Optically pure benzyl-4-chlorophenyl C-glucoside derivatives
A benzyl and trimethylsilyl technology, applied in the field of optically pure C-glycoside derivatives of benzyl-4-chlorophenyl, can solve the problem of increasing research and development risks, reducing drug efficacy and quality control difficulties, toxic and side effects And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
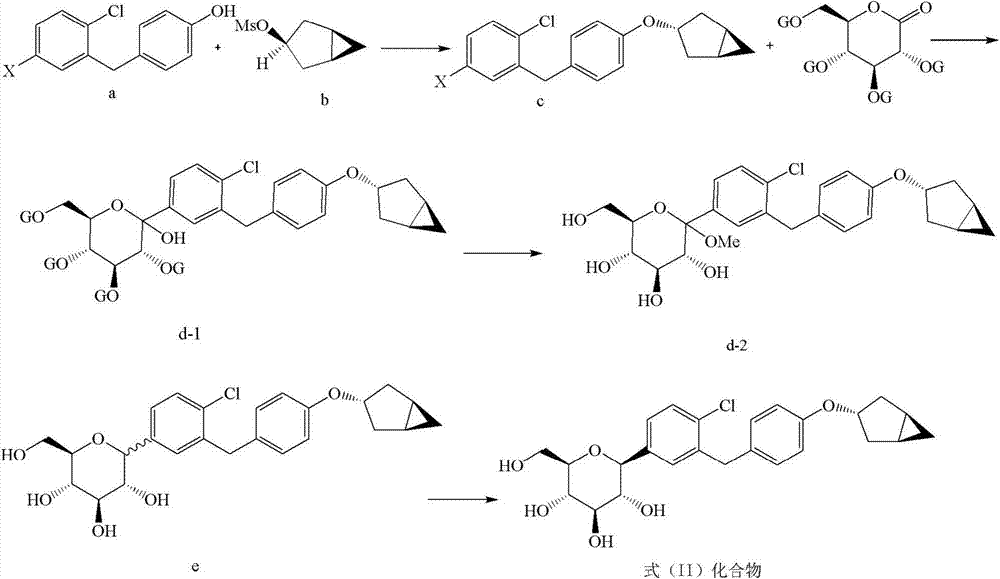
Examples
experiment example 1
[0069] Experimental Example 1 The in vitro activity test of the compound of the present invention
[0070] Test product: the compound of formula (II), formula (III), formula (IV) and formula (V) of the present invention, its chemical name and preparation method are shown in the preparation examples of each compound.
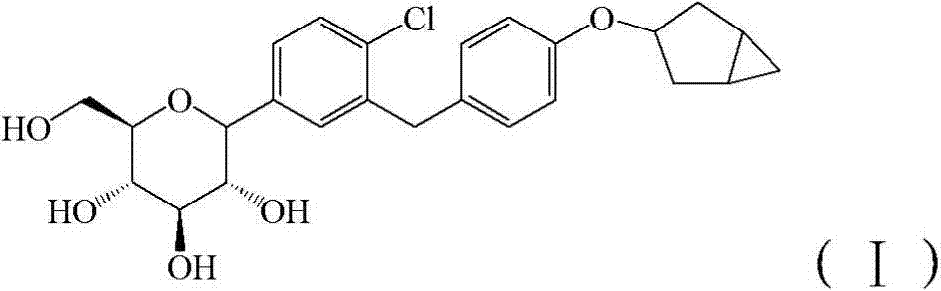
[0071] Control drug 1: compound 4 in patent WO2013 / 000275A1, self-made (refer to patent WO2013 / 000275A1 for the preparation method), and its structural formula is as follows:
[0072] Compound 4 is the compound of formula (I).
[0073] The meanings represented by the abbreviations of the following experiments are as follows:
[0074] NMG N-methylglucosamine (N-methyl-glucosamine)
[0075] KRH Krebs-Ringer-Henseleit
[0076] Experimental method: The human SGLT-2 and SGLT-1 sequences were transfected into Chinese hamster ovary cells for stable expression, and by inhibiting the cells to [ 14 C]-labeled-R-methyl-D-glucopyranoside (AMG) sodium-dependent absorpt...
experiment example 2
[0085] Experimental Example 2 Pharmacokinetics experiment in rats of compounds of the present invention
[0086] Test animals: 6-8 week old male SD rats (purchased from Beijing Weitong Lihua Experimental Animal Technology Co., Ltd.), 3 rats / compound, body weight 200-240g.
Embodiment 1
[0088] Control drug 1: compound 4 in patent WO2013 / 000275A1, self-made (refer to patent WO2013 / 000275A1 for the preparation method), and its structural formula is as follows:
[0089] Compound 4 is the compound of formula (I).
[0090] Control drug 2: compound 22 in patent WO2013 / 000275A1, self-made (refer to patent WO2013 / 000275A1 for the preparation method), and its structural formula is as follows:
[0091] Compound 22.
[0092] Solvent: 0.5% MC (methylcellulose) solution + 0.1% SDS (sodium dodecyl sulfate).
[0093] experimental method:
[0094] See Table 2 for the administration method of intragastric administration (PO)
[0095] Rat PK (pharmacokinetics) administration method of table 2 compound
[0096]
[0097] Blood collection: 0.17 hours, 0.5 hours, 1 hour, 2 hours, 4 hours, 6 hours, 8 hours, 24 hours, 30 hours, 48 hours, 54 hours, 72 hours, take about 200 μL whole blood at each time point, at low temperature High-speed centrifuge (5415R, Eppendorf) wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com