Dispersible tablet for treating cold and its preparing process
A technology for dispersible tablets and colds, which is applied in the field of dispersible tablets for treating colds and its preparation technology, and can solve the problems of high incidence of colds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Acetaminophen 325g Pseudoephedrine hydrochloride 30g
[0014] Dextromethorphan Hydrobromide 15g Chlorpheniramine Maleate 2g
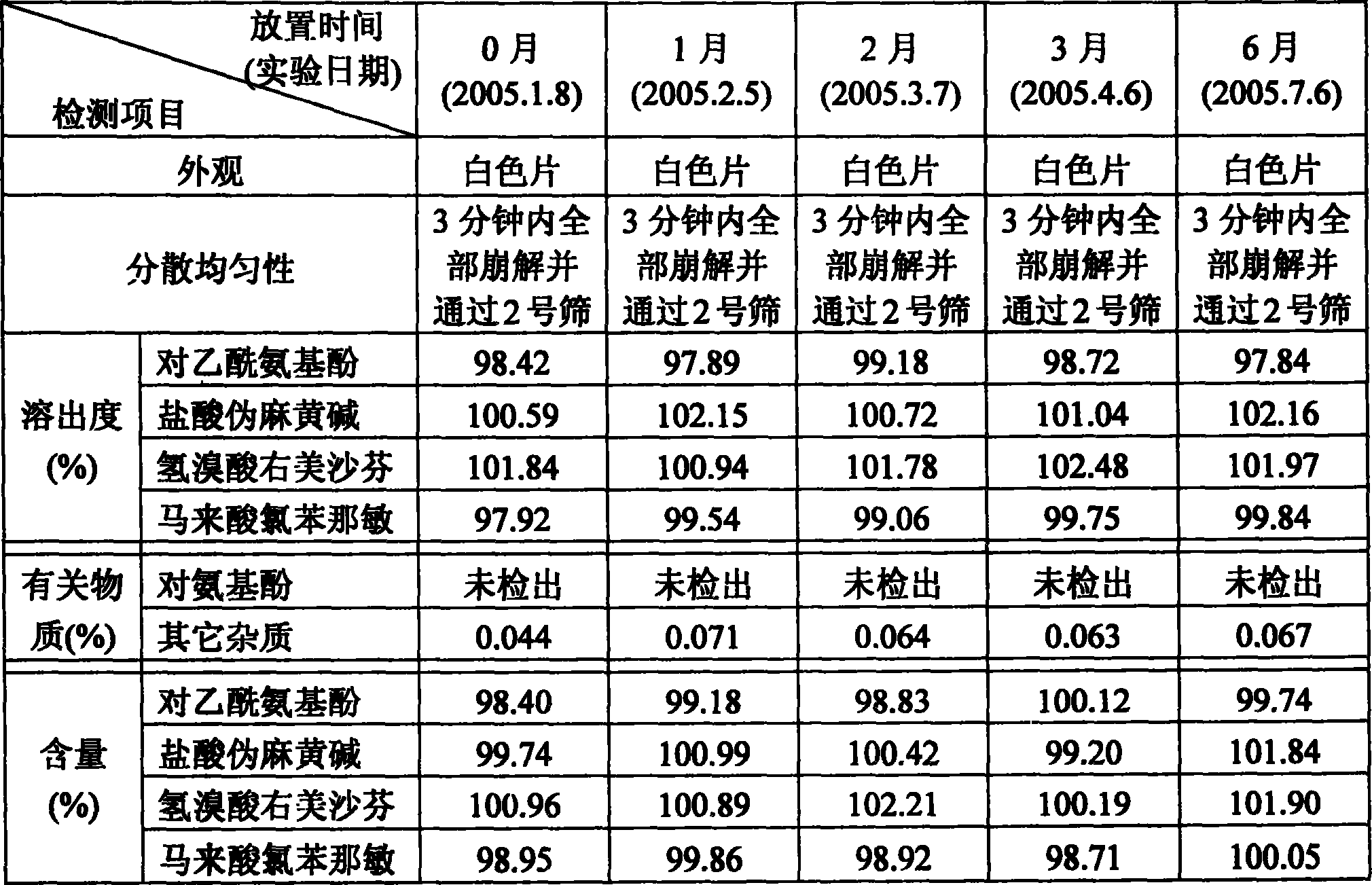
[0015] The preparation method of the present invention: respectively pulverize acetaminophen, pseudoephedrine hydrochloride, dextromethorphan hydrobromide and chlorpheniramine maleate, pass through a 100-mesh sieve, and set aside. Weigh the prescription amount povidone K 30 , add an appropriate amount of water to prepare a 10% solution, as a binder, and set aside. Weigh the prescribed amount of paracetamol fine powder, pseudoephedrine hydrochloride fine powder, dextromethorphan hydrobromide fine powder, chlorpheniramine maleate fine powder, microcrystalline cellulose, low-substituted hydroxypropyl cellulose, aspirin For Patan, mix evenly with equal increments, add binder to make soft material, granulate with 35 mesh sieve, dry at 60°C, granulate with 35 mesh sieve, add prescription amount of crospovidone and micropowder silica gel , magnesium ...
Embodiment 2
[0017] Acetaminophen 325g Pseudoephedrine hydrochloride 30g
[0018] Dextromethorphan Hydrobromide 15g Chlorpheniramine Maleate 2g
[0019] The preparation method of the present invention: respectively pulverize acetaminophen, pseudoephedrine hydrochloride, dextromethorphan hydrobromide and chlorpheniramine maleate, pass through a 100-mesh sieve, and set aside. Weigh the prescription amount povidone K 30 , add an appropriate amount of water to prepare a 10% solution, as a binder, and set aside. Weigh the prescribed amount of paracetamol fine powder, pseudoephedrine hydrochloride fine powder, dextromethorphan hydrobromide fine powder, chlorpheniramine maleate fine powder, microcrystalline cellulose, low-substituted hydroxypropyl cellulose, aspirin For Patan, mix evenly with equal increments, add binder to make soft material, granulate with 35 mesh sieve, dry at 60°C, granulate with 35 mesh sieve, add prescription amount of crospovidone and micropowder silica gel , magnesium ...
Embodiment 3
[0021] Acetaminophen 325g Pseudoephedrine hydrochloride 30g
[0022] Dextromethorphan Hydrobromide 15g Chlorpheniramine Maleate 2g
[0023] The preparation method of the present invention: respectively pulverize acetaminophen, pseudoephedrine hydrochloride, dextromethorphan hydrobromide and chlorpheniramine maleate, pass through a 100-mesh sieve, and set aside. Weigh the prescription amount povidone K 30 , add an appropriate amount of water to prepare a 10% solution, as a binder, and set aside. Weigh the prescribed amount of paracetamol fine powder, pseudoephedrine hydrochloride fine powder, dextromethorphan hydrobromide fine powder, chlorpheniramine maleate fine powder, microcrystalline cellulose, low-substituted hydroxypropyl cellulose, aspirin For Patan, mix evenly with equal increments, add binder to make soft material, granulate with 35 mesh sieve, dry at 60°C, granulate with 35 mesh sieve, add prescription amount of crospovidone and micropowder silica gel , magnesium ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com