Method for detecting contents of baicalin, forsythin, indirubin and glycyrrhizic acid in Qingreling granules
A technology of baicalin and Qingreling, which is applied in the field of content determination of traditional Chinese medicine preparations, can solve problems such as difficult to reflect the quality of medicines, and achieve the effects of saving time and testing costs, improving quality control standards, and controlling quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
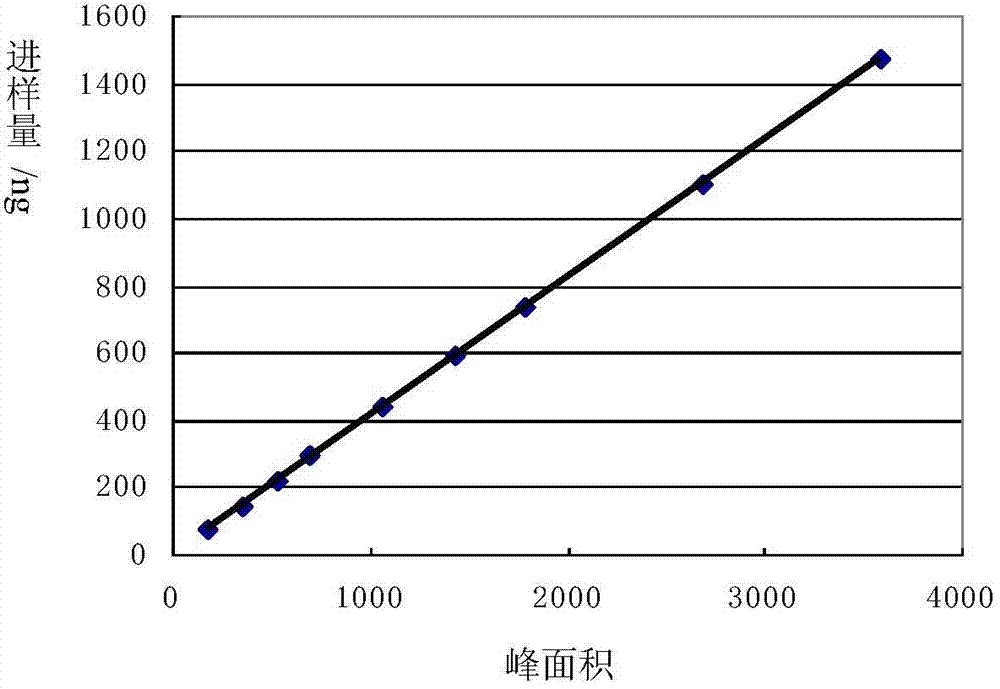
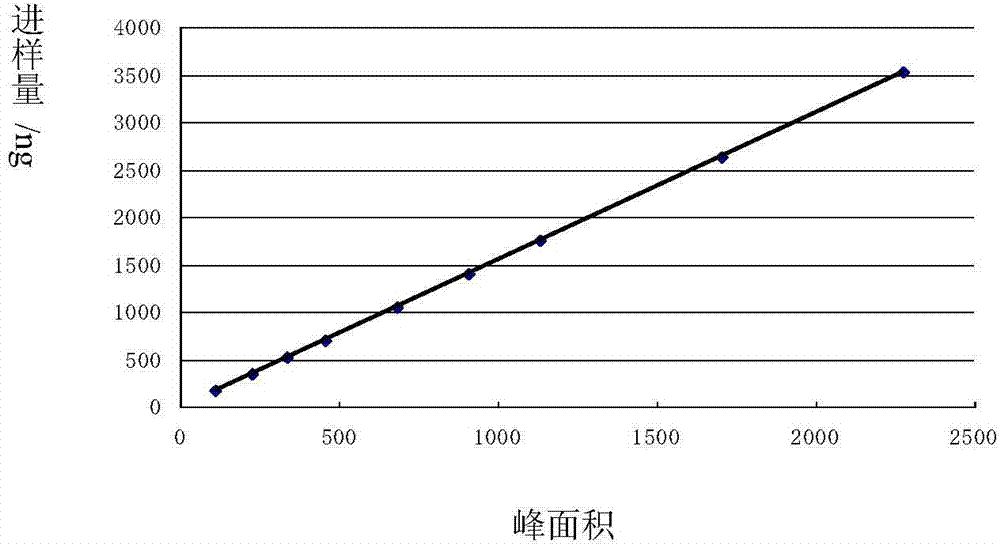
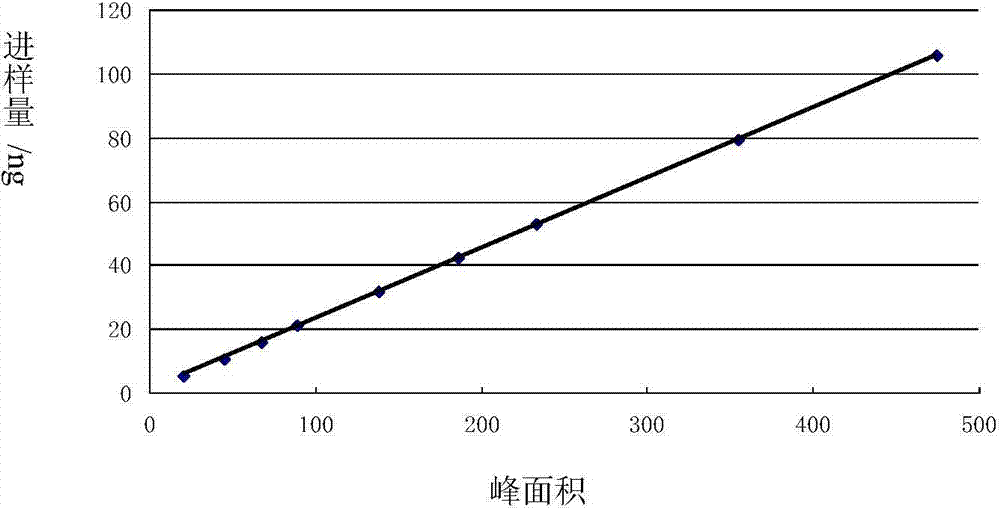
Image
Examples
Embodiment 1
[0060] Example 1: The content of baicalin, forsythin, indirubin and glycyrrhizic acid in Qingreling granules was determined by high performance liquid chromatography.
[0061] (1) HPLC conditions
[0062] Instrument name: Agilent 1100 high performance liquid chromatograph
[0063] Chromatographic conditions: Column: ZORBAX SB-phenyl (column length 250mm, inner diameter 4.6mm), filler: phenyl-bonded silica gel (particle size 5μm), flow rate: 1.0ml / min, column temperature: 25°C
[0064] DAD UV detector detection wavelength: 280nm, 277nm, 289nm, 252nm
[0065] Use 0.5% glacial acetic acid aqueous solution as mobile phase A and acetonitrile as mobile phase B, and perform gradient elution according to Table 1.
[0066] Table 1 Gradient elution conditions
[0067] time (minutes)
Mobile phase A (%)
Mobile phase B (%)
0~20
80→72
20→28
20~35
72→57
28→43
35~50
57
43
[0068] (2) Preparation of standard solution:
...
Embodiment 2
[0086] Precisely weigh the baicalin standard, add 70% ethanol aqueous solution by volume, and prepare a standard solution containing 36 μg of baicalin per 1 ml.
[0087] Precisely weigh the standard substance of forsythin, add 70% ethanol aqueous solution by volume, and prepare a standard solution containing 100 μg of forsythin per 1 ml.
[0088] Precisely weigh the indirubin standard substance, add 70% ethanol aqueous solution by volume, and prepare a standard solution containing 2.8 μg of indirubin per 1 ml.
[0089] Accurately weigh the ammonium glycyrrhizinate standard, add 70% ethanol aqueous solution by volume to prepare a standard solution containing 120 μg of ammonium glycyrrhizinate per 1ml. (weight of glycyrrhizic acid = weight of ammonium glycyrrhizinate / 1.0207).
[0090] The volume concentration 50% ethanol aqueous solution in the step (3) in Example 1 was replaced with a volume concentration 70% ethanol aqueous solution, and the ultrasonic time was replaced from ...
Embodiment 3
[0092] The 50% ethanol aqueous solution of volume concentration in the preparation of the sample to be tested in Example 1 was replaced with the solution shown in Table 2, and the sample to be tested A was detected. Peak, record the peak area, the results are shown in Table 2.
[0093] It can be seen from Table 2 that the ethanol aqueous solution used in the preparation of the sample solution to be tested is preferably a 50% ethanol solution or a 70% ethanol solution. The reason is that baicalin, forsythin, baicalin, forsythin, The chromatographic peak areas of indirubin and glycyrrhizic acid were the largest when extracted with 50% ethanol solution and 70% ethanol solution.
[0094] Table 2 Influence of solvent on absorption peak
[0095]
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com