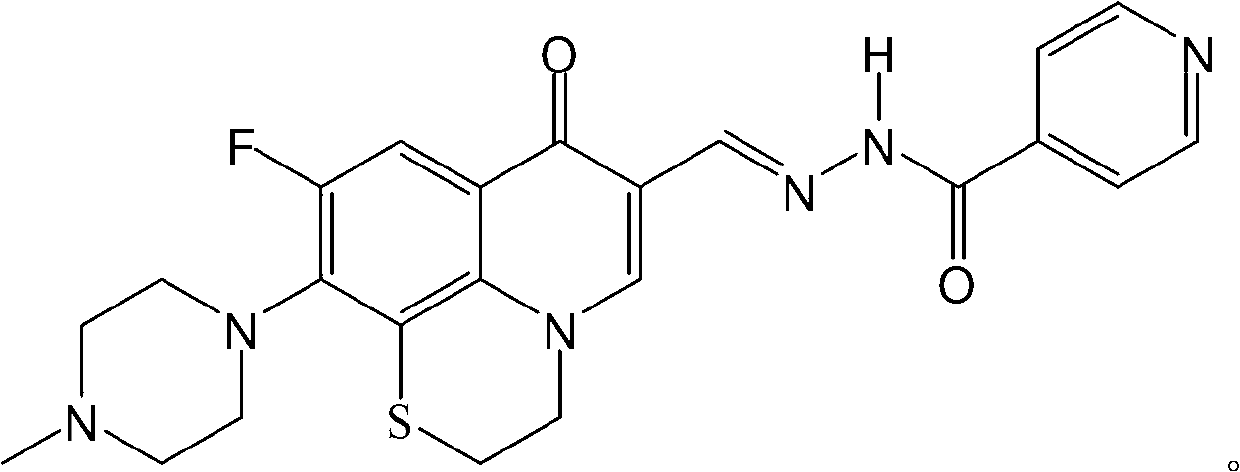
Fluoroquinolone acetal isoniazone, and preparation method and application thereof
A technology of fluoroquinolone aldehyde and quinolone aldehyde, which is applied in the field of fluoroquinolone derivative compounds, can solve problems such as phototoxicity, poor efficacy of tuberculosis, and affect animal cartilage development, and achieve the effects of reducing toxic side effects and drug resistance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
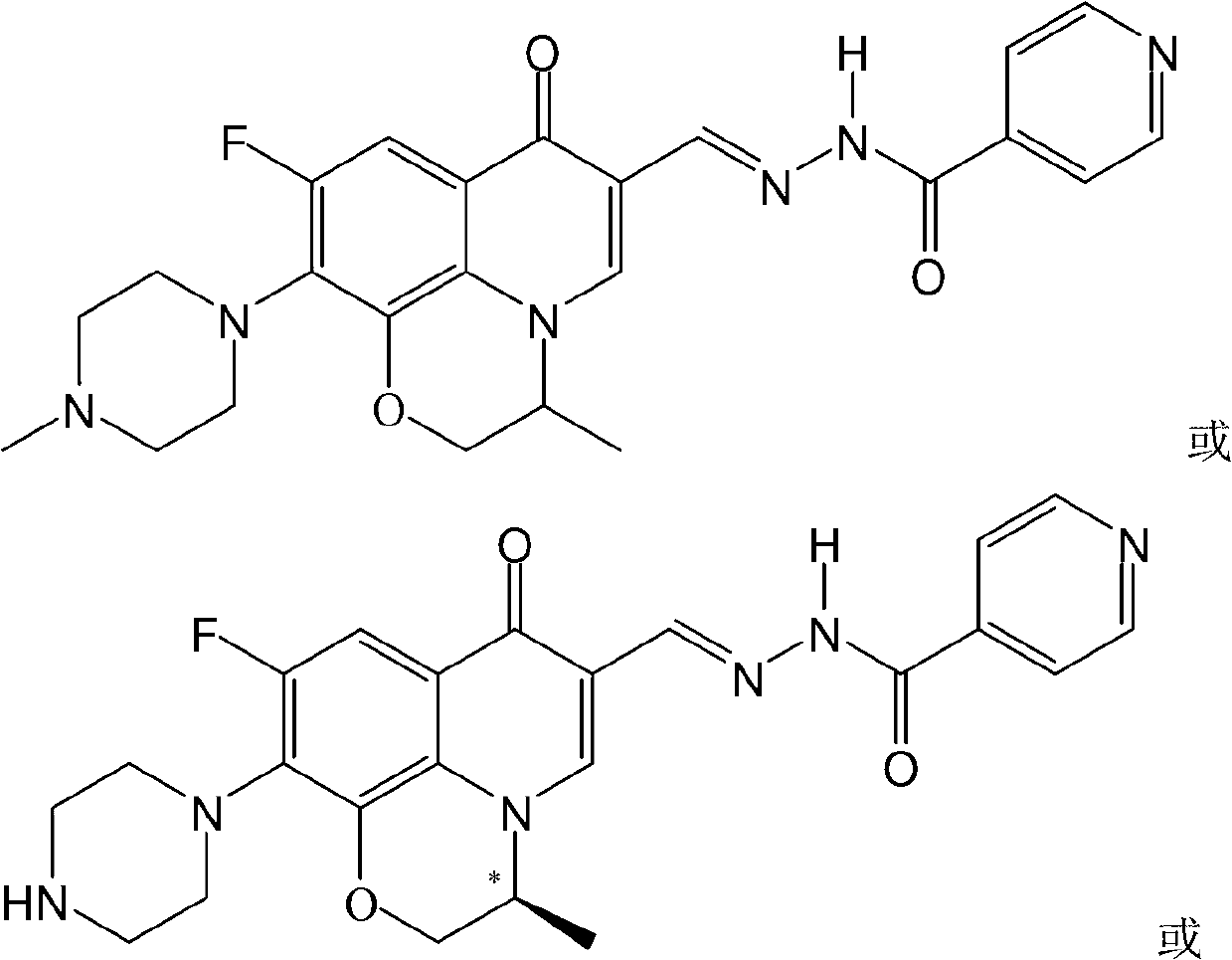
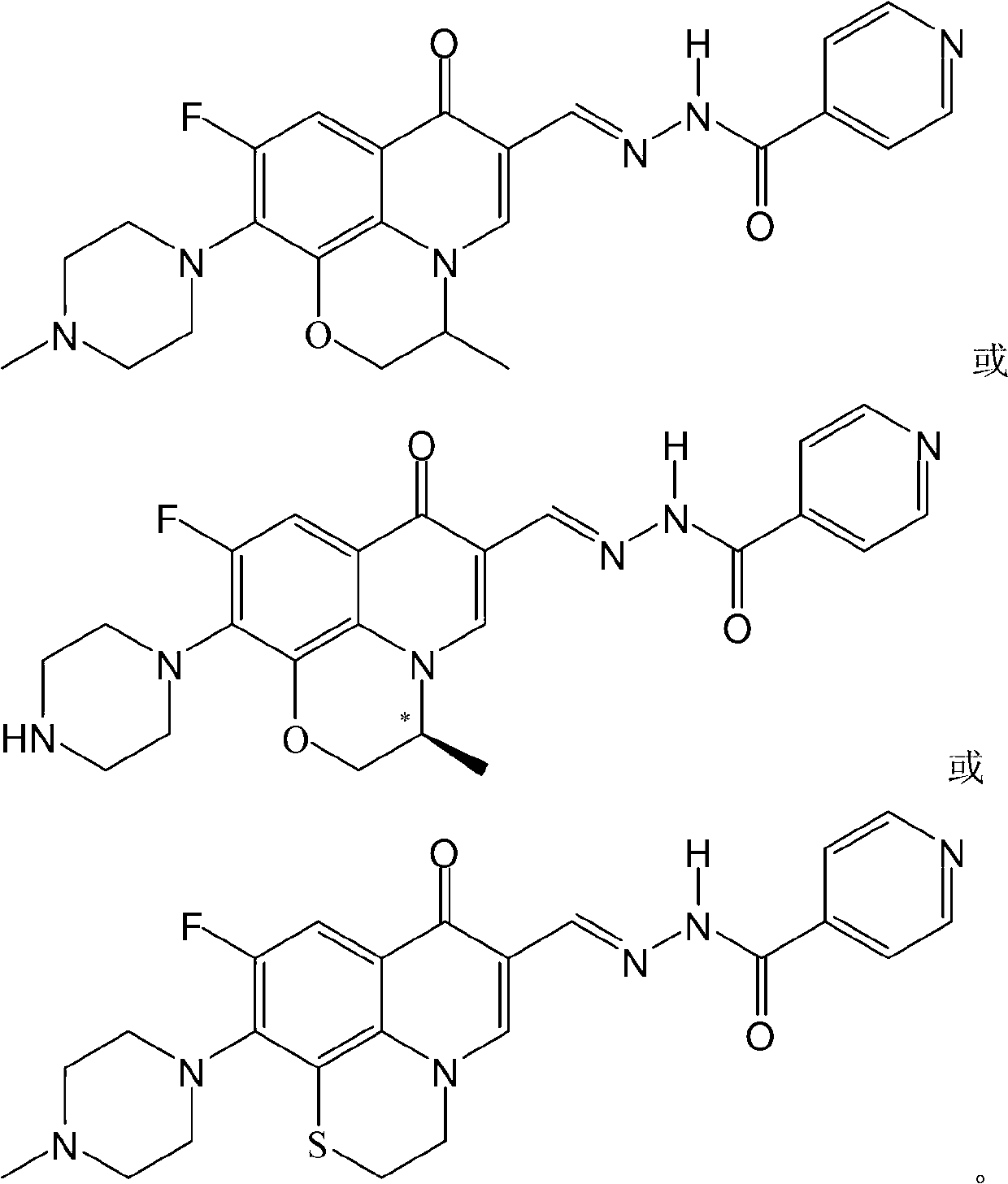
[0032] The fluoroquinolone aldehyde isonicotinoid hydrazone in this example is (S)-N'-[6-fluoro-7-(4-methylpiperazin-1-yl)-1,8-(2,1-oxopropane Base)-quinoline (1H)-4-one-3-methylene]isoniazone, its chemical structural formula is:
[0033]
[0034] That is, R in formula I 1 for methyl(S), R 2 is a hydrogen atom, R 3 is methyl, R 4 is a hydrogen atom, and X is an oxygen atom.
[0035] The preparation method of the fluoroquinolone aldehyde isonicotinoid hydrazone of the present embodiment is: take (S)-6-fluoro-1,8-(2,1-oxopropyl)-7-(4-methylpiperazine-1 -yl)-3-formyl-4(1H)-quinolinone 0.35g (1mmol), absolute ethanol 12ml, heated to dissolve, then add isoniazid 0.14g (1mmol), reflux for 3h. After standing overnight, the resulting solid was collected by filtration, washed with absolute ethanol, and dried to obtain 0.37 g of the fluoroquinolone aldehyde isonicotinoid hydrazone compound of Example 1, with a yield of 81%, m.p.211.9-214.3°C. 1 HNMR (DMSO-d 6 , 400MHz) δ: 11.9...
Embodiment 2
[0037] The fluoroquinolone aldehyde isonicotinoid hydrazone in this embodiment is N'-[6-fluoro-7-(4-methylpiperazin-1 base)-1,8-(2,1-oxopropyl)-quinoline (1H)-4-keto-3-methylene]isonicotinamide, its chemical structural formula is:
[0038]
[0039] That is, R in formula I 1 is methyl, R 2 is a hydrogen atom, R 3 is methyl, R 4 is a hydrogen atom, and X is an oxygen atom.
[0040] The preparation method of the fluoroquinolone aldehyde isonicotinoid hydrazone of the present embodiment is: take 6-fluoro-1,8-(2,1-oxypropyl)-7-(4-methylpiperazin-1-yl)- 0.35g (1mmol) of 3-formyl-4(1H)-quinolinone and 15ml of absolute ethanol were heated until dissolved, then 0.14g (1mmol) of isoniazid was added, and refluxed for 3h. After standing overnight, the resulting solid was collected by filtration, washed with absolute ethanol, and dried to obtain 0.38 g of the fluoroquinolone aldehyde isonicotinoid hydrazone compound of Example 2, with a yield of 83%, m.p.293.5-295.6°C. 1 HNMR (DMS...
Embodiment 3
[0042] The fluoroquinolone aldehyde isonicotinoid hydrazone of the present embodiment is (S)-N'-[6-fluoro-7-piperazin-1-yl-1,8-(2,1-oxopropyl)-quinoline ( 1H)-4-keto-3-methylene]isoniazone, its chemical structural formula is:
[0043]
[0044] That is, R in formula I 1for methyl(S), R 2 is a hydrogen atom, R 3 is a hydrogen atom, R 4 is a hydrogen atom, and X is an oxygen atom.
[0045] The preparation method of the fluoroquinolone aldehyde isonicotinoid hydrazone of the present embodiment is: take (S)-6-fluoro-1,8-(2-methylethyleneoxy)-7-(piperazin-1-yl) - 0.33g (1mmol) of 3-formyl-4(1H)-quinolinone, 12ml of absolute ethanol, heated until dissolved, then added 0.14g (1mmol) of isoniazid, and heated to reflux for 3h. After standing overnight, the resulting solid was collected by filtration, washed with absolute ethanol, and dried to obtain 0.34 g of the fluoroquinolone aldehyde isonicotinoid hydrazone compound of Example 3, with a yield of 76% and m.p.>300°C. 1 HNMR (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com