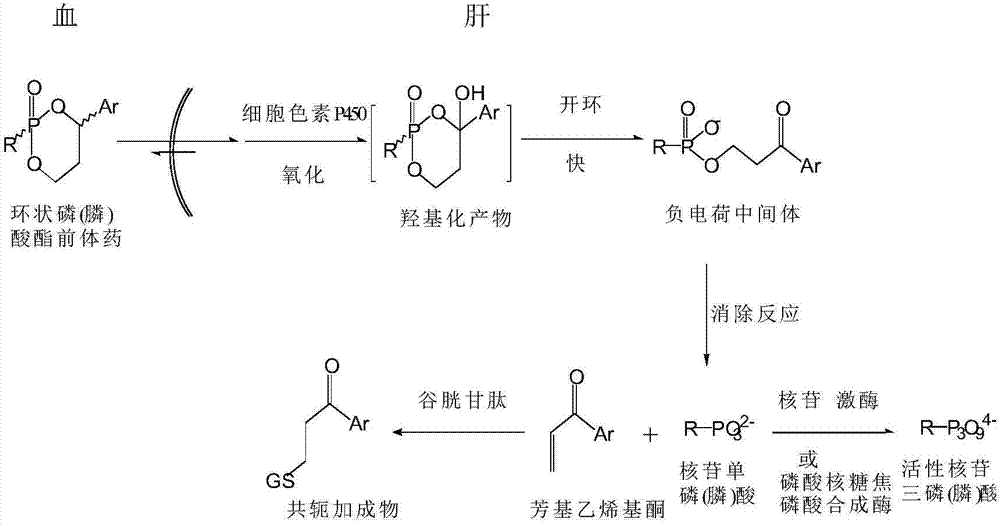
Tenofovir cyclic phosphonate compound and pharmaceutically acceptable salt thereof, and preparation methods and applications thereof
A technology of vircyclondronate and compounds, applied in the field of chemical pharmaceuticals, to achieve the effect of avoiding kidney toxicity, high safety, and good antiviral effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
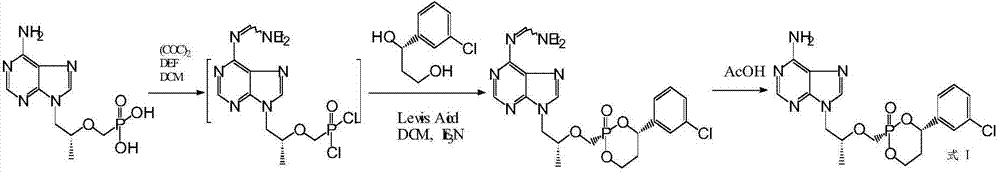
[0066] Example 1 The preparation of formula I compound
[0067] The preparation method of formula I compound comprises the following steps:
[0068] 1) Slowly add 150 ml of oxalyl chloride dropwise under nitrogen protection at 15-25°C with stirring to a mixture consisting of 150 g of tenofovir, 60 g of N,N-diethylformamide and 2 liters of dichloromethane. In the mixture, stir and reflux for 4 to 6 hours, then cool to 15 to 25°C for later use;
[0069] 2) Add 62 ml of titanium tetrachloride dropwise to 100 g of (S)-1-(3-chlorophenyl)-1,3-propanediol and 2 liters of In the solution that methyl chloride was formed, after adding dropwise 310 milliliters of triethylamine, stirred for 30 minutes, obtained reaction mixture;
[0070] 3) Add the reaction mixture prepared in step 2) to the reaction mixture prepared in step 1), stir at 20-25°C for 1-2 hours, then add 2 liters of saturated saline, and stir for 30 minutes , static layering, the aqueous layer was extracted five times w...
Embodiment 2
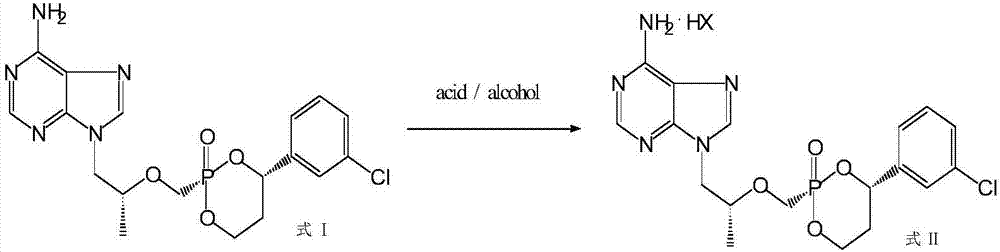
[0074] Example 2 Preparation of formula I compound mesylate
[0075] The preparation method of formula I compound mesylate may further comprise the steps:
[0076] 1) Add 85 grams of methanesulfonic acid and 900 milliliters of ethanol to the distillation residue obtained in Step 4) of Example 1, heat to reflux until clarified, cool down to 50°C, stir for 4-6 hours, and then stir at 0-5°C After 1-2 hours, filter under reduced pressure to obtain the crude product of the compound mesylate of formula I;
[0077] 2) Add 500 ml of ethanol to the obtained crude mesylate of the compound of formula I, heat to reflux until completely dissolved, cool down to 50°C, stir for 4-6 hours, then cool down to 5°C and stir for 1-2 hours, filter under reduced pressure , washed with cold ethanol, and dried at 45-50°C to obtain 175 g of the mesylate salt of the compound of formula I as a light yellow solid; cis / trans: 99.6:0.4.
[0078] δ( 1 HNMR, DMSO-d 6 ): 1.28 (d, J=7.5Hz, 3H), 1.95-2.20 (...
Embodiment 3
[0079] Example 3 Preparation of formula I compound fumarate
[0080] The preparation method of formula I compound fumarate, comprises the steps:
[0081] 1) Add 90 g of fumaric acid and 900 ml of ethanol to the distillation residue obtained in Step 4) of Example 1, heat to reflux until clarified, cool down to 50°C and stir for 4-6 hours, then cool down to 0-5°C Stir for 1-2 hours, and filter under reduced pressure to obtain the crude product of compound fumarate of formula I;
[0082] 2) Add 500 ml of ethanol to the obtained crude product of compound fumarate of formula I, heat to reflux until completely dissolved, cool down to 50°C and stir for 4-6 hours, then cool down to 5°C and stir for 1-2 hours, filter under reduced pressure , washed with cold ethanol, and dried at 45-50°C to obtain 182 grams of fumarate salt of the compound of formula I as a light yellow solid; cis / trans: 99.5:0.5.
[0083] δ( 1 HNMR, DMSO-d 6 ): 1.28 (d, J=7.5Hz, 3H), 1.95-2.20 (m, 2H), 2.68-2.75 (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com