Gastralgia dispersed tablet and preparation
A dispersible tablet and prescription technology, applied in the field of Anwei dispersible tablet and its preparation, can solve problems such as difficulty in taking, unable to meet the medication needs of patients with acute epigastric pain, and slow disintegration speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1 Orthogonal experiment to optimize the granulation process of Anwei Dispersible Tablets
[0029] Orthogonal experimental design and results
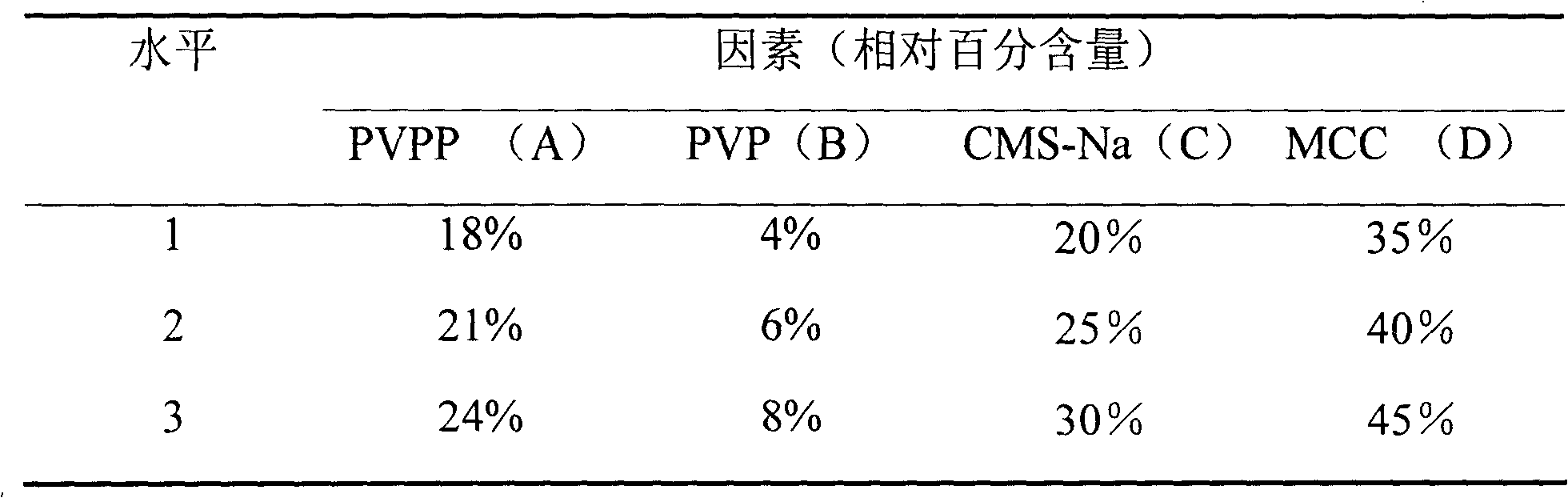
[0030] Investigate the four factors of the relative percentages of PVPP, PVP, CMS-Na and MCC in the used excipients (that is, the percentage of the excipients used in the total amount of excipients in the prescription), and use the dispersion uniformity as the investigation index to determine the amount of each excipient , the results are shown in Table 1 to Table 3 below.
[0031] Table 1 Experimental factor level table
[0032]
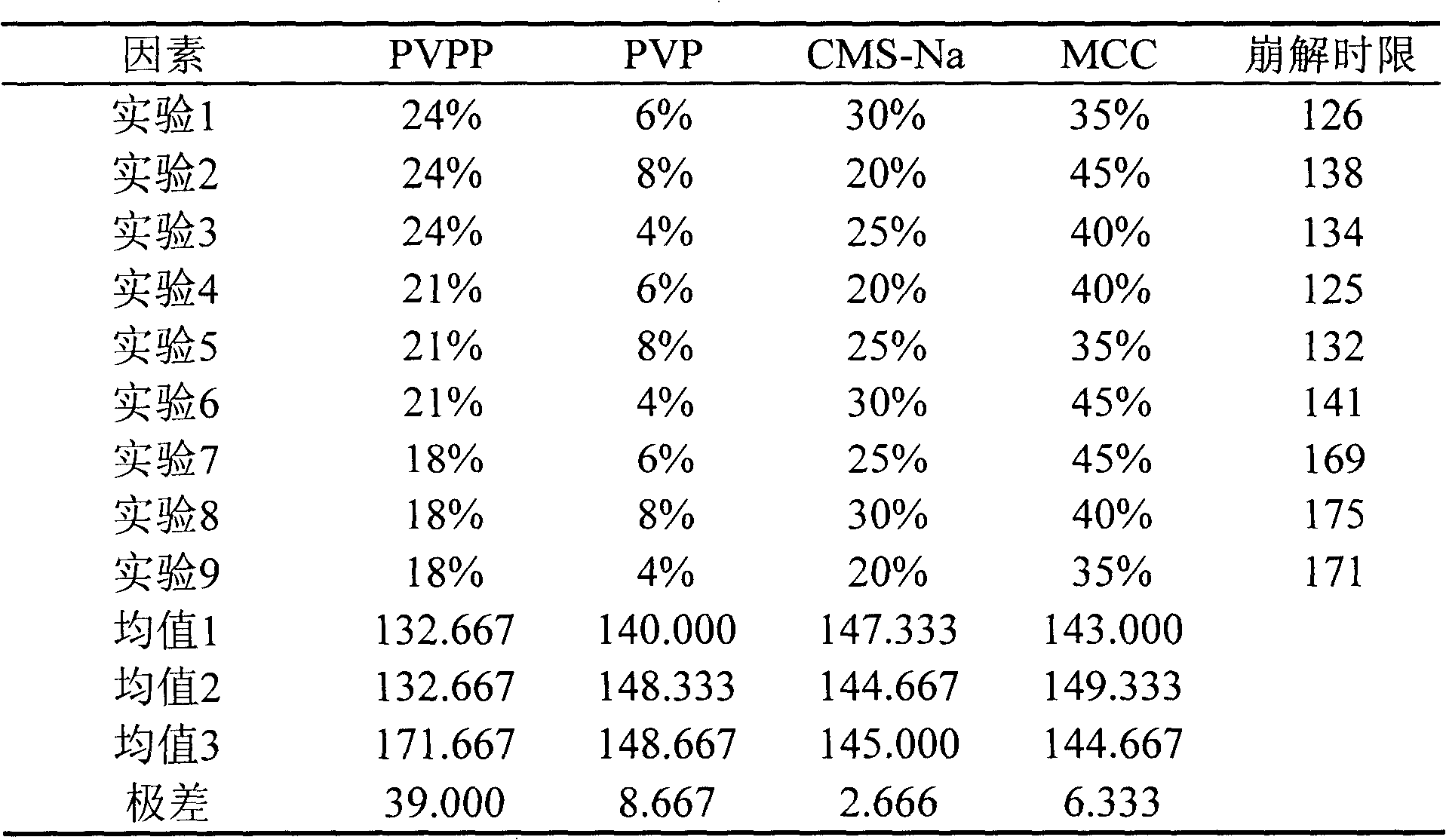
[0033] Table 2 Intuitive analysis results
[0034]
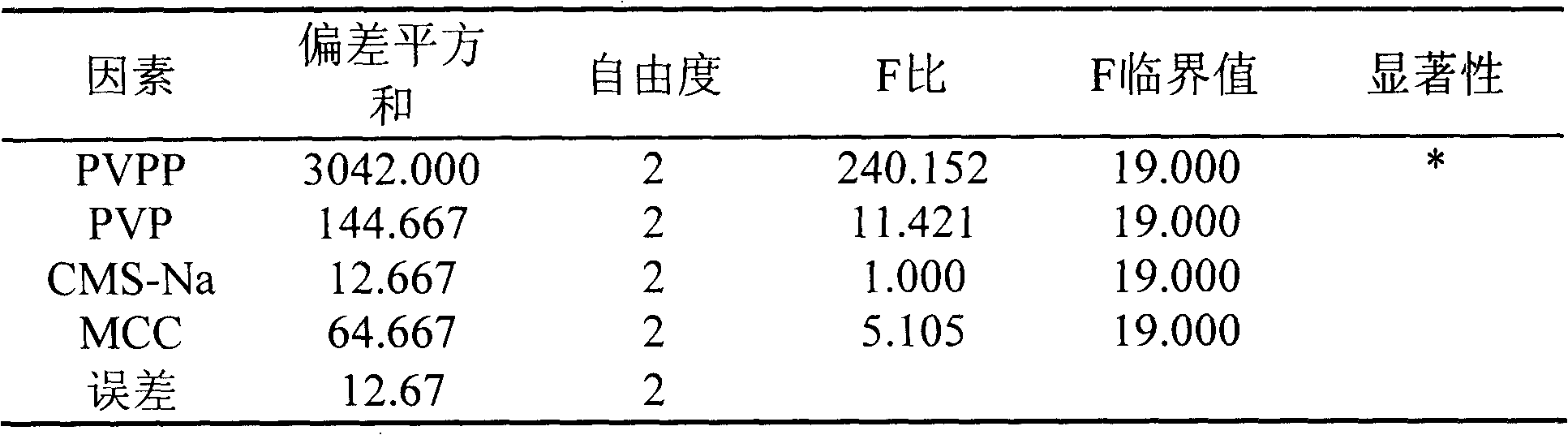
[0035] Table 3 ANOVA results
[0036]
[0037] *Indicates that the factor is significant.
[0038] From the above results, it can be seen that the four factors affecting the tablet granulation process are: PVPP>PVP>MCC>CMS-Na, the optimal granulation process conditions: A 2 B 1 C 2 D. 3 , namely PVPP21%, PVP6%, MCC40%, CMS-Na...
Embodiment 2
[0039] Example 2 The best implementation of this invention is: the three herbs in the prescription are tested and qualified respectively for future use; vinegar-made corydalis (10.50%), shelled sea octopus (41.67%), calcined alum (31.17%), and cross-linked Polyvinylpyrrolidone (3.50%), microcrystalline cellulose (6.67%), sodium lauryl sulfate (2.17%), sodium carboxymethyl starch (3.33%) are mixed evenly, with polyvinylpyrrolidone (1%) as the viscosity The mixture is used to prepare soft materials, granulated with a No. 2 sieve, dried at 60°C, sized with a No. 2 sieve, and compressed into tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com