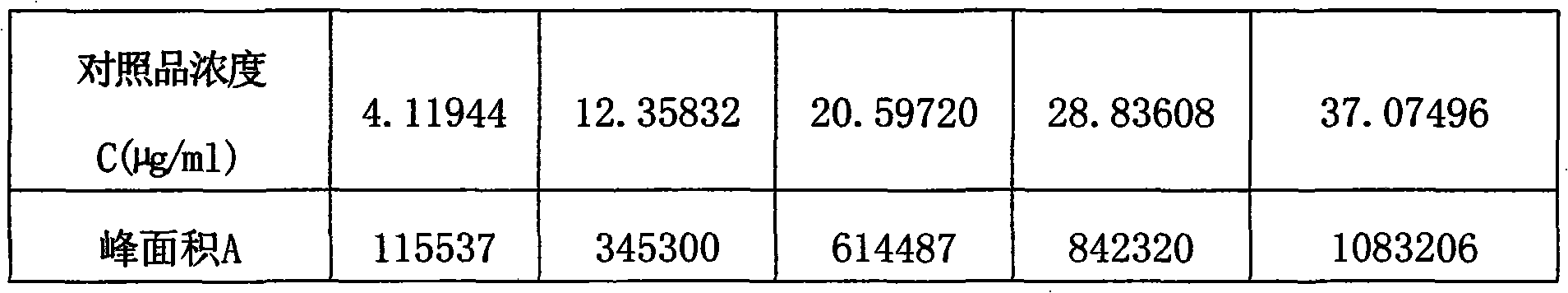
The invention provides a method for measuring content of ginsenoside in Aidi preparation, which is to measure the content of the effective component, namely the ginsenoside Rg1, Re and Rb1 contained in ginseing in the preparation by taking ginsenoside Rg1, Re and Rb1 comparison products as contrast. Compared with the prior art, the method adopts the high performance liquid chromatography for gradient elusion of two kinds of mobile phases, improves the method for measuring the content of the ginseng, has high precision, good repeatability, good stability, high recovery rate and accurate and reliable measurement result, improves the quality control standard of the Aidi preparation, and can effectively control product quality so as to ensure clinical efficacy of the Aidi preparation.
Owner:GUIZHOU YIBAI PHARMA CO LTD
Quality inspection method of cough pills
ActiveCN101732553AImprove quality control standardsHigh level of quality standardsInorganic boron active ingredientsComponent separationChemical reactionMagnolol
The invention relates to a quality inspection method of cough pills prescribed in Volume 11 of Drug Standard of Ministry of Public Health of the People's Republic of China (Chinese Traditional Patent Formulation Preparations). The quality inspection method comprises microscopic identification of cough pills, a thin-layer chromatography identification method of ephedrine hydrochloride, magnolol and dried orange peel, a thin-layer chromatography qualitative identification method of bitter orange and a content determination method of ephedrine hydrochloride in the cough pills. The quality inspection method raises the quality control standards of cough pills and establishes content determination inspection indexes of main drugs in a preparation and a inspection method thereof; and meanwhile, the quality inspection method deletes physical and chemical reaction identification of poor specialization and adds the thin-layer chromatography qualitative identification method of ephedrine hydrochloride, magnolol, dried orange peel and bitter orange, thereby ensuring a higher quality standard level of compound preparations of cough pills.
Owner:KUNMING CHINESE MEDICINE FACTORY
Quality control method of yinzhihuang preparation
InactiveCN101264148AGood dispersionShort disintegration timeComponent separationDigestive systemClinical efficacyQuality control
The invention provides a quality control method for anti-hepatitis-jaundice preparation, the anti-hepatitis-jaundice preparation is made of artemisia capillaris extraction, gardenia extraction, and baicalin and honeysuckle extraction; compared with the prior art, the quality control method has the advantages of high precision, good reproducibility, high recovery, improvement of the quality control standard of the anti-hepatitis-jaundice preparation, and ability to ensure the clinical effect of the preparation.
Owner:GUIZHOU YIBAI PHARMA CO LTD
Gastralgia dispersed tablet and preparation
InactiveCN101297869AImprove quality control standardsAdd Quantitative DeterminationDigestive systemPill deliveryDuodenal ulcerEructation
The invention discloses a dispersible gastralgia tablet and a preparation method thereof, the invention carries out the technological reform of the formulation based on the existing ordinary tablets, thus overcoming the shortcomings of heavier fishy smell, difficult administration, slow speed of disintegration and slow onset of action of the original formulation, enriching the types of the drugs, improving the quality standards and further meeting and ensuring the demands on the drugs by the people. The drug has the effects of activating qi-flowing, activating blood circulation, inhibiting acid and stopping pain, which is used for gastralgia, acid regurgitation, belching eructation and epigastric distension caused by qi-stagnancy and blood stasis, as well as gastric and duodenal ulcers and chronic gastritis with the symptoms. The efficacy is significant. The usage and carrying are convenient, which is a clinically popular, safe, stable and effective drug and an ideal product for the modern pharmaceutical enterprises.
Owner:福建省医学科学研究所
Qili dispersing tablet for treating traumatic injuries, and its prepn. method
InactiveCN1537572ARaise quality standardsImprove product qualityAntipyreticAnalgesicsInjury causeTraumatic Hemorrhage
A Chinese medicine 'injury Qili dispersable tablet' for treating the bone fracture, injury and traumatic hemorrhage is an improvement to existing same medicine in the form of tablet or powder. Its advantages are correct dosage, high curative effect and high safety.
Owner:毛友昌
Quality control method of Chinese medicine preparation for menstruction regulating and pain relieving
InactiveCN1985940AImprove quality control standardsAvoid product qualityComponent separationAntipyreticClinical efficacyThin layer chromatographic
The quality control method for menstruation regulating and pain relieving Chinese medicine preparation includes partial or all the items of character identification and content measurement. The identification includes the thin layer chromatographic identification of white peony root, licorice and corydalis tuber in the preparation; and the content measurement is to measure the content of white peony root and penoniflorin in the preparation. Compared with available technology, the present invention has increased thin layer chromatographic identification of white peony root, licorice and corydalis tuber and content measurement of penoniflorin, and possesses high precision, high reproducivity, high stability, high recovery rate, accurate measurement and capacity of raising the quality of menstruation regulating and pain relieving Chinese medicine preparation.
Owner:GUIZHOU YIBAI PHARMA CO LTD
Weibao pillets and their preparing method
InactiveCN1471950ARaise quality standardsImprove product qualityDigestive systemUnknown materialsCurative effectPeptic ulcer
A Chinese medicine "Weibao tablet" for treating indigestion, peptic ulcer, chronic gastritis, etc is prepared from 8 Chinese-medicinal materials through pulverizing, adding auxiliaries and tabletting. Its advantages are high curative effect and no by-effect.
Owner:毛友昌
Method for measuring content of components of prince's-feather herb
InactiveCN101822723AImprove quality control standardsGood precisionComponent separationAntipyreticLuteolinGlucoside
The invention provides a method for measuring content of components of prince's-feather herb and belongs to the technical field of medicament content measurement. High performance liquid chromatography is adopted to measure the content of eight compounds, namely catechin, homo-orientin, orientin, quercetin-3-O-(2'-O-alpha-rhamnose)-betal-glucal, texifolin, luteolin-7-O-beta-D-glucoside, quercetin and kaempferide-3-0-beta-3-D-glucoside, in the prince's-feather herb under the same chromatographic condition. Compared with the conventional single component content measuring method, the method of the invention is more integral, characteristic and stable, and provides test basis for further improving the quality of the prince's-feather herb and the re-development and application of the prince's-feather herb. The invention also provides the methods for preparing reference solution and test sample solution.
Owner:GUIYANG MEDICAL UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com