Method for detecting quality of blood fat recovery capsule
A quality inspection method, the technology of Xuezhikang Capsules, is applied in the direction of measuring devices, instruments, scientific instruments, etc., and can solve problems such as difficult to distinguish counterfeit products, endanger life safety, and content determination indicators cannot fully reflect and control the quality of preparations, etc., to improve Quality control standards, reproducible results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example
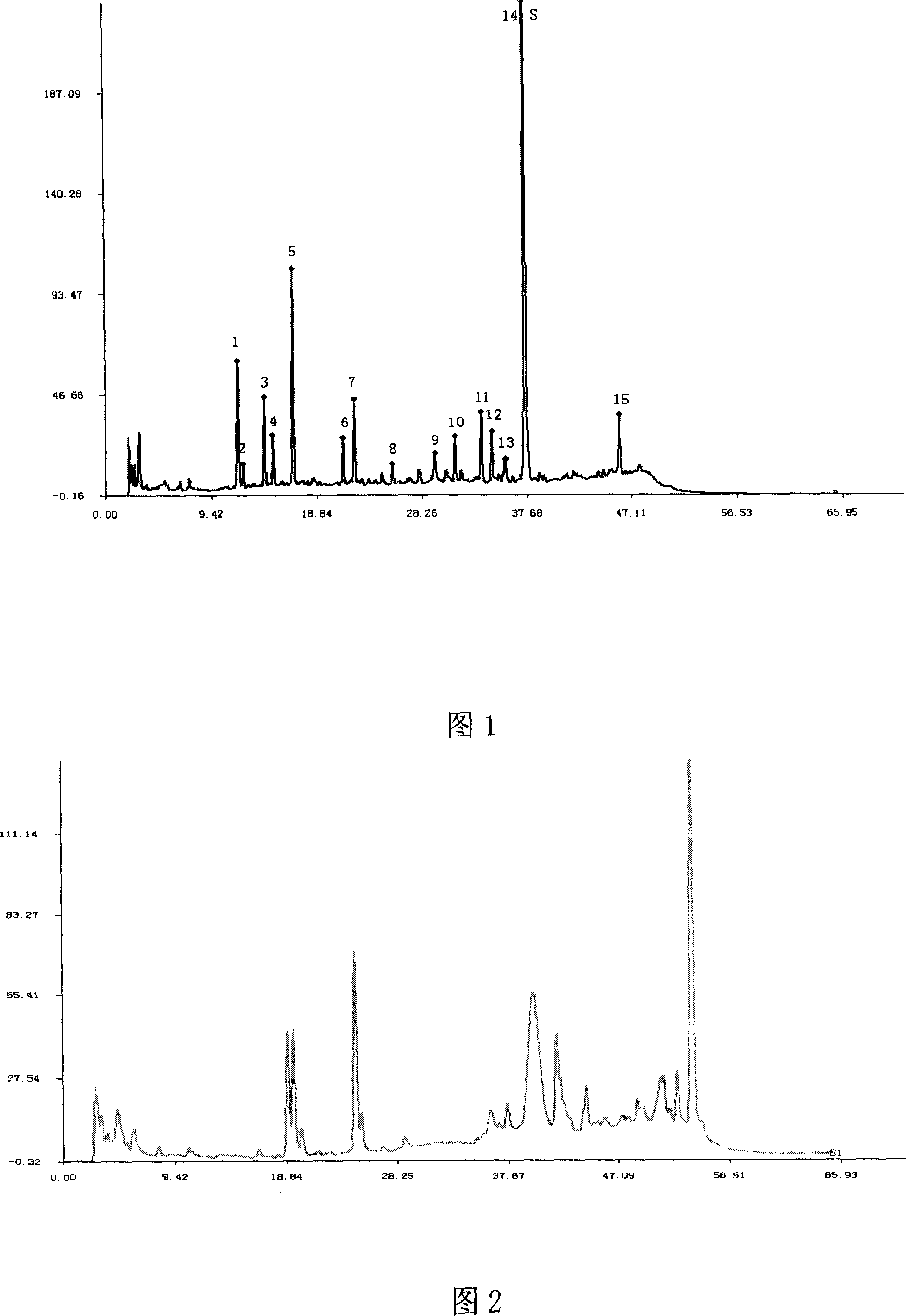
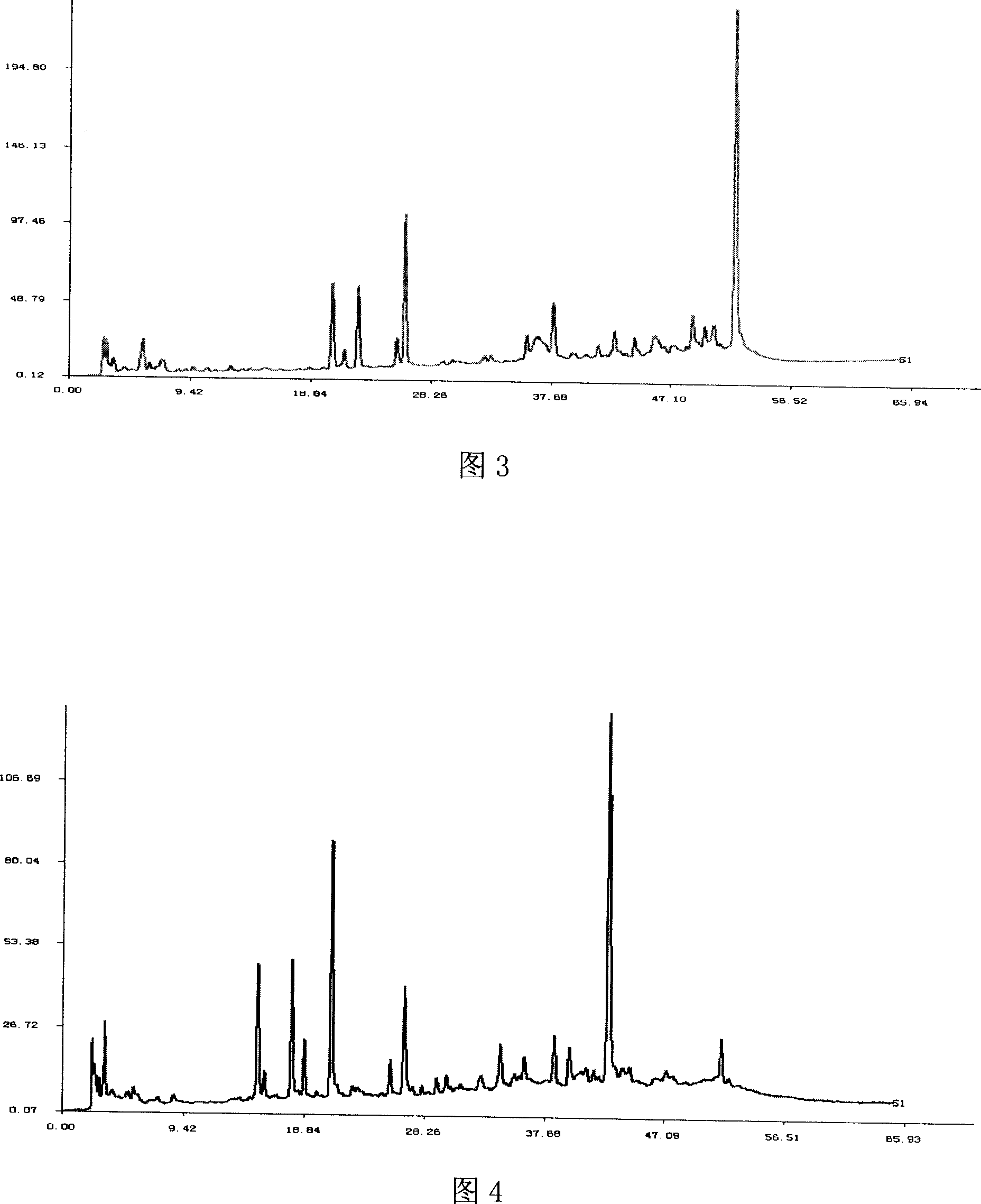
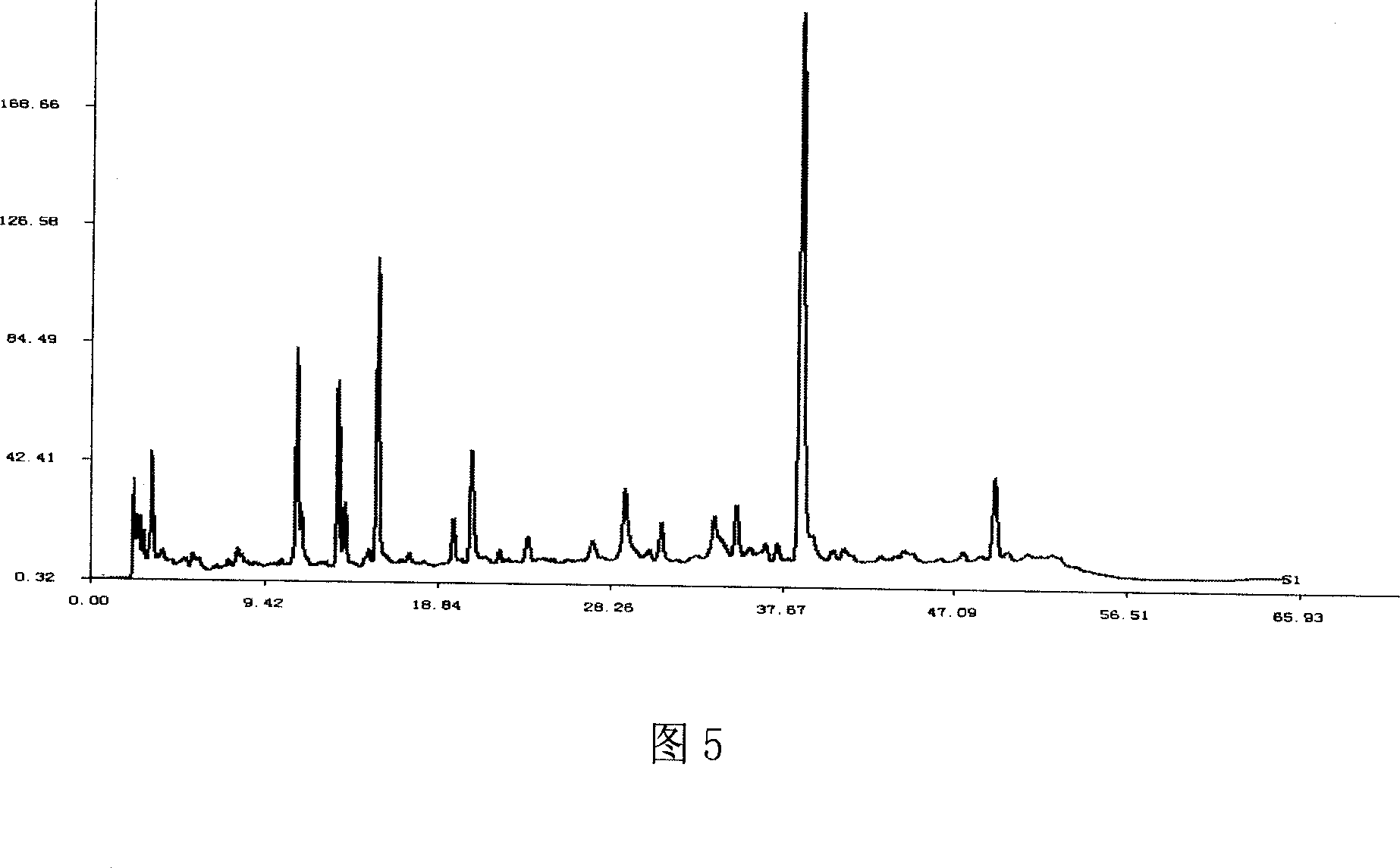
[0027] Experimental example Study on the quality detection method of Xuezhikang capsule fingerprint
[0028] 1. Preparation of the test solution
[0029] Xuezhikang capsule is the 75% ethanol extract of red yeast rice. Comparing the three extraction solvents of methanol, 75% ethanol and acetonitrile, the fingerprints of the three extracts are the same, but the color of the acetonitrile extract is much lighter. In the chromatogram, t R The dissolution of pigment components R The common fingerprint peaks > 10min are similar. In order to reduce column pollution and prolong column life, acetonitrile was used as the extraction solvent.
[0030] Compared with ultrasonic extraction and heating reflux extraction, the former method is simple and reproducible.
[0031] Comparing the chromatograms of ultrasonic extraction for 5min, 10min, and 20min, after 10min, the extraction rate of each substance in the fingerprint chromatogram did not change much, so the extraction time was determin...
Embodiment 1
[0055] Embodiment 1: Xuezhikang capsule quality detection method
[0056] Using fingerprint detection method:
[0057] Instrument used: Waters 600-996-717 high performance liquid chromatography (gradient lag time is 2.75min), Millennium 32 Chromatographic workstation; column: Apollo C 18 (4.6mm*250mm, 5μ) analytical column, TIANHE (P / N: TH0011, 5μ, C 18 ) guard column;
[0058] The method detects as follows:
[0059] Determine according to high performance liquid chromatography (Appendix VID of the Pharmacopoeia of the People's Republic of China), combined with fingerprint requirements;
[0060] Chromatographic conditions and system adaptability test, octadecylsilane bonded silica gel was used as filler; acetonitrile was used as mobile phase A, and 0.05% phosphoric acid was used as mobile phase B for gradient elution; detection wavelength was 256nm; column temperature was room temperature ;The flow rate is 1.0ml / min;
[0061] Preparation of the reference substance soluti...
Embodiment 2
[0065] Embodiment 2: Xuezhikang capsule quality detection method
[0066] Using fingerprint detection method:
[0067] Instrument used: Waters 600-996-717 high performance liquid chromatography (gradient lag time is 2.75min), Millennium 32 Chromatographic workstation; column: Apollo C 18 (4.6mm*250mm, 5μ) analytical column, TIANHE (P / N: TH0011, 5μ, C 18 ) guard column;
[0068] The method detects as follows:
[0069] Determine according to high performance liquid chromatography (Appendix VID of the Pharmacopoeia of the People's Republic of China), combined with fingerprint requirements;
[0070] Chromatographic conditions and system adaptability test, octadecylsilane bonded silica gel was used as filler; acetonitrile was used as mobile phase A, and 0.05% phosphoric acid was used as mobile phase B for gradient elution. The mobile phase gradient elution program was: 0 The volume ratio of mobile phase A increased linearly from 25% to 84% in ~45 minutes, and the volume ratio ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com