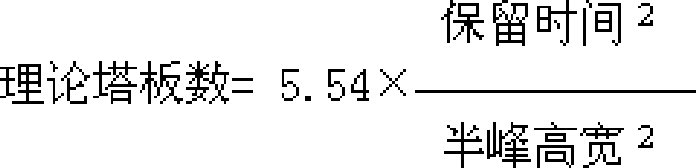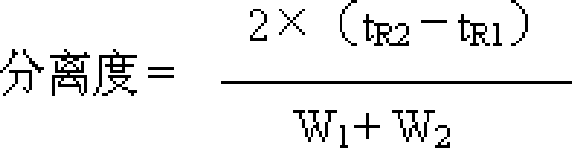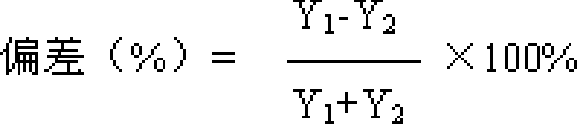Quality control method of Chinese medicine preparation for menstruction regulating and pain relieving
A quality control method and technology of traditional Chinese medicine preparations, applied in non-central analgesics, pharmaceutical formulas, medical preparations containing active ingredients, etc., can solve problems such as difficult product quality control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0222] Embodiment 1 of the present invention: the quality control method of Tongjingning capsules comprises:
[0223] Properties: Its content is light yellowish brown to brown granules, with little gas and slightly bitter taste.
[0224] Identification: (1) Take 10g of the contents of the capsule, add 50ml of ether, extract under reflux for 30 minutes, discard the ether solution, evaporate the ether from the dregs, add 50ml of ethanol, extract under reflux for 30 minutes, filter, evaporate the filtrate to dryness, add water to the residue 20ml dissolved, extracted twice with water-saturated n-butanol, 20ml each time, combined n-butanol solution, washed with n-butanol saturated water 20ml, dehydrated, n-butanol solution was evaporated to dryness, and the residue was dissolved in 1ml of ethanol, used as Test solution; take another paeoniflorin reference substance, add ethanol to make a solution containing 2mg per 1ml, as the reference solution; according to the Chinese Pharmacop...
Embodiment 2
[0234] Embodiment 2 of the present invention: the quality control method of Tongjingning granule comprises:
[0235] Properties: The product is light yellow to yellow-brown granules; sweet, slightly bitter or slightly bitter.
[0236] Identification: (1) Take 20g of granules, add 80ml of ether, reflux extraction for 40 minutes, discard the ether solution, evaporate the ether from the dregs, add 80ml of ethanol, reflux extraction for 40 minutes, filter, evaporate the filtrate to dryness, add 25ml of water to dissolve the residue , extracted 3 times with water-saturated n-butanol, 30ml each time, combined n-butanol solution, washed with n-butanol saturated water 30ml, dehydrated, n-butanol solution was evaporated to dryness, and the residue was dissolved in 2ml ethanol as the test sample solution; take another paeoniflorin reference substance, add ethanol to make a solution containing 3mg per 1ml, as the reference substance solution; according to the Chinese Pharmacopoeia TLC te...
Embodiment 3
[0246]Embodiment 3 of the present invention: the quality control method of Tongjingning tablet comprises:
[0247] Properties: Light yellow-brown to tan after removing the coating; slight gas, slightly bitter taste.
[0248] Identification: (1) Take 10g of the tablet after removing the coating, add 120ml of ether, extract by reflux for 50 minutes, discard the ether solution, evaporate the ether from the dregs, add 120ml of ethanol, extract by reflux for 50 minutes, filter, and evaporate the filtrate to dryness , the residue was dissolved in 30ml of water, extracted 4 times with water-saturated n-butanol, 40ml each time, the n-butanol solution was combined, washed with n-butanol saturated water 40ml, the water was removed, the n-butanol solution was evaporated to dryness, and the residue was dissolved in 4ml of ethanol , as the test solution; take another paeoniflorin reference substance, add ethanol to make a solution containing 4mg per 1ml, as the reference substance solution...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com