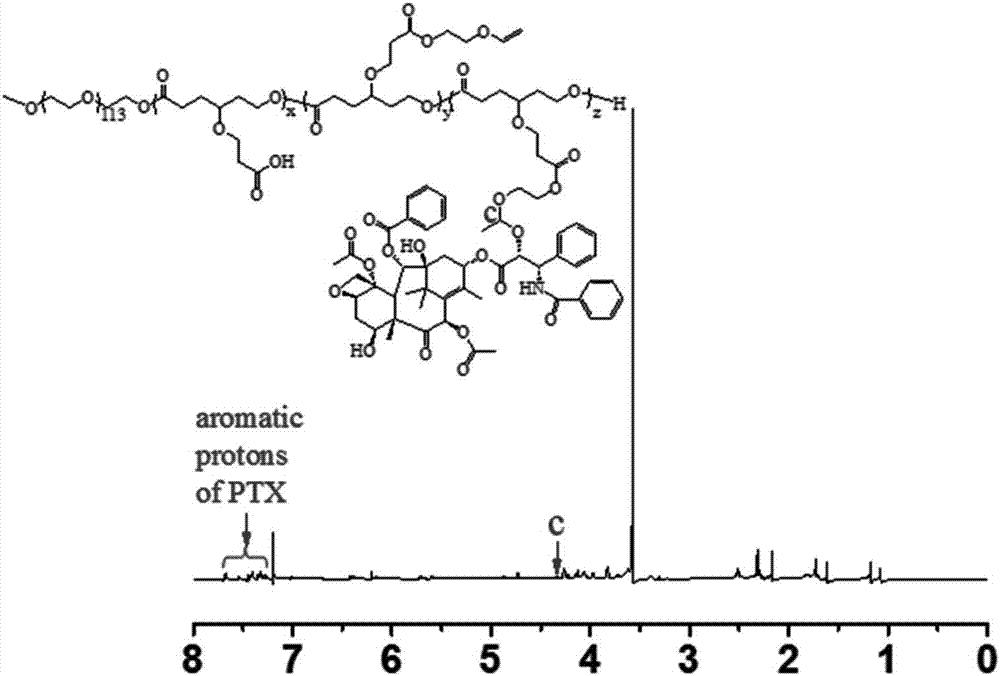
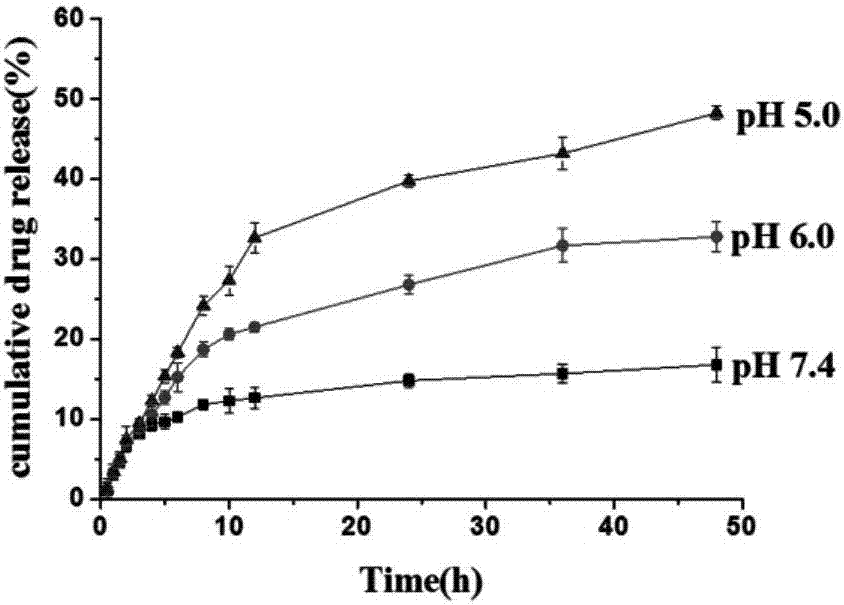
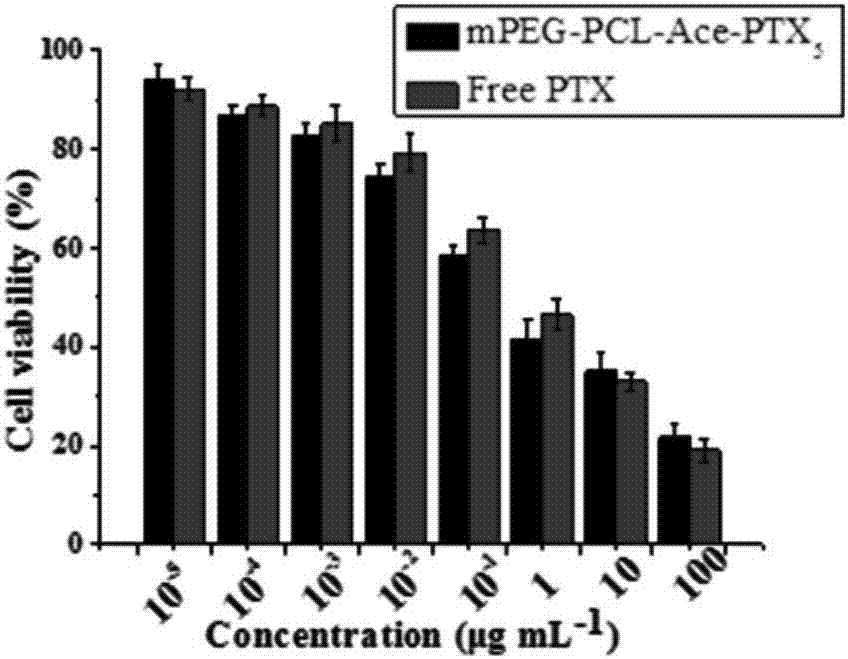
Acid-sensitive paclitaxel prodrug and preparing method thereof and prodrug nano-micelle
A nano-micelle and paclitaxel technology, which is applied in the field of prodrug nano-micelle and acid-sensitive paclitaxel prodrug, can solve the problems of high toxicity and side effects, low bioavailability, strong hydrophobicity of paclitaxel, etc., and achieve good biocompatibility Sexuality, improvement of effect, strong effect of killing tumor cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] 1) Polymerization reaction:
[0059] Add 1.29g of functionalized caprolactone monomer, 0.5g of polyethylene glycol monomethyl ether 2000 and 0.0506g of stannous octoate into a 10mL polymerization reactor. The reaction was terminated by formic acid to obtain a diblock copolymer mixture. After dissolving the reaction mixture with 5 mL of dichloromethane, the mixed solution was poured into 50 mL of methanol in an ice-water bath. The dissolution-precipitation process was repeated three times, and the filter residue was collected and vacuum-dried in a vacuum oven at 40° C. to obtain a diblock copolymer (III).
[0060] 2) Hydrolysis of ester bond
[0061] Under nitrogen protection, 1.79 g of diblock copolymer (III) was dissolved in 100 mL of dichloromethane. At 0°C, under stirring, 7.75 mL of trichloroacetic acid was slowly added dropwise to the solution. After the addition was complete, stirring was continued at 0°C for 4 hours. The solvent was evaporated under reduced ...
Embodiment 2
[0068] 1) Polymerization reaction:
[0069] Add 1.032g of functionalized caprolactone monomer, 0.4g of polyethylene glycol monomethyl ether 5000 and 0.0162g of stannous octoate into a 10mL polymerization reactor. The reaction was terminated by formic acid to obtain a diblock copolymer mixture. After dissolving the reaction mixture with 5 mL of dichloromethane, the mixed solution was poured into 50 mL of methanol in an ice-water bath. The dissolution-precipitation process was repeated three times, and the filter residue was collected and vacuum-dried in a vacuum oven at 40° C. to obtain a diblock copolymer (III).
[0070] 2) Hydrolysis of ester bond
[0071] Under nitrogen protection, 1.432 g of diblock copolymer (III) was dissolved in 100 mL of dichloromethane. 7.75 mL of trifluoroacetic acid was slowly added dropwise to the solution under stirring condition at 0°C. After the addition was complete, stirring was continued at 0°C for 4 hours. The solvent was evaporated unde...
Embodiment 3
[0077] 1) Polymerization reaction:
[0078] Add 1.032g of functionalized caprolactone monomer, 0.5g of polyethylene glycol monomethyl ether 10000 and 0.0101g of stannous octoate into a 10mL polymerization reactor. The reaction was terminated by formic acid to obtain a diblock copolymer mixture. After dissolving the reaction mixture with 5 mL of dichloromethane, the mixed solution was poured into 50 mL of methanol in an ice-water bath. The dissolution-precipitation process was repeated three times, and the filter residue was collected and vacuum-dried in a vacuum oven at 40° C. to obtain a diblock copolymer (III).
[0079] 2) Hydrolysis of ester bond
[0080] Under nitrogen protection, 1.532 g of diblock copolymer (III) was dissolved in 100 mL of dichloromethane. 7.75 mL of p-toluenesulfonic acid was slowly added dropwise to the solution under stirring condition at 0°C. After the addition was complete, stirring was continued at 0°C for 4 hours. The solvent was evaporated u...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com