An Inhalable Rapamycin Formulation for Treating Age-Related Conditions
a technology of rapamycin and inhalation, which is applied in the direction of drug compositions, respiratory disorders, anti-noxious agents, etc., can solve the problems of affecting the quality of life, and increasing cancer risk, so as to reduce the toxicity of rapamycin and the associated drug concentration, inhibit the signaling of mtor, and reduce the effect of toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Aqueous Aerosol Formulation
[0195]An exemplary aqueous formulation of rapamycin was prepared using the following components.
ComponentAmount (g)Mass Fraction (w / w)rapamycin0.10.01% ethanol25025%propylene glycol25025%polysorbate 800.020.002% water50050%Total1000
[0196]Blending Procedure: in a 1000 ml amber volumetric flask, blend 250 propylene glycol with 250 ethanol until uniform. Then sequentially dissolve first 100 mg rapamycin then 20 mg polysorbate 80 in the propylene glycol and ethanol solution. Add water to bring the volumetric to 1000 ml and stir or sonicate until uniform and all the rapamycin is dissolved. Store at controlled temperature away from light.
example 2
Dry Powder Formulation
[0197]Batch 06RP68.HQ00008 and 06RP68.HQ00009. These two formulations are each a blend of micronized drug (rapamycin) particles dispersed onto the surface of lactose carrier particles. The final composition of each batch comprises 1% (w / w) drug particles having a mean diameter of about 2.60 microns and 3.00 microns, respectively. Drug particles having a suitable size range are made by wet polishing (06RP68.HQ00008) or jet milling (06RP68.HQ00009), as described below. While this example used 1% (w / w) rapamycin, a range 0.5 to 20% is practicable. The carrier particles consist of a blend of two carriers, Respitose® SV003, present at 95.5% (w / w) and having particle sizes of about 30 to 100 microns (equivalent spherical diameter), and Respitose® LH300 (Lactohale 300) present at 5.5% (w / w) and having particle sizes less than 10 microns (equivalent spherical diameter). After blending, the blends were assayed to confirmed homogeneity and drug content of 1%.
[0198]To red...
example 3
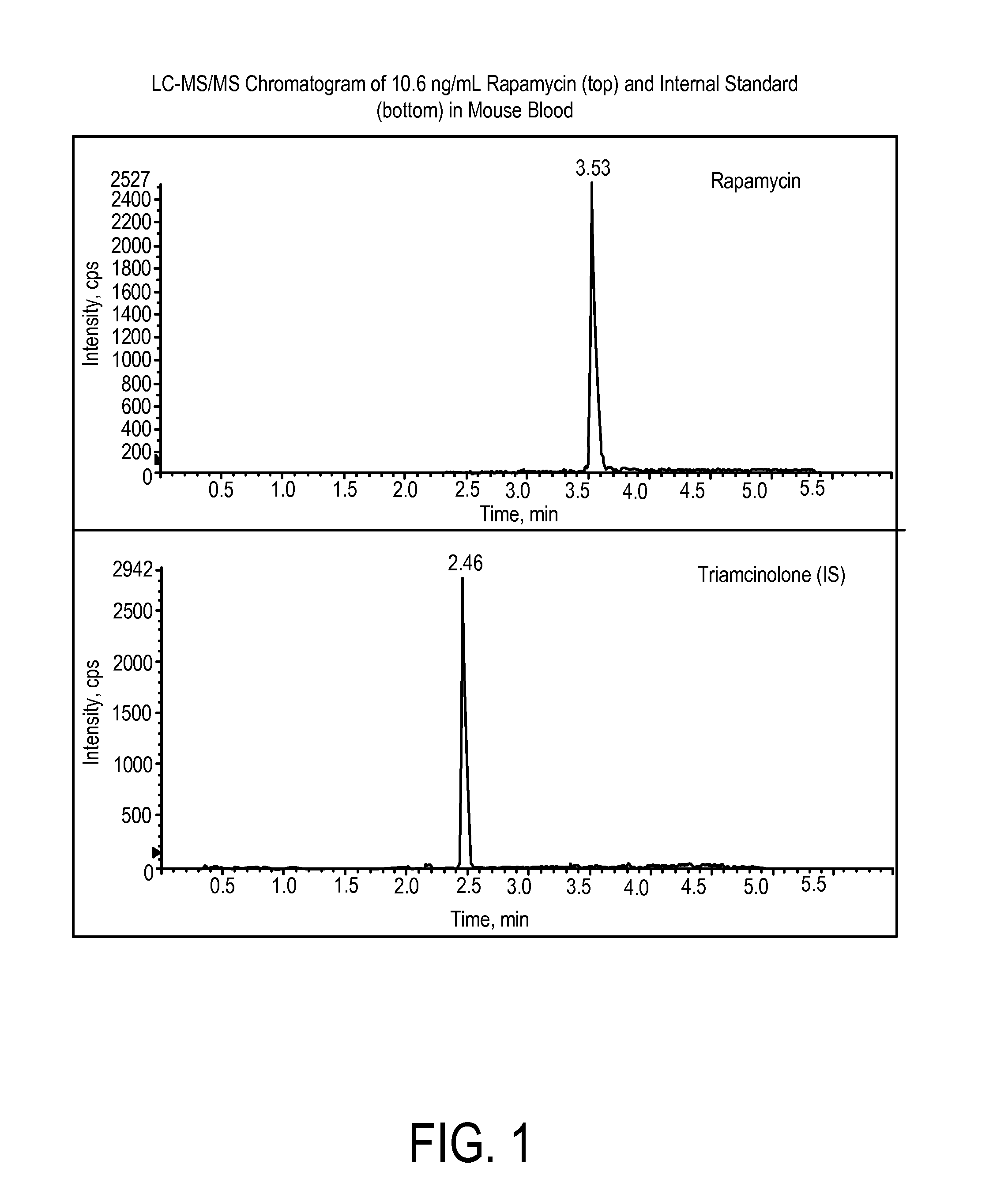
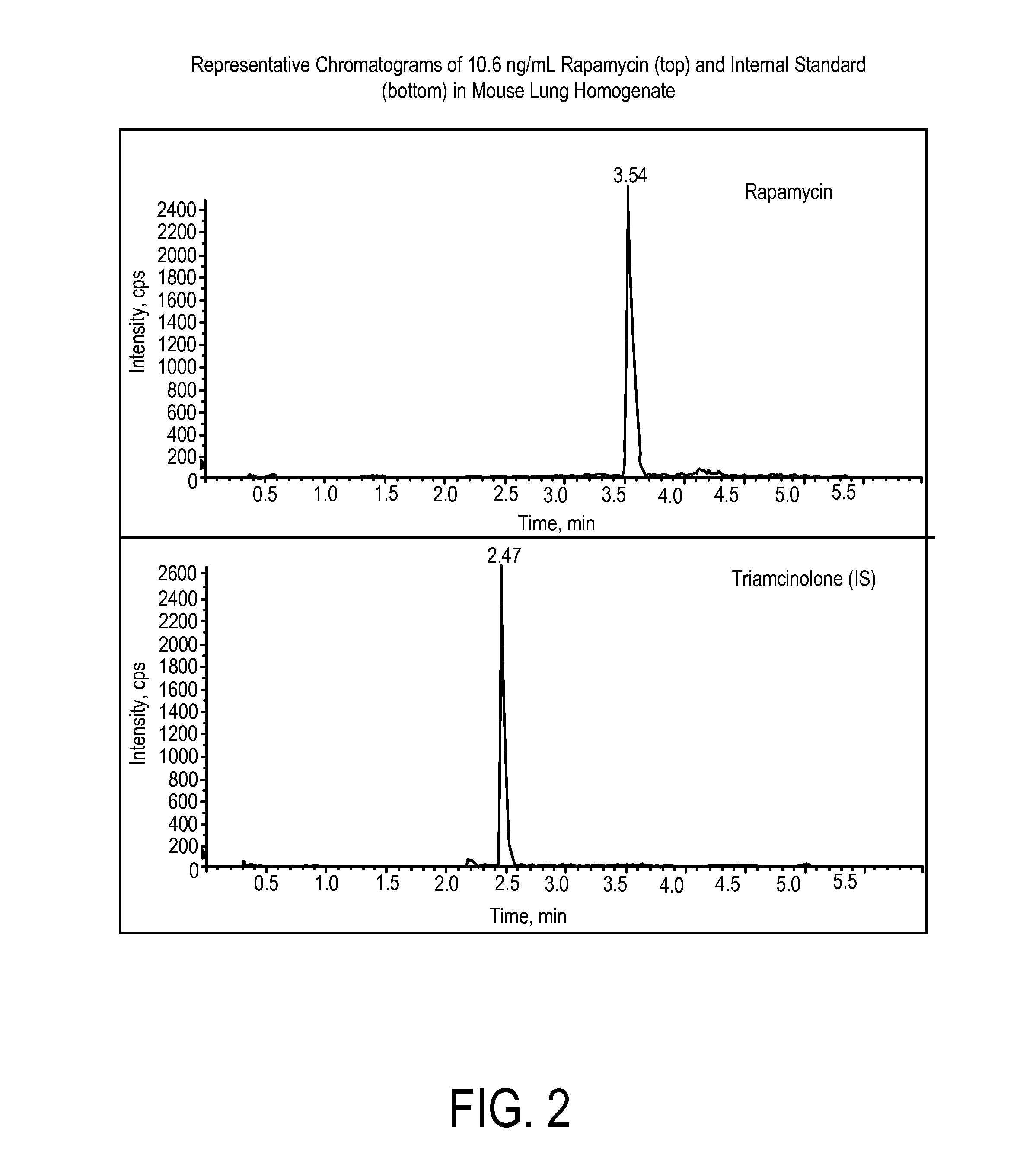
Determination of Rapamycin in Lung and Blood Following Administration by Oropharyngeal Aspiration (OPA) and Oral Gavage to C57BL6 Mice
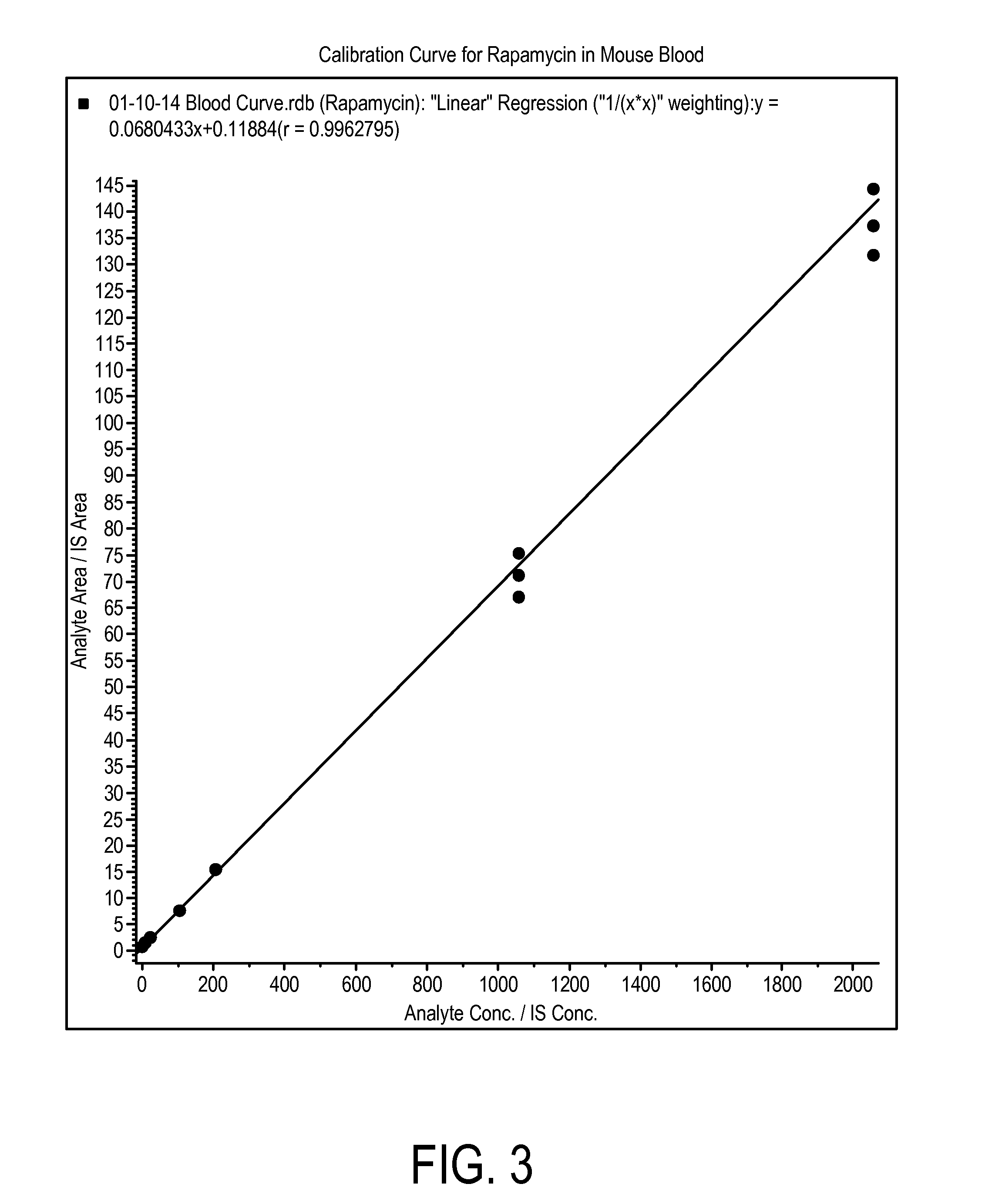
[0201]This study was conducted to evaluate the concentration of rapamycin in male C57BL / 6 mice after administration of rapamycin at a very high target dose of 1 mg / kg by gavage and oropharyngeal aspiration (OPA). A method for the analysis of rapamycin in mouse blood and lung homogenate was developed using liquid chromatography with tandem mass spectrometry detection (LC-MS / MS). Calibration curves of rapamycin using triplicate concentrations were analyzed between 1 ng / mL and 2000 ng / mL in mouse blood, and between 2 ng / mL and 20,000 ng / mL in mouse lung homogenate. Accuracy, precision and linearity were within expected ranges.
[0202]In pilot studies, the efficiency of vehicle delivery to the lungs via oropharyngeal aspiration with a volume of 50 μL per mouse was evaluated by administration of Evans Blue dye. The presence of blue dye only in lungs was veri...
PUM
| Property | Measurement | Unit |
|---|---|---|
| mean diameter | aaaaa | aaaaa |
| mean diameter | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com