Water soluble medicament sustained-release tablets and preparation method thereof
A technology of water-soluble drugs and sustained-release tablets, which is applied in pharmaceutical formulations, drug combinations, drug delivery, etc., can solve problems such as blockage, difficult release, and no sustained-release tablets, so as to reduce toxic and side effects, increase compliance, and treat good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
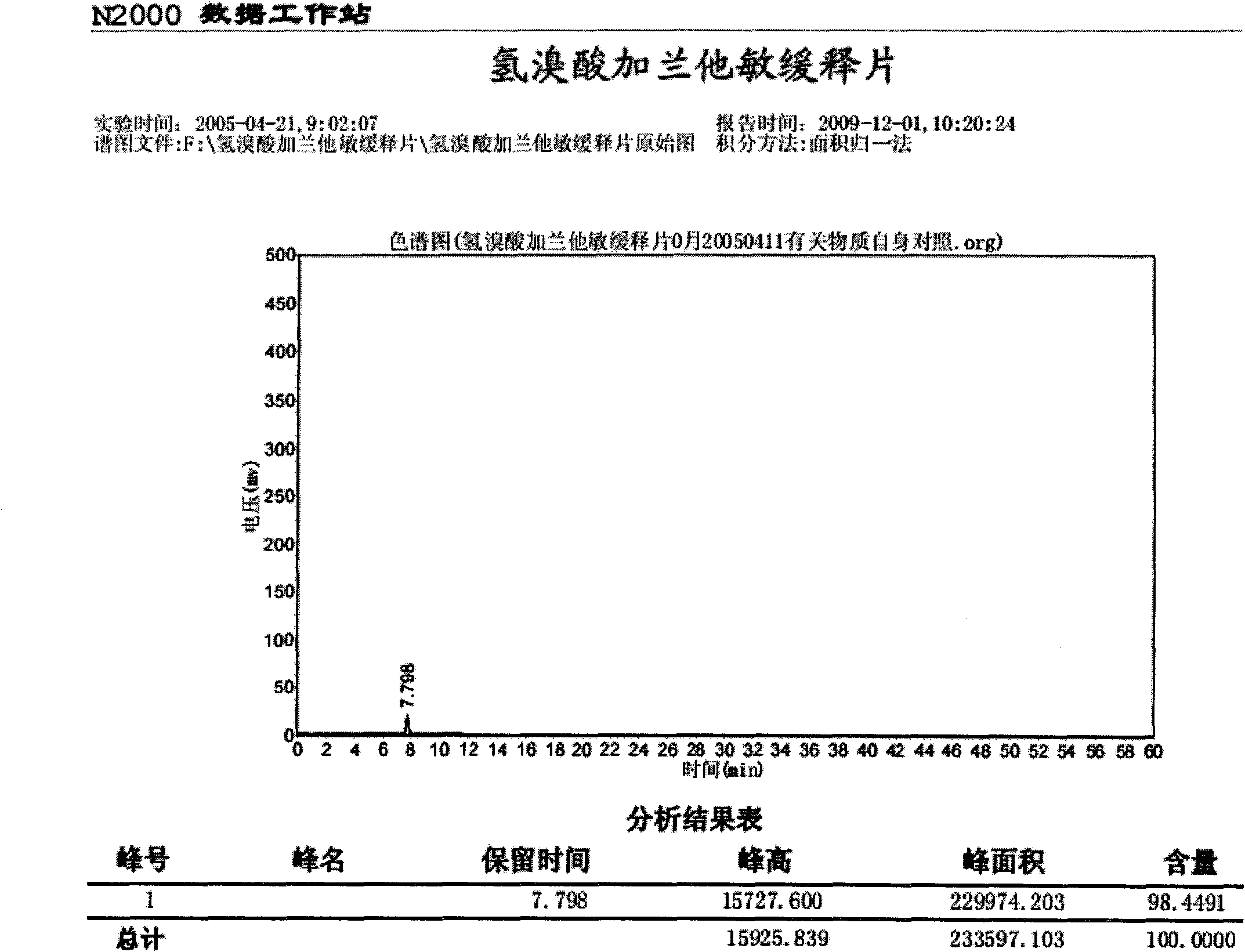
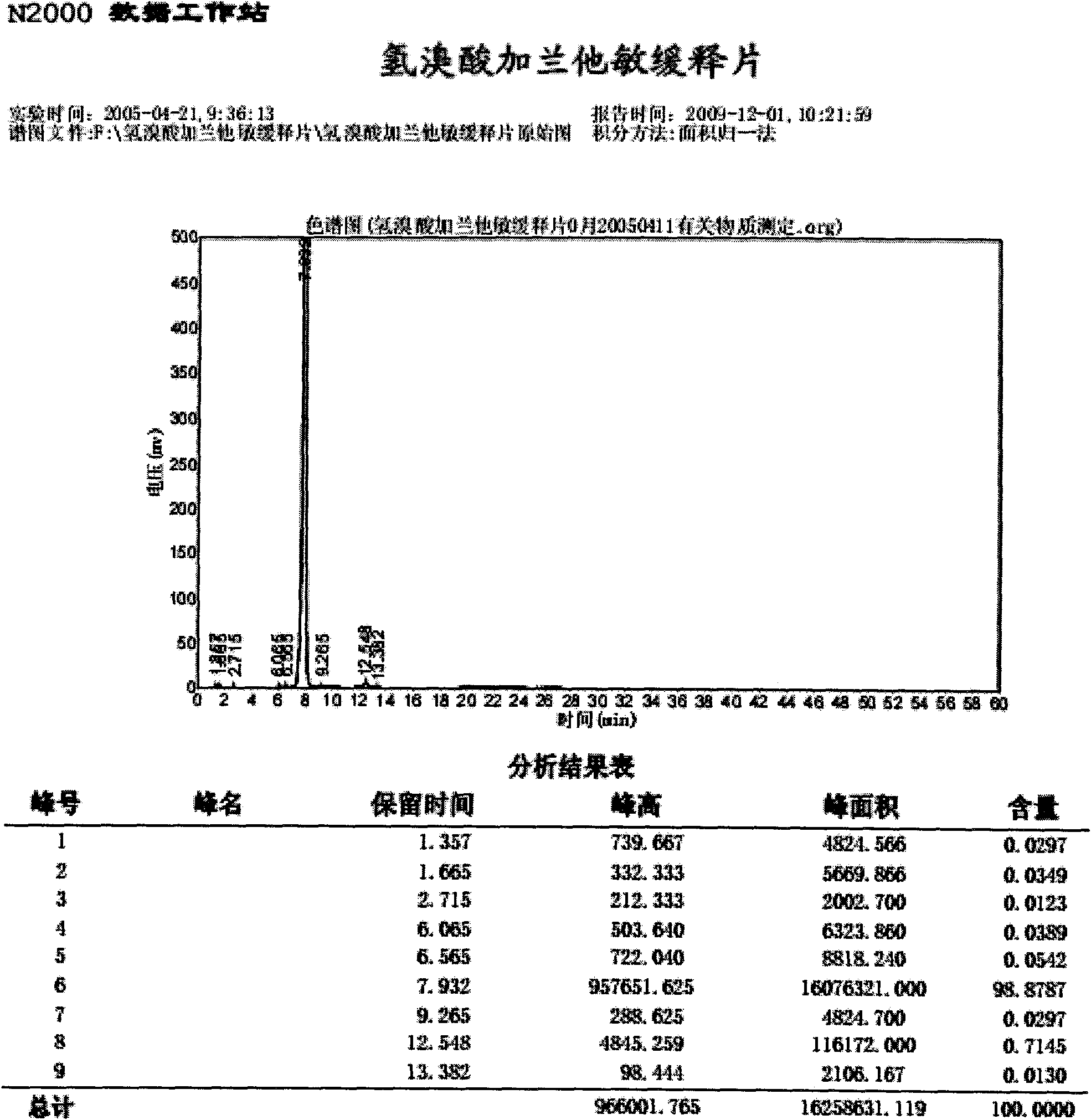
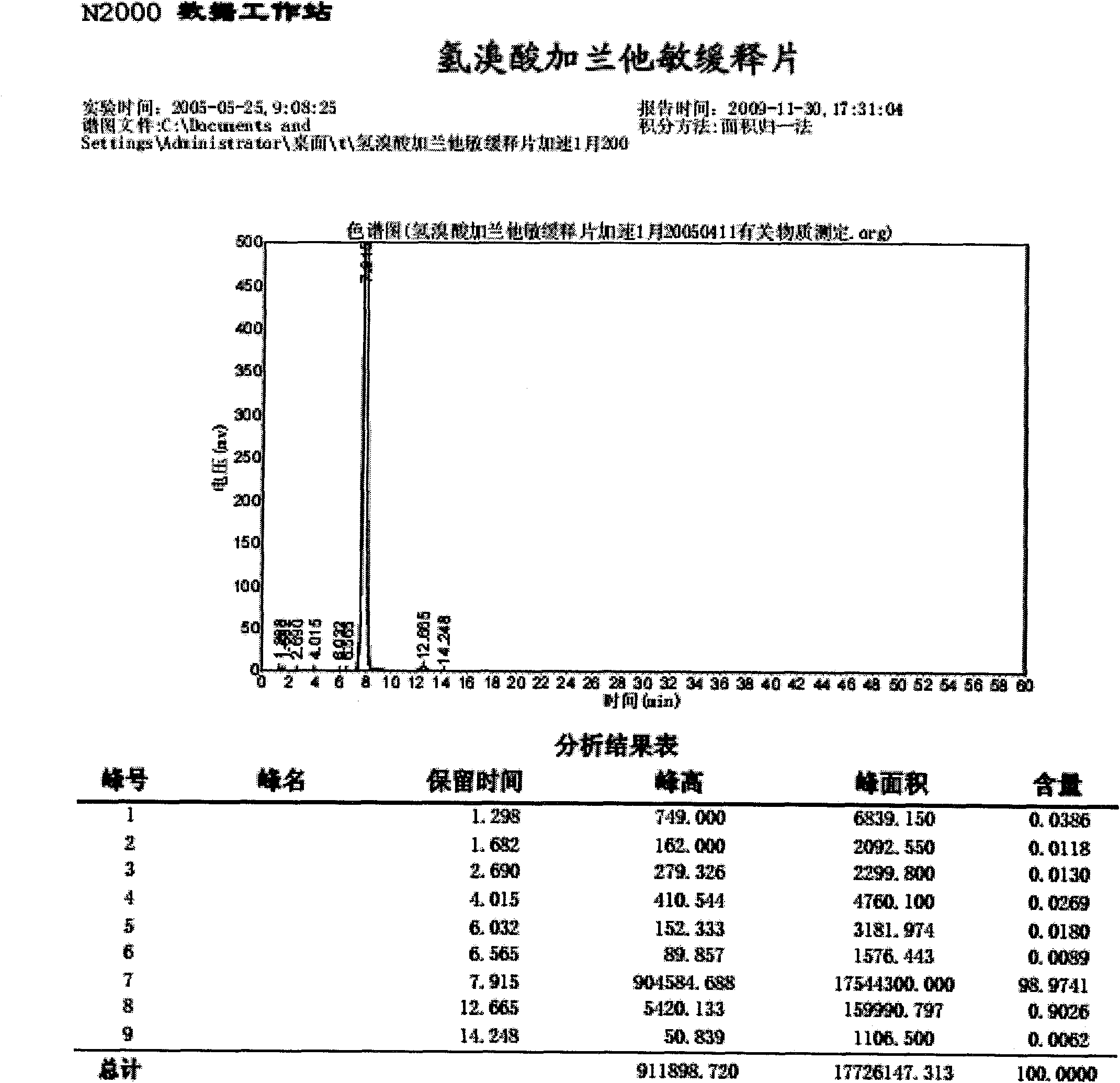
[0036] Embodiment 1: Preparation of Galantamine Hydrobromide Sustained-release Tablets:
[0037] The formula is: each 1000 tablets is made of the following components:
[0038] Galantamine Hydrobromide 10.0g
[0039] Octadecanol 25.0g
[0040] Utechy L100-55 9.0g
[0042] Lactose 8.0g
[0043] 60% ethanol 14ml
[0044] The above-mentioned galantamine hydrobromide refers to galantamine hydrobromide extracted from the plant Lycoris, which was purchased from Zhejiang Yixin Pharmaceutical Co., Ltd. Stearyl alcohol was purchased from Shanghai Sinopharm Group (imported repackaging); Utech L100-55 was purchased from Shanghai Texiang Pharmaceutical Technology Co., Ltd. (imported repackaging, Germany Rohm); talcum powder was purchased from Guangxi Long Bought from Sheng County Sanmen Talcum Powder Factory.
[0045] The preparation process is as follows:
[0046] (1) The raw materials and auxiliary materials are first passed through an 80-mesh sieve; ...
Embodiment 2
[0053] Embodiment 2: prepare captopril sustained-release tablet:
[0054] The formula is: each 1000 tablets is made of the following components:
[0055] Captopril 12.5g
[0056] Octadecanol 27.0g
[0057] Utech L100-55 10.5g
[0059] Lactose 8.0g
[0060] 60% ethanol 15ml
[0061] The above-mentioned captopril was purchased from Wuhan Hezhong Biochemical Manufacturing Co., Ltd. Stearyl alcohol was purchased from Shanghai Sinopharm Group (imported repackaging); Utech L100-55 was purchased from Shanghai Texiang Pharmaceutical Technology Co., Ltd. (imported repackaging, Germany Rohm); talcum powder was purchased from Guangxi Long Bought from Sheng County Sanmen Talc Powder Factory.
[0062] The preparation process is as follows:
[0063] (1) The raw materials and auxiliary materials are first passed through an 80-mesh sieve;
[0064] (2) Pass 10.5g of Eutech L100-55, 27.0g of stearyl alcohol, 5.0g of talc and 8.0g of lactose through a 60-mesh ...
Embodiment 3
[0070] Embodiment 3: preparation metoprolol tartrate sustained-release tablet:
[0071] The formula is: each 1000 tablets is made of the following components:
[0072] Metoprolol Tartrate 100.0g
[0073] Octadecanol 80.0g
[0074] Utech L100-55 50.0g
[0075] Talc powder 20.0g
[0076] Lactose 10.0g
[0077] 3% carboxypropyl methylcellulose alcohol solution (80%, V / V) 32ml
[0078] The above-mentioned metoprolol tartrate was purchased from Wuhan Hezhong Biochemical Manufacturing Co., Ltd. Stearyl alcohol was purchased from Shanghai Sinopharm Group (imported repackaging); Utech L100-55 was purchased from Shanghai Texiang Pharmaceutical Technology Co., Ltd. (imported repackaging, Germany Rohm); talcum powder was purchased from Guangxi Long Bought from Sheng County Sanmen Talc Powder Factory.
[0079] The preparation process is as follows:
[0080] (1) The raw materials and auxiliary materials are first passed through an 80-mesh sieve;
[0081] (2) Pass 50.0g of Eutech L...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com