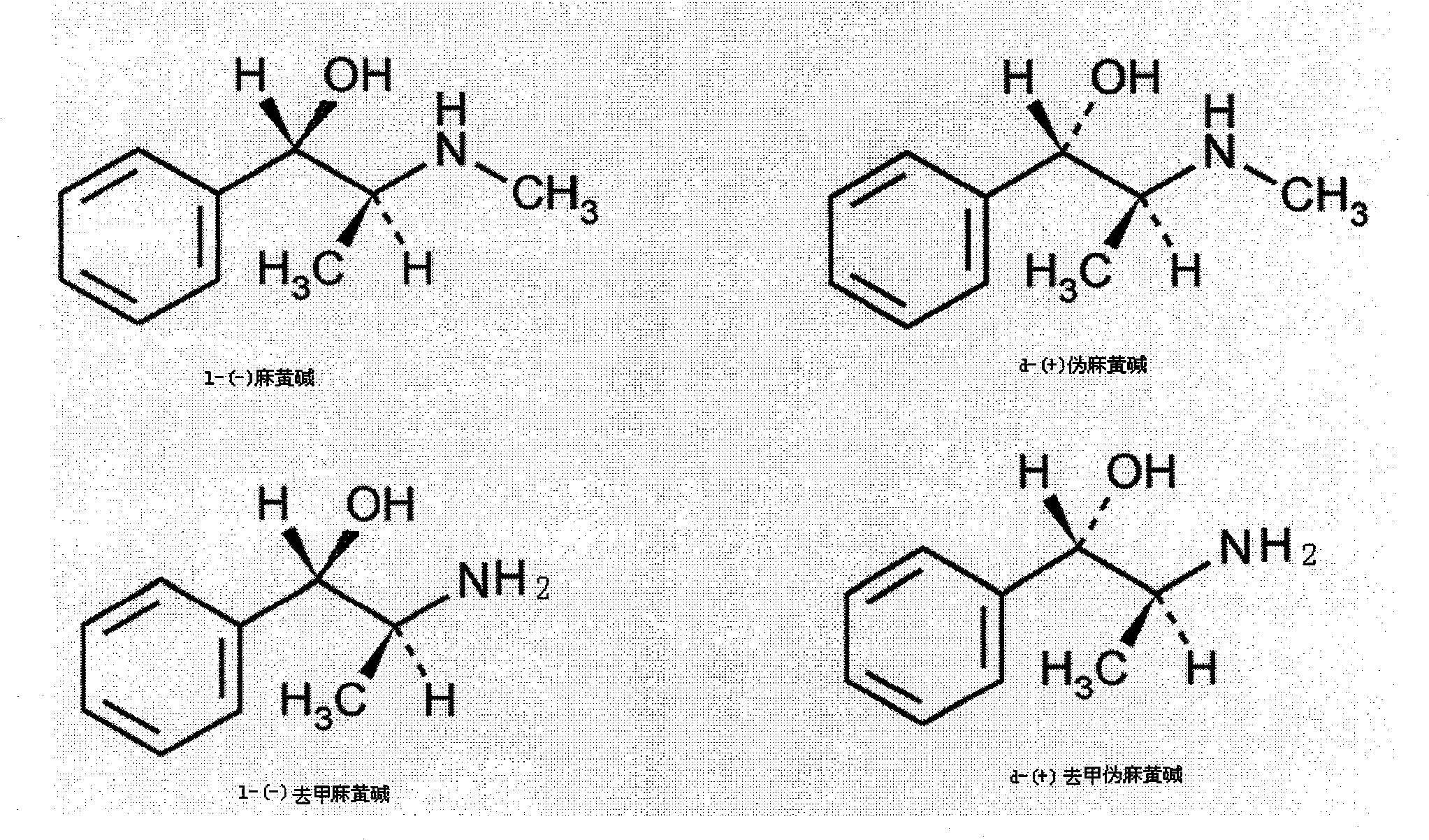
Preparation method of L-(-)-ephedrine chloride and d-(+)-pseudoephedrine hydrochloride
A technology of pseudoephedrine hydrochloride and ephedrine hydrochloride, which is applied in the field of medicine and chemical industry, can solve the problems of high price of D-arabonic acid, the restriction of the company's production of pseudoephedrine hydrochloride, and the inability to adapt to large-scale industrial production, so as to achieve weak vasoconstriction of the whole body, eliminate mucosal congestion, The effect of raising blood pressure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
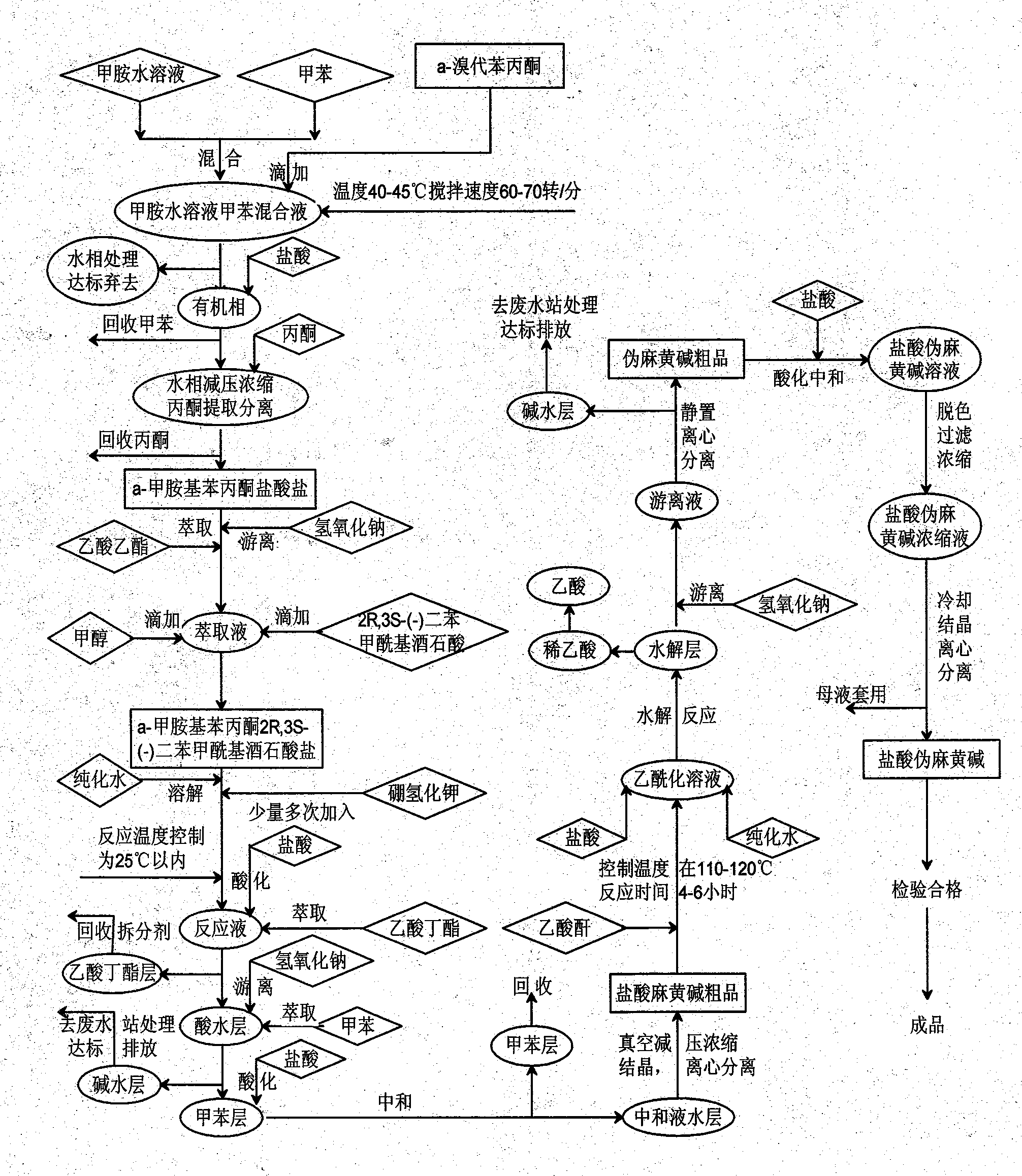
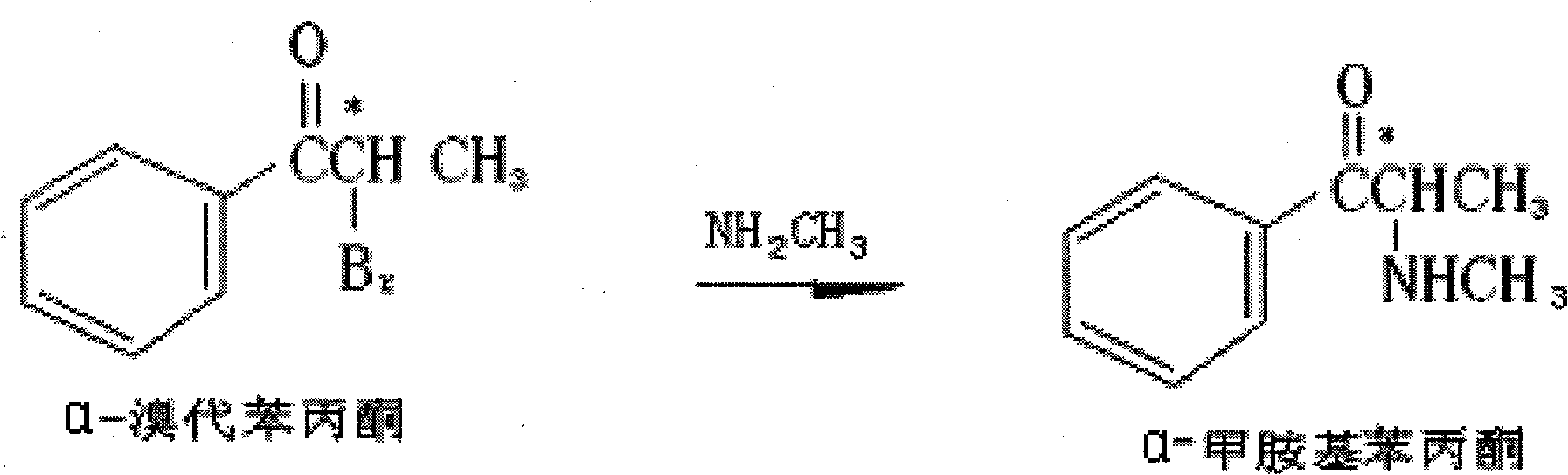
[0098] 1. In a 2000ml rotary glass evaporation reactor, add 1100ml of toluene solution and 160ml of methylamine aqueous solution, keep the temperature of the water bath at 45°C, start stirring, control the stirring speed at 65 rpm, add α-bromopropiophenone dropwise 210 g, and then added dropwise 15% aqueous sodium hydroxide solution prepared by 50 g of sodium hydroxide, after addition, reacted for two hours, stopped heating, and cooled to room temperature. Separate the organic phase with an extractor, extract the aqueous phase twice with toluene (300ml*2 times), and combine the organic phases. 1500 ml of 15% hydrochloric acid aqueous solution was added dropwise to the organic phase, stirred for 1 hour, the aqueous phase was separated, evaporated under reduced pressure to syrupy state. Add 550ml of acetone to shake, let it stand overnight, and filter out the white solid, which is the hydrochloride of α-methylaminopropiophenone (yield: 74%).
[0099] 2. In a 1000ml glass reactor,...
Embodiment 2
[0102] 1. In a 2000ml rotary glass evaporation reactor, add 1000ml of toluene solution and 150ml of methylamine aqueous solution, keep the temperature of the water bath at 40°C, start stirring, control the stirring speed at 60 rpm, and add α-bromopropiophenone dropwise 210.0 g, then added dropwise a 15% aqueous sodium hydroxide solution prepared by 40 g of sodium hydroxide, after the addition was complete, reacted for two hours, stopped heating, and cooled to room temperature. Separate the organic phase with an extractor, extract the aqueous phase twice with toluene (300ml*2 times), and combine the organic phases. 1000ml of 15% hydrochloric acid aqueous solution was added dropwise to the organic phase, stirred for 1 hour, the aqueous phase was separated, evaporated under reduced pressure to syrupy state. Add 500ml of acetone to shake, let stand overnight, filter out the white solid, which is the hydrochloride of α-methylaminopropiophenone (yield: 73%);
[0103] 2. Take 100g o...
Embodiment 3
[0106] 1. In a 2000ml rotary glass evaporation reactor, add 1100ml of toluene solution and 165ml of methylamine aqueous solution, keep the temperature of the water bath at 43°C, start stirring, control the stirring speed at 65 rpm, and add α-bromopropiophenone dropwise 210 g, then added dropwise 15% aqueous sodium hydroxide solution prepared by 45 g of sodium hydroxide, after addition, reacted for two hours, stopped heating, and cooled to room temperature. Separate the organic phase with an extractor, extract the aqueous phase twice with toluene (300ml*2 times), and combine the organic phases. 1200 ml of 15% hydrochloric acid aqueous solution was added dropwise to the organic phase, stirred for 1 hour, the aqueous phase was separated, evaporated under reduced pressure to syrupy state. Add 600ml of acetone to shake, let stand overnight, filter out the white solid, which is the hydrochloride of α-methylaminopropiophenone (yield: 76%);
[0107] 2. In a 1000ml glass reactor, weig...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com