Quality control method of concentrated-type oral liquid for treating cough and asthma of children
A quality control method and technology for Kechuanling oral liquid are applied in the field of quality control of traditional Chinese medicine oral liquid, which can solve the problems of inaccurate quality control and imperfect drug quality standards.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
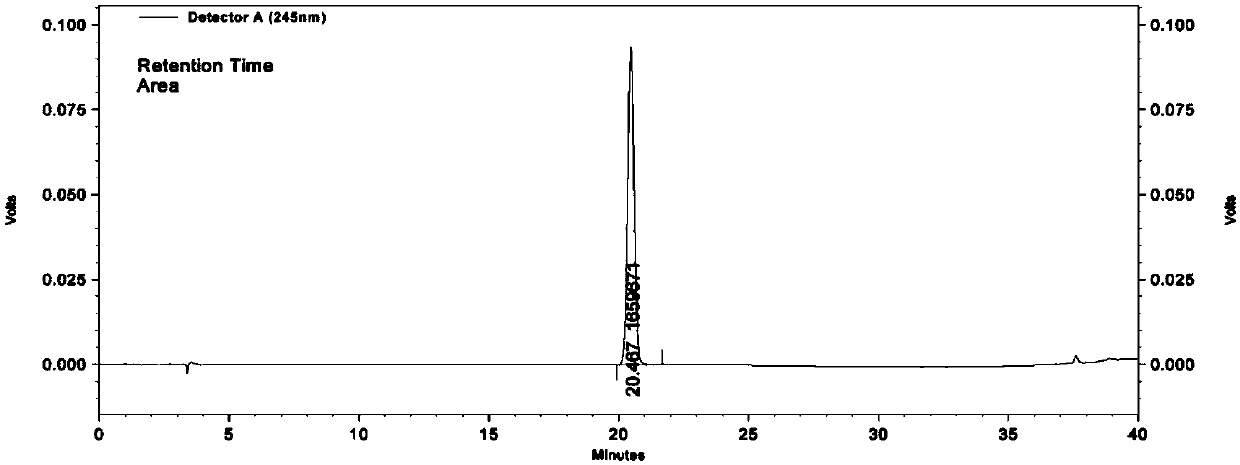
[0150] High performance liquid chromatography identification method of Radix isatidis in Xiaoer Kechuanling Oral Liquid
[0151] 1) Preparation of the test solution
[0152] Take 10mL of the oral solution, shake and extract twice with ethyl acetate, 20mL each time, combine the ethyl acetate solution, evaporate the ethyl acetate in it to dryness, and accurately configure the residue into 5mL methanol solution to obtain the test solution ;
[0153] 2) Preparation of reference substance solution
[0154] Take (R,S)-Gaoyichun 10.80mg, add methanol to a 10mL volumetric flask, and shake well; accurately measure 3mL, add methanol to a 100mL volumetric flask, and shake well to prepare the reference substance solution 2;
[0155] 3) Preparation of the reference medicinal material solution
[0156] Take 1.05g of Radix Radix, add 50mL of water, decoct for 2h, let cool, filter, take 10mL of the filtrate, and prepare the reference medicinal material solution with the preparation method...
Embodiment 2
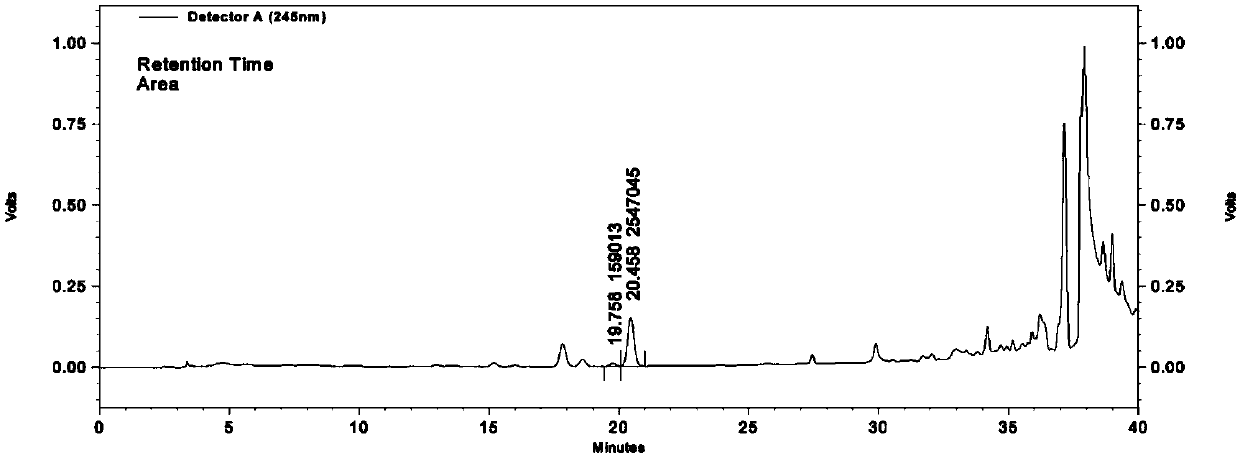
[0172] Detection method and methodological investigation of ephedrine hydrochloride and pseudoephedrine hydrochloride content
[0173] 1. Instruments and reagents
[0174] 1.1 Reference substances: ephedrine hydrochloride (National Institute for the Control of Pharmaceutical and Biological Products), pseudoephedrine hydrochloride (National Institute for the Control of Pharmaceutical and Biological Products).
[0175] 1.2 Reagents: Acetonitrile is chromatographically pure, water is purified water, and other reagents are analytically pure.
[0176] 1.3 Instruments: Agilent 1100 high performance liquid chromatograph, ultraviolet detector; AS10200A ultrasonic oscillator. LABOROTA-4000 rotary evaporator. Chromatographic column: phenomenonex, packing particle size 4μm, length 250mm, inner diameter 4.6mm;
[0177] 2. Preparation of the test solution
[0178] Precisely measure 10mL of oral liquid and put it in a separatory funnel, add 1mL of ammonia water, shake well; shake and ex...
Embodiment 3
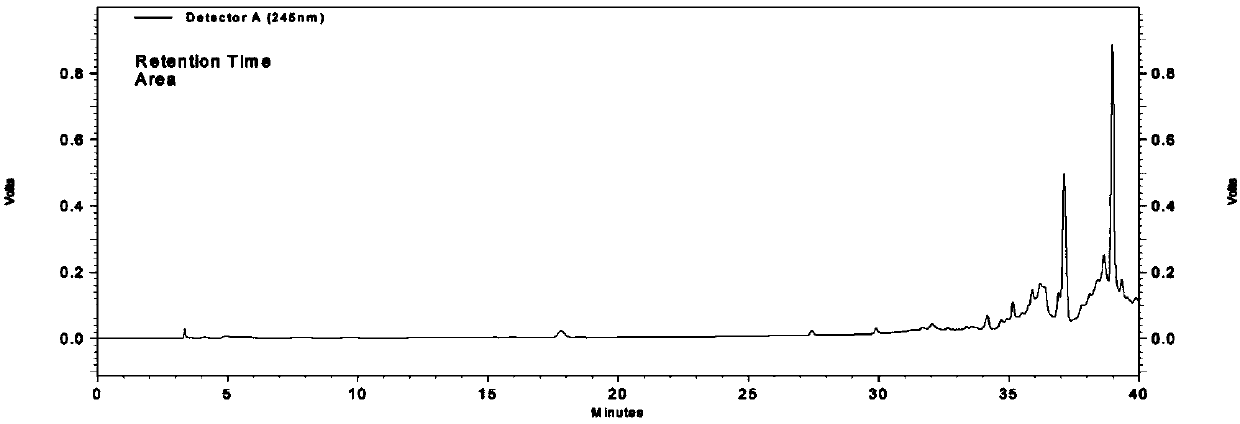
[0232] The identification of ephedra includes the following steps:
[0233] 1) Preparation of the test solution
[0234] Take 10 mL of oral liquid, add 1 mL of ammonia water, extract with ether, add 1 mL of 0.6 mol / L ethanol hydrochloric acid solution to the ether phase, evaporate the solvent to dryness, dissolve the residue with 1 mL of methanol, and use it as the test solution;
[0235] 2) Preparation of reference substance solution
[0236] Get ephedrine hydrochloride reference substance 1.10mg, add methanol 1mL to dissolve, as reference substance solution;
[0237] 3) Preparation of the reference medicinal material solution
[0238] Take 1.05g of the ephedra reference medicinal material, add water to decoct for 1h, concentrate to 40mL, cool, and then prepare the reference medicinal material solution according to the preparation method of the test solution described in step 1);
[0239] 4) Preparation of negative control solution
[0240] According to the prescription p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com