Quality detection method of five-flavor manna medicine bath preparation
A quality inspection method and preparation technology, which can be applied to measuring devices, instruments, scientific instruments, etc., can solve problems such as difficulty in ensuring the safety and effectiveness of preparations, no quality control means for Wuwei Ganlu preparations, and inability to accurately control the quality of ephedra.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0052] Experimental example 1: identification experiment (method adopts the method in embodiment 1)
[0053] Wuwei Ganlu medicinal bath powder is provided by Qinghai Jinhe Tibetan Medicine Pharmaceutical Co., Ltd.
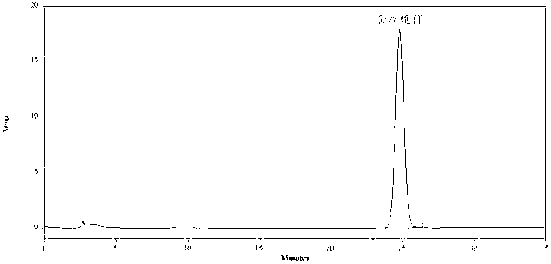
[0054] A. TLC identification of ephedra
[0055] Take 5g of the finely ground powder of this product, add 50ml of 1% aqueous hydrochloric acid solution by volume and fraction ratio, ultrasonically treat for 30min, filter, adjust the pH value of the filtrate to 10 with ammonia water, extract it with 20ml of dichloromethane, and extract dichloromethane The methane solution was evaporated to dryness, and the residue was dissolved by adding 2ml of absolute ethanol, which was used as the test solution. Another ephedra reference drug 1g, according to the preparation method of the test solution in the same way to prepare the reference drug solution. Take the ephedra-deficiency negative control substance prepared according to the prescription ratio, and prepare the negat...
experiment example 2
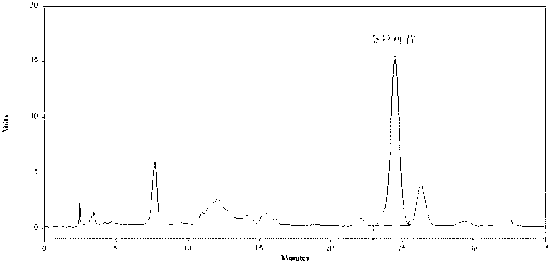
[0070] Experimental example 2: Ephedra content determination experiment
[0071] 1. Instruments, reagents and test samples
[0072] Instruments: Hitachi L-2100 pump, Hitachi L-2400 ultraviolet detector, Shimadzu AUW-220D electronic balance.
[0073] Reference substance: ephedrine hydrochloride reference substance (National Institute for the Control of Pharmaceutical and Biological Products) batch number: 171241-201007, pseudoephedrine hydrochloride reference substance (National Institute for the Control of Pharmaceutical and Biological Products) batch number: 171237-200807; ) Batch number: 121051-200704.
[0074] Sample: Wuwei Ganlu Herbal Bath Powder (Qinghai Jinhe Tibetan Medicine Pharmaceutical Co., Ltd.) Batch number: 20100701, 20100702, 20100703.
[0075] 2. Selection of detection wavelength
[0076] Take the mixed solution of ephedrine hydrochloride reference substance and pseudoephedrine hydrochloride reference substance, scan in the wavelength range of 190~400nm, an...
experiment example 3
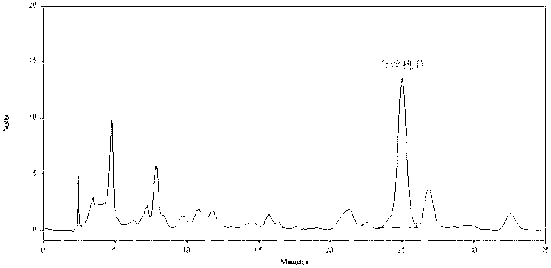
[0138] Experimental Example 3: Determination of the content of Rhododendron lanceolata
[0139] 1. Instruments, reagents and test samples
[0140] Instruments: Hitachi L-2100 pump, Hitachi L-2400 ultraviolet detector, Shimadzu AUW-220D electronic balance.
[0141] Reference substance: hyperoside reference substance (National Institute for the Control of Pharmaceutical and Biological Products) batch number: 111521-201004, and rhododendron reference substance (National Institute for the Control of Pharmaceutical and Biological Products) batch number: 121394-200401.
[0142] Sample: Wuwei Ganlu Herbal Bath Powder (Qinghai Jinhe Tibetan Medicine Pharmaceutical Co., Ltd.) Batch number: 20100701, 20100702, 20100703.
[0143] 2. Selection of detection wavelength
[0144] Take the hyperin reference substance solution, scan in the wavelength range of 190~500nm, and select 360nm as the detection wavelength according to the ultraviolet absorption spectrum.
[0145] 3. Mobile phase sel...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com