Acid sensitive polymer prodrug, nanoparticles of prodrug and application of nanoparticles
A technology of acid-sensitive polymers and nanoparticles, applied in the field of medical materials, can solve the problems of easy excretion of human body side effects, low cell endocytosis efficiency, low bioavailability, etc., to achieve strong killing of cancer cells and drug resistance Sexual cancer cells, improve the therapeutic effect, improve the effect of endocytic efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
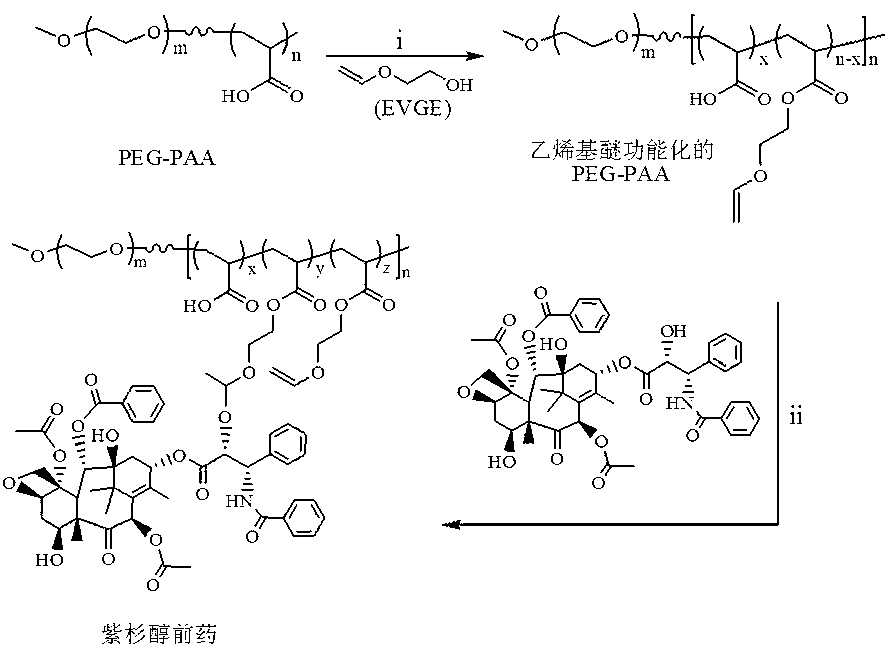
[0048] paclitaxel prodrug PEG 113 -P(AA 20 - g -PTX) preparation, attached figure 1 Synthesize a schematic diagram for it:
[0049] in N 2 Under protection, the initiator AIBN (3.12 mg, 0.019 mmol), macromolecular RAFT reagent PEG-DMP (0.48 g, 0.095 mmol), acrylic acid (163 μL, 2.375 mmol) and 6 mL of solvent 1,4-dioxane Rings were added to the 25mL sealed reactor, and continued to flow for 30 minutes under N 2 , and then the reactor was placed in an oil bath at 70°C and stirred for 48 hours. After the reaction, it was precipitated in glacial ether, and the sample was vacuum-dried for 24 hours to obtain the target diblock copolymer PEG 113 -PAA 20 , and the yield was 85%. 1 H NMR (400 MHz, D 2 O): PEG (-CH 2 -CH 2 -O-: δ 3.63; CH 3 -O-: δ 3.38), PAA (-CH 2 -CH-COO-): δ 2.37; -CH 2 -CH-COO-: δ 1.6-1.9). M n ( 1 H NMR) = 6.4 kg / mol.
[0050] Polymer PEG 113 -PAA 20 (0.3 g, 0.93 mmol COOH), dissolved in 10 mL of anhydrous 1,4-dioxane, added to a 50 ml two-nec...
Embodiment 2
[0053] paclitaxel prodrug PEG 113 -P(AA 20 - g -PTX) preparation
[0054] in N 2 environment, the PEG with a vinyl ether functionalization degree of 12 prepared in Example 1 113 -PAA 20 (100.0 mg, equv. 164.8 μmol EGVE unit) was dissolved in 10 mL of anhydrous DMF, and then sequentially added PTX (70.4 mg, 82.4 μmol), p -TSA (0.32 mg, 1.65 μmol), freshly dried over 4? molecular sieves. After reacting for 4 days, the reaction solution was filtered and then dialyzed with DMF (molecular weight cut-off 3500) to remove unreacted paclitaxel. After dialysis in DMF for 24 hours, it was transferred to water for dialysis for 48 hours. After dialysis, it was freeze-dried to obtain a white solid with a yield of about 73.5%. NMR and HPLC test results showed that the content of PTX in the prodrug was about 27.6 wt.%.
Embodiment 3
[0056] paclitaxel prodrug PEG 113 -P(AA 20 - g -PTX) preparation
[0057] in N 2 environment, the PEG with a vinyl ether functionalization degree of 12 prepared in Example 1 113 -PAA 20 (100.0 mg, equv. 164.8 μmol EGVE unit) was dissolved in 10 mL of anhydrous DMF, and then sequentially added PTX (140.8 mg, 164.8 μmol), p -TSA (0.32 mg, 1.65 μmol), freshly dried over 4? molecular sieves. After reacting for 4 days, the reaction solution was filtered and then dialyzed with DMF (molecular weight cut-off 3500) to remove unreacted paclitaxel. After dialysis in DMF for 24 hours, it was transferred to water for 48 hours for dialysis. After dialysis, it was freeze-dried to obtain a white solid with a yield of about 78.2%. NMR and HPLC test results showed that the content of PTX in the prodrug was about 42.8 wt.%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com