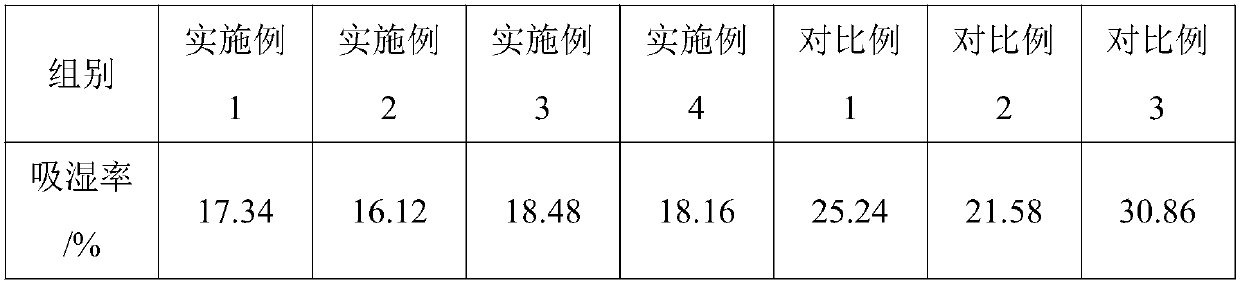
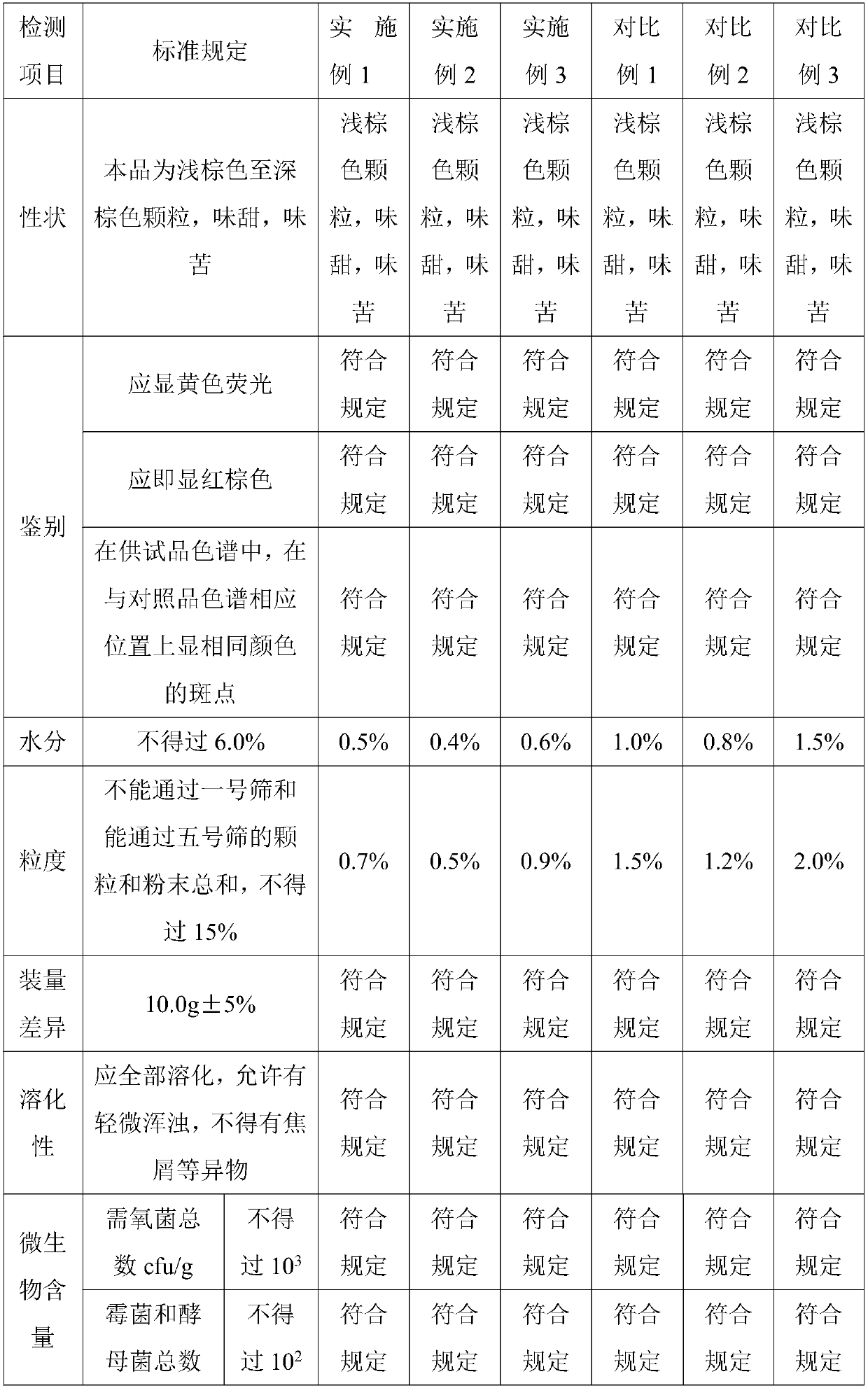
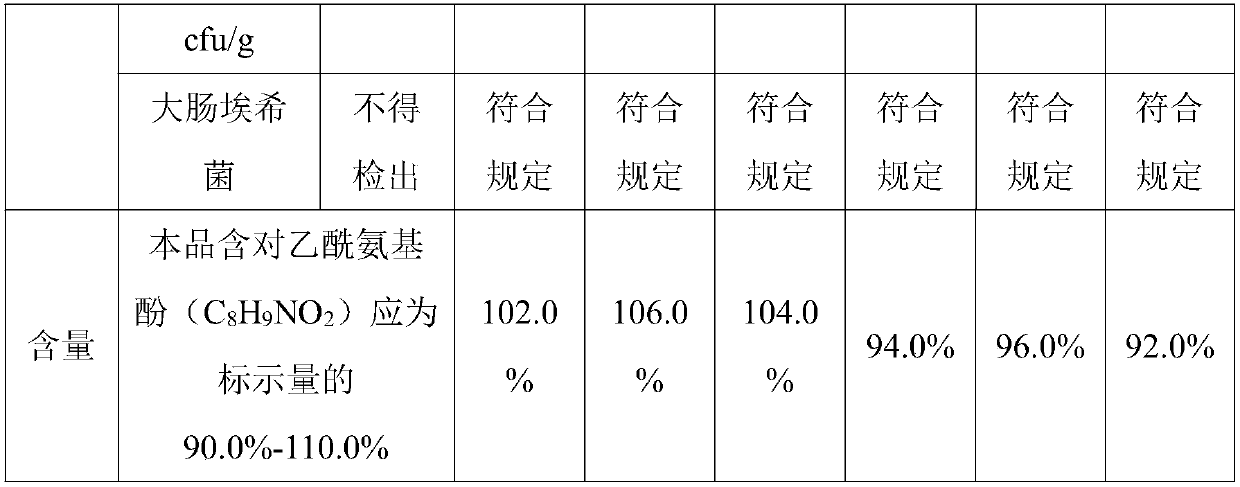
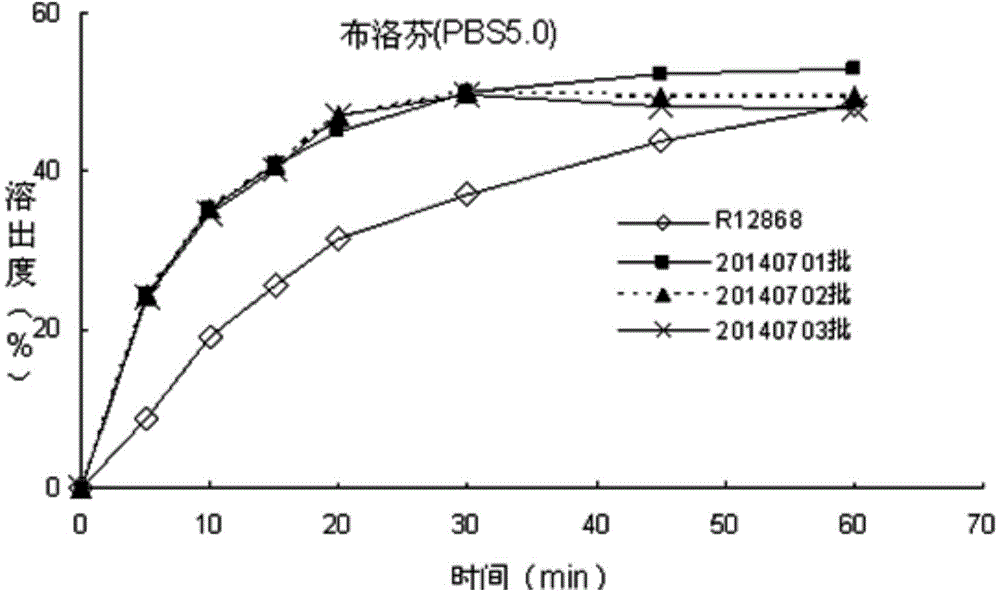
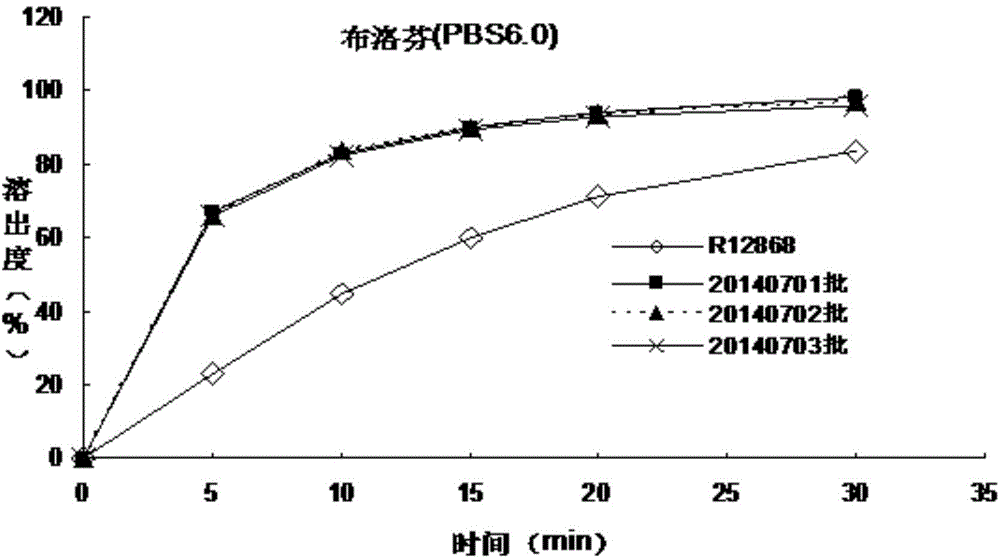
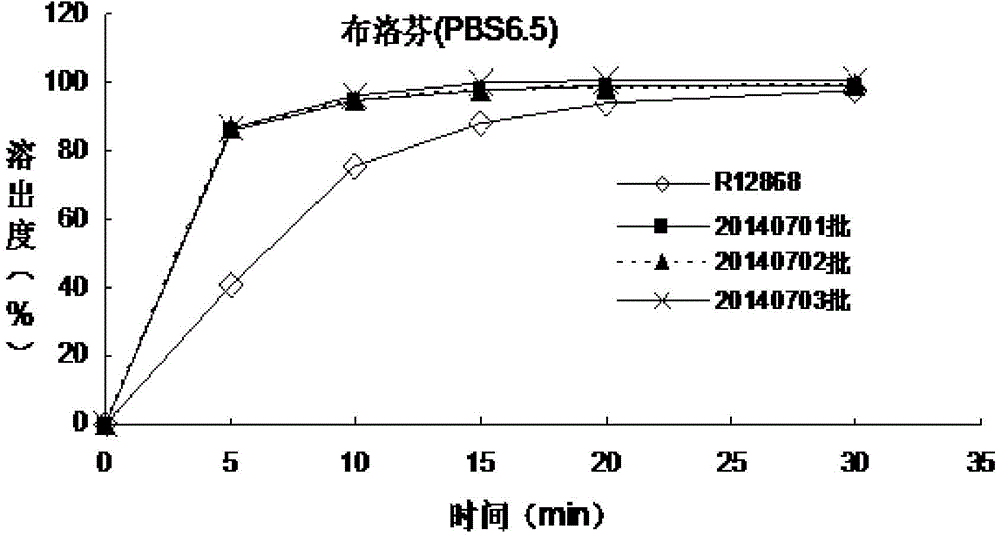
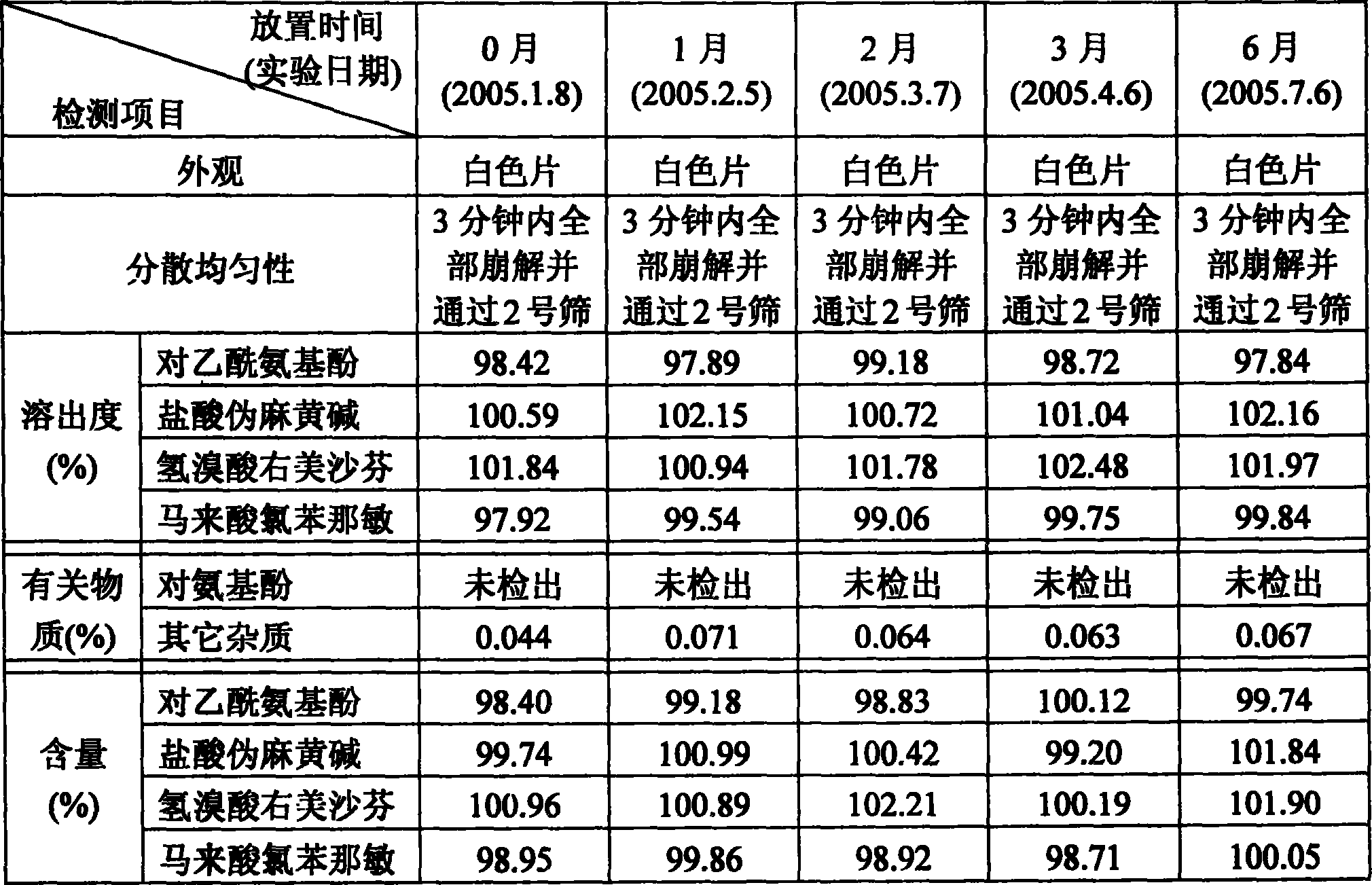
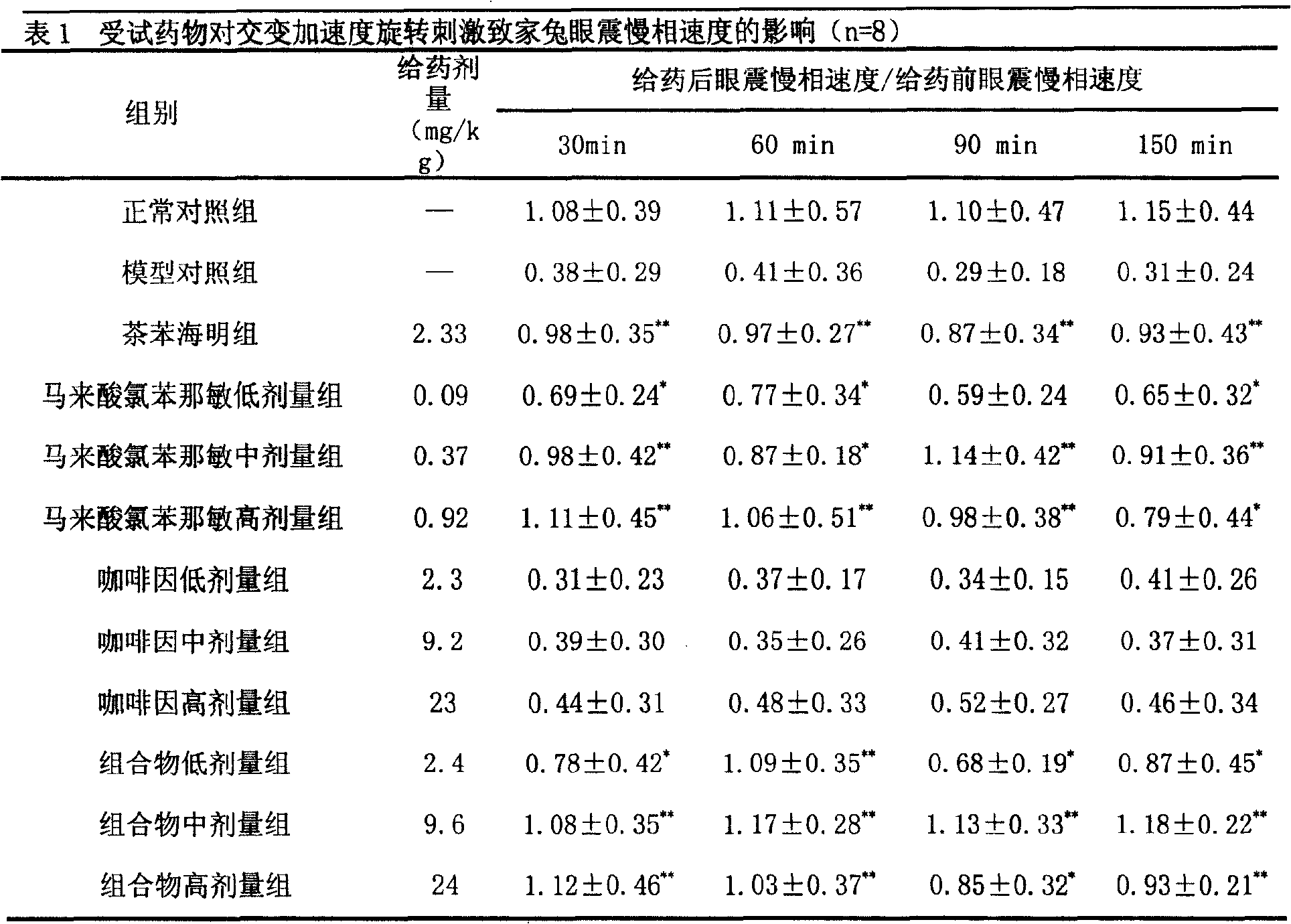
Patents
Literature
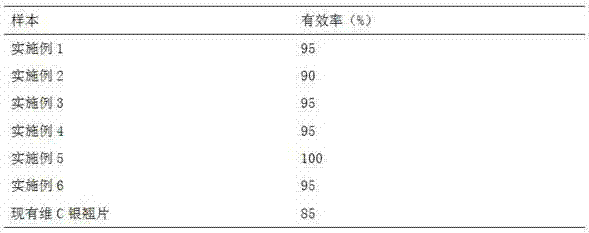
297 results about "Chlorpheniramine Maleate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A synthetic alkylamine derivative used in allergic reactions, hay fever, rhinitis, urticaria, and asthma, antihistamine Chlorpheniramine Maleate acts as a competitive histamine H1 receptor antagonist, and displays anticholinergic and mild sedative effects as well. (NCI04)
Oxymetazoline HCI and/or chlorpheniramine maleate nasal spray compositions
InactiveUS6316483B1Extend nasal muco-cilia clearance timeBiocidePharmaceutical delivery mechanismNasal sprayWater soluble
Aqueous nasal spray compositions comprising a medicament and an aqueous carrier comprising water soluble polymers selected from the group consisting of polyvinylpyrrolidone and mixtures thereof.
Owner:MSD CONSUMER CARE INC
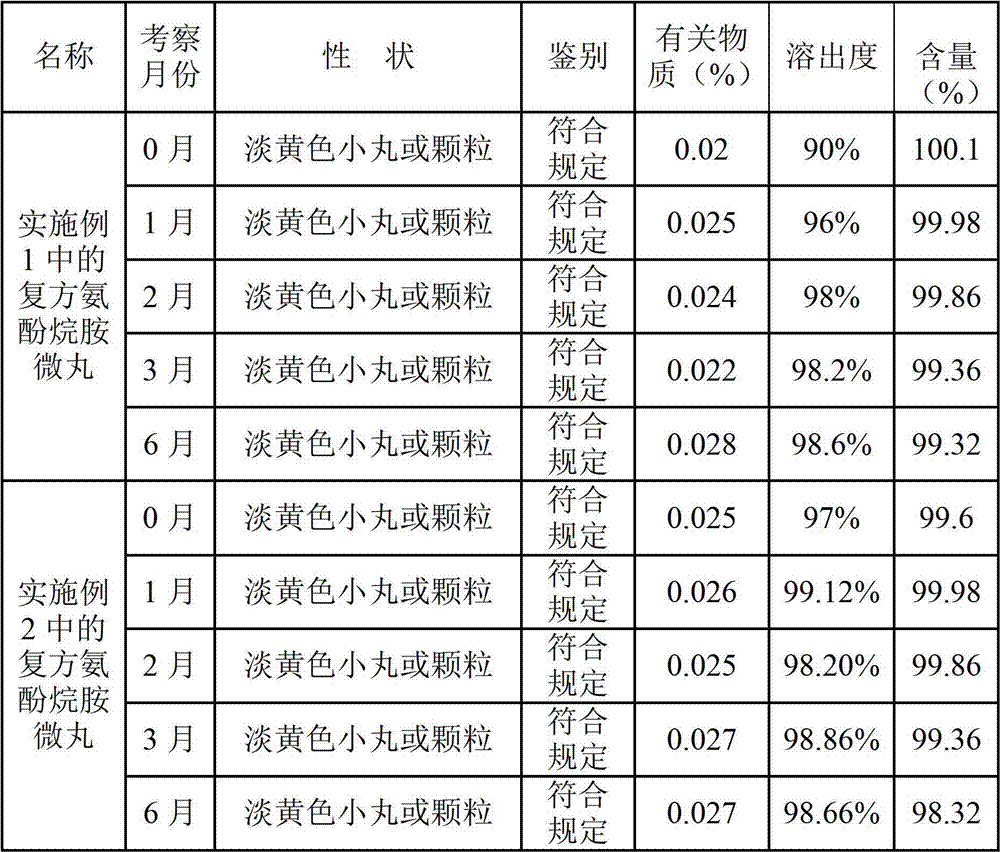
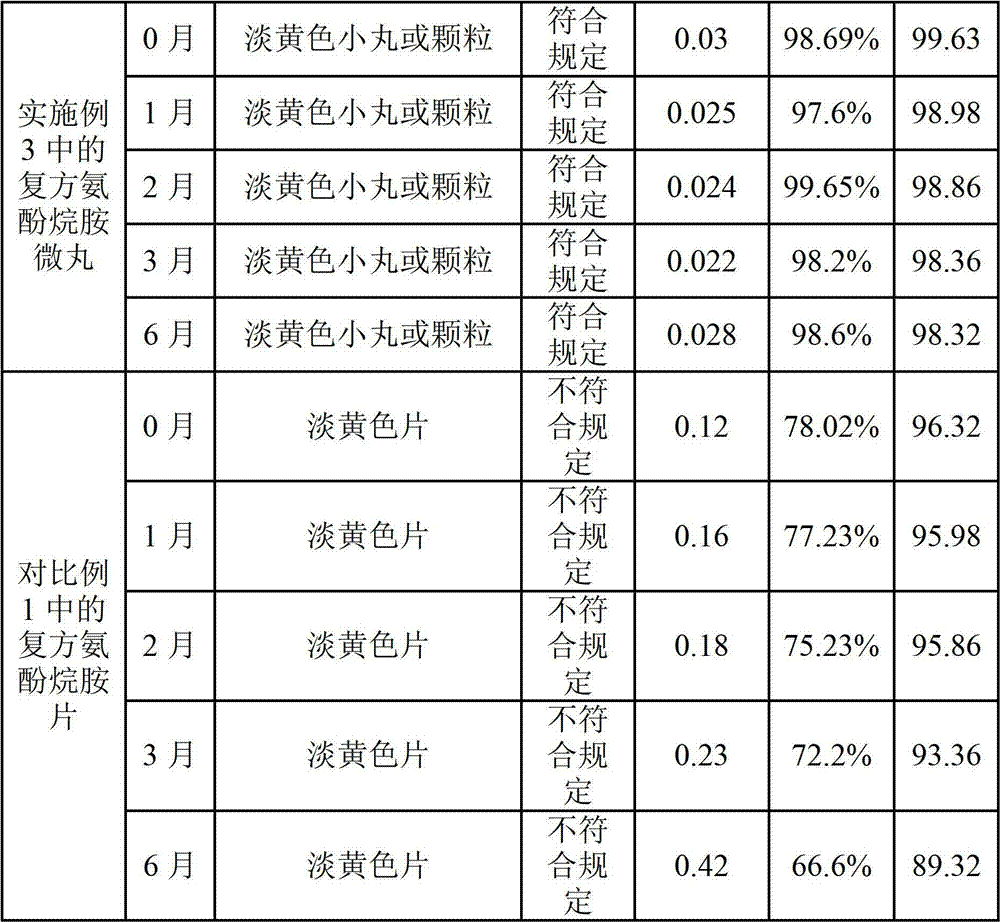
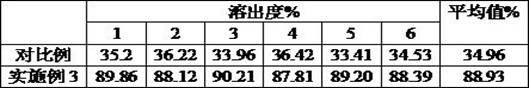
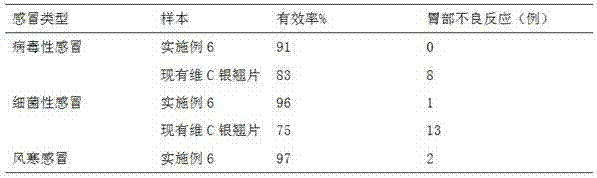
Preparation method of compound paracetamol and amantadine pellets
ActiveCN102861106AEvenly distributedEasy to fillNervous disorderAntipyreticDissolutionCalculus bovis
The invention discloses a preparation method of compound paracetamol and amantadine pellets. The compound paracetamol and amantadine pellet is mainly prepared from the following raw materials: chlorpheniramine maleate, calculus bovis factitious, caffeine, amantadine hydrochloride, acetaminophen and dextrin, and the mass ratio of the above raw materials is 1 to 5 to 7.5 to 50 to 125 to (4-5); the preparation method comprises the following steps: adding the chlorpheniramine maleate and the caffeine in the form of solution into a dextrin aqueous solution, spraying and packing the mixed solution on the mixed masterbatches consisting of the acetaminophen, the amantadine hydrochloride and the calculus bovis factitious, and then spraying the rest of dextrin and the rest of mixed powder to obtain the compound paracetamol and amantadine pellet. The method is simple to operate, liable to control and suitable for industrial production, and the obtained pellet has the advantages of low related substances, good content uniformity and dissolution rate and the like.
Owner:HAINAN HULUWA PHARMA GRP CO LTD
Medicine for relieving cough and asthma
The present invention relates to a specific medicine with the functions of relieving inflammation, clearing away heat and toxic material, supplementing spleen and kidney, disinhibiting qi of lung, suppressing cough and calming panting, and is made up by using Chinese medicinal material portion and Western medicine portion, in which the Chinese medicinal material portion contains 18 Chinese medicinal materials of houttuynia, isatis root, Sichuan fritillaria bulb, ephedra, apricot kernel, stemona root, mulberry root bark, ginseng and others, and its Western medicine portion includes midecamycin, chlorpheniramine maleate, aminophylline and compound tablets of licorice.
Owner:高守荣 +1
Compound Ganmaoling granules and preparation method thereof
InactiveCN104383332ADispelling wind is strongGood treatment effectDispersion deliveryPill deliveryTreatment effectChrysanthemum Flower
The invention relates to the field of medicines, and in particular relates to compound Ganmaoling granules and a preparation method thereof. The compound Ganmaoling granules disclosed by the invention are prepared by decocting, mixing and blending 16 natural raw materials including honeysuckle, rheum officinale, herba viticis, schizonepeta, radix scrophulariae, fructus forsythiae, wild chrysanthemum flower, cape jasmine flower, evodia lepta, astragalus membranaceus, rhizoma phragmitis, cusiae, chufa, holly root, wight osyris root and leaf and mint with acetaminophen, chlorpheniramine maleate and caffeine through a special preparation method. The compound Ganmaoling granules disclosed by the invention have the effects of relieving the exterior syndrome with drugs pungent in flavour and cool in property, clearing heat and removing toxicity, and are obvious in curative effect; and furthermore, the compound Ganmaoling granules disclosed by the invention are rapid to take effect and are capable of treating anemopyretic cold rapidly and effectively.
Owner:华润三九(郴州)制药有限公司
Eye medicine for preventing and treating asthenopia and allergy
InactiveCN1839834AOvercome the disadvantages of taking single medicineCompatibility is reasonableSenses disorderAnhydride/acid/halide active ingredientsMentholAllergy
Disclosed is a compound preparation for treating and preventing eye strain and allergy, which comprises (by weight percent) taurine 0.1-99.9%, naphazoline hydrochloride 0.01-99.9%, vitamin B6 0.01-99.9%, chlorpheniramine maleate 0.01-99.9%, and right amount of auxiliary therapeutic medicaments such as menthol, sodium hyaluronate, glycerine and medicinal findings such as preservative agent and buffering agent.
Owner:崔彬
Preparing method of paracetamol, caffein, atificial cow-bezoar and chlorphenamine maleate capsule content functional pellet
InactiveCN102614220AImprove liquiditySmall weight differenceUnknown materialsPharmaceutical non-active ingredientsChlorobenzenePharmaceutical Aids
A preparing method of a paracetamol, caffein, atificial cow-bezoar and chlorphenamine maleate capsule content functional pellet includes: respectively performing micronization process on bulk pharmaceutical chemicals of acetaminophen, caffeine, chlorpheniramine maleate and calculus bovis factitious, taking 23%-52% of bulk pharmaceutical chemicals by weight, adding auxiliary materials into the bulk pharmaceutical chemicals to prepare a raw material primary kernel, placing the primary kernel into a sugar coating pot, and performing spraying coating on the raw material medicine primary kernel with spray and balance bulk pharmaceutical chemicals to obtain bulk pharmaceutical chemical pellets; mixing the auxiliary materials and fillers to prepare a resolving-assisting pellet; and mixing the bull pharmaceutical chemical pellets including 250mg of acetaminophen, 15mg of caffeine, 1mg of chlorpheniramine maleate and 10mg of calculus bovis factitious, and using the resolving-assisting pellet serving as the balance for filling, so that the total weight of the capsule content is 340mg. The preparing method has the advantages of: adopting micronization processing and being good in pellet liquidity, small in weight difference in a filling process and easy to fill; performing granulation separately and being capable of strengthening functions of the bulk pharmaceutical chemicals and stable in medicine composition; and being fast in disintegration and dissolving out, capable of fast achieving blood concentration of treating and good in treating effect.
Owner:辽宁王牌速效制药有限公司
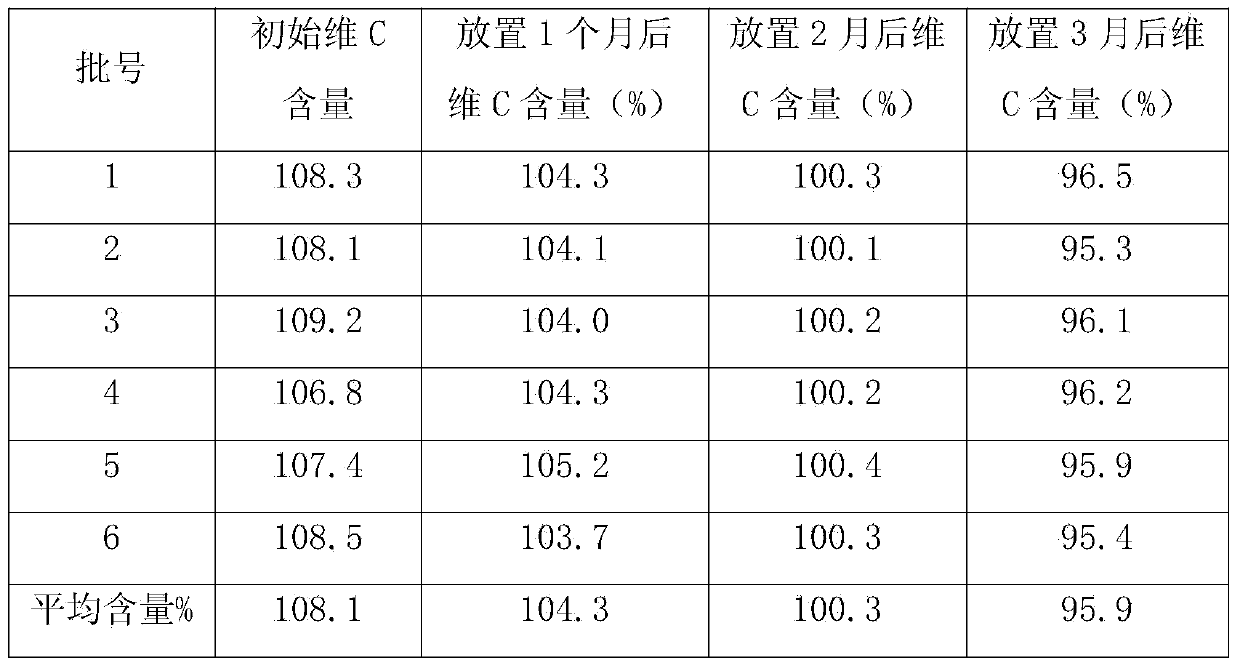
Novel vitamin C yinqiao tablet
ActiveCN103169891ASignificant effectWide range of indicationsAntibacterial agentsHydroxy compound active ingredientsDiseaseVitamin C
The invention provides a novel vitamin C yinqiao tablet. The novel vitamin C yinqiao tablet is prepared from the following medicines in parts by weight: 60-360 parts of honeysuckle flowers, 60-360 parts of fructus forsythia, 24-144 parts of schizonepeta, 30-180 parts of fermented soybeans, 24-144 parts of lophatherum gracile, 36-216 parts of burdocks, 36-216 parts of reed rhizomes, 36-216 parts of platycodon grandiflorum, 30-180 parts of liquorices, 0.5-2 parts of chlorpheniramine maleate, 35-210 parts of paracetamol, 5-100 parts of vitamin C and 0.5-2 parts of peppermint oil. The novel vitamin C yinqiao tablet disclosed by the invention can be used for preventing pathogenic microorganisms including bacterial cold, viral cold as well as upper respiratory tract infection caused by mycoplasma and Chlamydia. Moreover, the novel vitamin C yinqiao tablet has a remarkable curative effect to the wind-cold type common cold and the wind-heat type common cold and is extensive in adaptation disease range.
Owner:HUBEI WUDANG JINDING PHARMA
Artificial cowbezoar and chlorpenaleate capsule and preparation method thereof
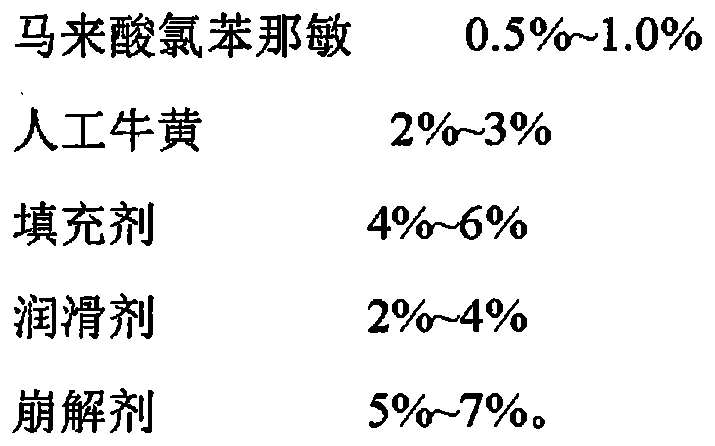
The invention relates to an artificial cowbezoar and chlorpenaleate capsule and a preparation method thereof. The artificial cowbezoar and chlorpenaleate capsule is prepared from the following raw materials in percentage by weight: 68-75 percent of paracetamol, 4-6 percent of caffeine, 0.5-1.0 percent of chlorpheniramine maleate, 2-3 percent of artificial calculus bovis, 4-6 percent of a filler, 2-4 percent of a lubricant and 5-7 percent of a disintegrant. The artificial cowbezoar and chlorpenaleate capsule is reasonable in formula, high in drug dissolution, simple in preparation method without pollution, remarkable in efficacy and easy in popularization.
Owner:SHANGHAI HUAYUAN ANHUI RENJI PHARMA
Capsule for treating rhinitis
InactiveCN101297862ADefinite curative effectHigh cure rateCapsule deliveryRespiratory disorderPeppermintsExisting Treatment
The invention pertains to the technical field of drugs for treatment for rhinitis, which can solve the problem of low effective rate of the existing treatment method of rhinitis. A rhinitis capsule is prepared by the following pharmaceutical raw materials: biond magnolia flower, siberian cocklour fruit, Szechuan lovage rhizome, dahurian angelica root, fineleaf schizonepeta herb, divaricate saposhnikovia root, Chinese thorowax root, baicalin, manchurian wildginger, platycodon root, peppermint, liquoric root, membranous milkvetch root, Chinese angelica, metronidazole, vitamin B1, chlorpheniramine maleate and prednisone acetate. The drug of the invention effectively combines the traditional Chinese medicine and the western medicine and learns from each other to offset the weaknesses; the clinical applications show that the efficacy is exact and the cure rate is high.
Owner:赵军海
Preparation method of Chinese herba preparation vitamin C yinqiao tablet
InactiveCN102614400AImprove stabilityImprove efficacyOrganic active ingredientsPharmaceutical delivery mechanismVitamin CPeppermints
The invention belongs to the field of medicines, relates to a Chinese herba tablet and a preparation method of the Chinese herba tablet, in particular to Chinese herba tablet vitamin C yinqiao tablet and a preparation method of the vitamin C yinqiao tablet. The preparation method of the vitamin C yinqiao tablet comprises the following steps: (1) preparing an essential oil wrappage: extracting fructus forsythiae, schizonepeta and honeysuckle to obtain essential oil, combining and mixing, wrapping essential oil mixed solution by beta-cyclodextrin to obtain a beta-cyclodextrin wrappage; (2) preparing dry extract power: adding water in the fructus forsythiae, the schizonepeta and dregs of the honeysuckle with lophatherum gracile, fermented soybean, rhizoma phragmitis, platycodon grandiflorum and liquorice and then decocting; and extracting burdock by ethanol, and then extracting and drying to obtain the dry extract powder; (3) preparing a tablet: mixing the dry extract powder, acetaminophen and micro particles of vitamin C uniformly to prepare particles, drying the particles, adding chlorpheniramine maleate and the beta-cyclodextrin wrappage in the particles, mixing uniformly, adding peppermint oil, pressing into the tablet, wrapping an isolation layer, and wrapping a sugar coating.
Owner:JIANGXI JIMINKEXIN PHARMA +1
Vitamin C Yinqiao tablets and preparation method thereof
InactiveCN104189588AIncrease polarityReduce adverse reactionsOrganic active ingredientsAntipyreticVitamin CGLYCYRRHIZA EXTRACT
The invention discloses vitamin C Yinqiao tablets. The vitamin C Yinqiao tablets are prepared in the following steps: adding 60-80 parts of folium isatidis, 70-90 parts of radix bupleuri, 70-90 parts of platycodon grandiflorus, 50-70 parts of astragalus, 40-60 parts of Cyrtomium fortunei, 30-60 parts of chrysanthemum and 40-60 parts of isatis root into a formula of the vitamin C Yinqiao tablets, adding a proper amount of auxiliary materials to be prepared into 1000 tablets, coating, thereby obtaining the vitamin C Yinqiao tablets. The invention also discloses a process for preparing the vitamin C Yinqiao tablets. According to the vitamin C Yinqiao tablets disclosed by the invention, the active ingredients of medicinal materials such as the radix bupleuri, liquorice, isatis root and folium isatidis are added into the prescription under the condition that the curative effect of the medicine is basically stable, and the adverse reaction of chlorpheniramine maleate, acetaminophen and vitamin C taken by patients can be relieved. Meanwhile, the stability, drug quality and drug effect of the vitamin C Yinqiao tablets in the production process are improved, the validity of the vitamin C Yinqiao tablets is prolonged, and the content of vitamin C is still larger than 95 percent after the vitamin C Yinqiao tablets are stored within 3 months at room temperature.
Owner:JIANGXI JIUHUA PHARMA
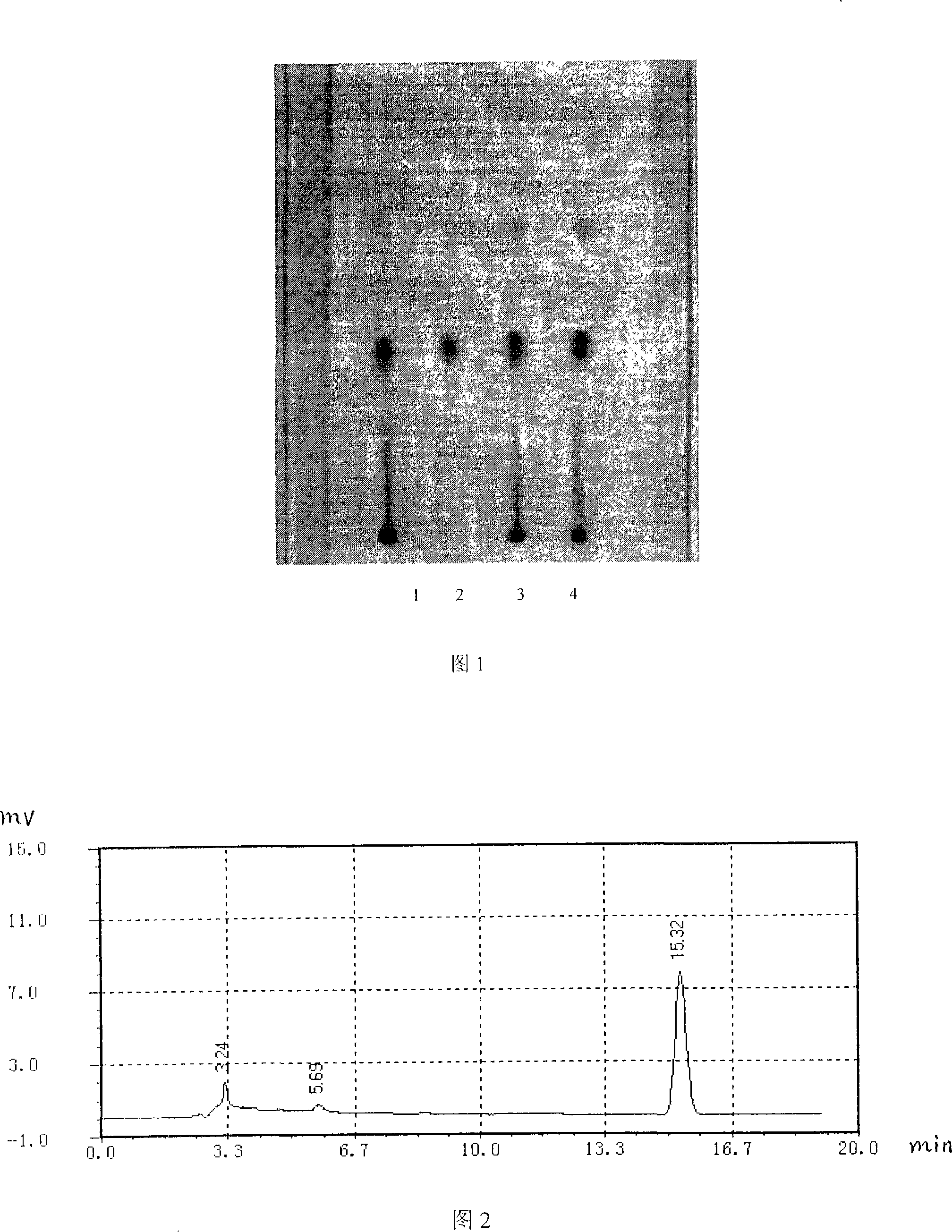
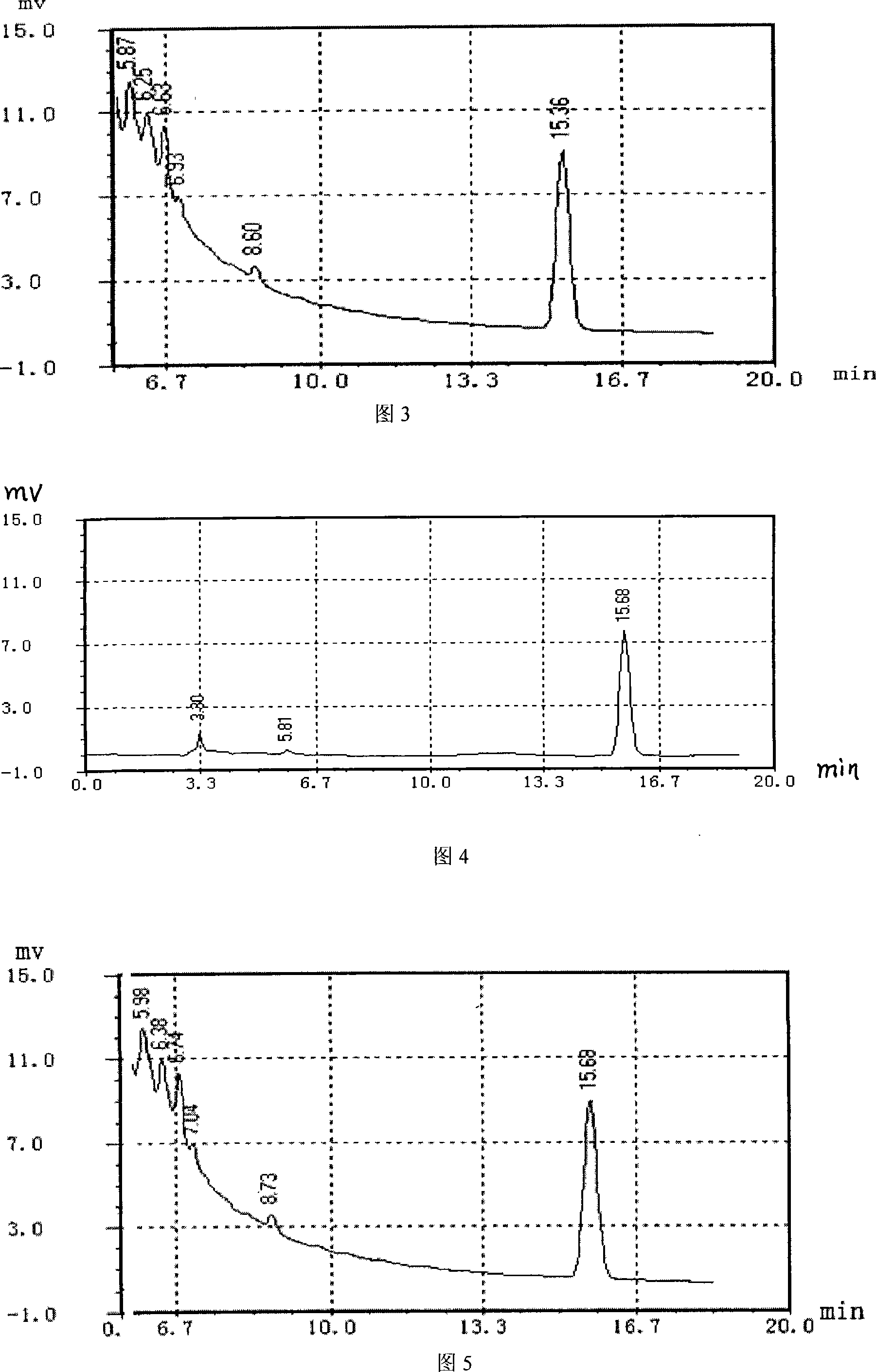
Mass control method of ketelin lozenge
InactiveCN101181357AQuality improvementEasy to separateOrganic active ingredientsComponent separationAdditive ingredientThin layer chromatographic
The invention relates to a quality control method of chlorpheniramine maleate of Keteling troche, which is characterized in that the method includes a chlorpheniramine maleate thin layer chromatographic method examination of the Keteling troche pressing grain, a content measurement of the chlorpheniramine maleate with high-efficiency liquid phase chromatography, a chlorpheniramine maleate thin layer chromatographic method examination of Keteling troche finished product and a content measurement of the chlorpheniramine maleate with high-efficiency liquid phase chromatography. The quality control method of the Keteling troche provided by the invention adopts the chromatography and the color developing conditions to lead the chlorpheniramine maleate to effectively separate from other ingredients, the test result has good repeatability; the content of the chlorpheniramine maleate can be precisely measured in the Keteling troche, and the quality of the Keteling troche finished product is effectively controlled.
Owner:广西药用植物园制药厂
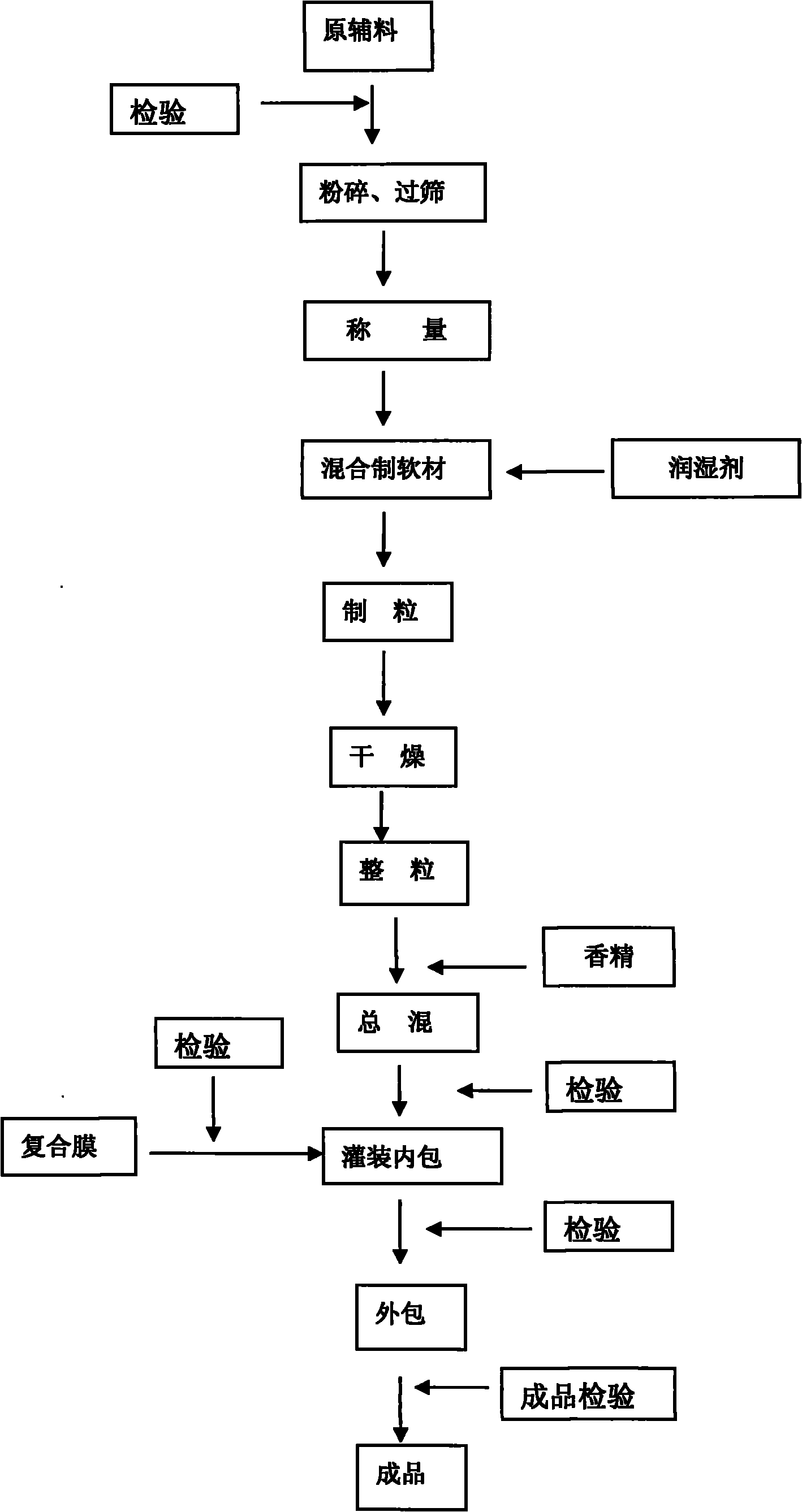
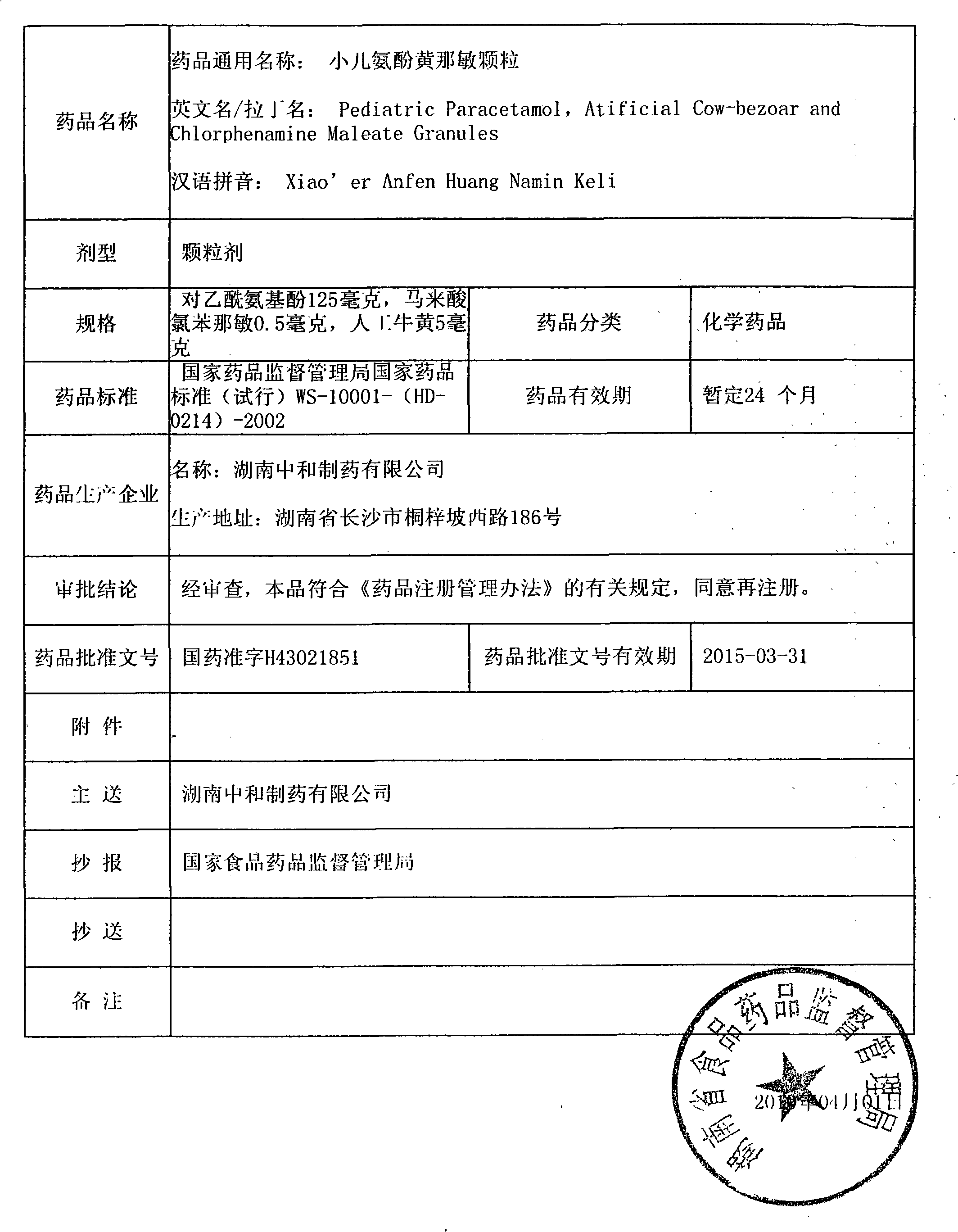
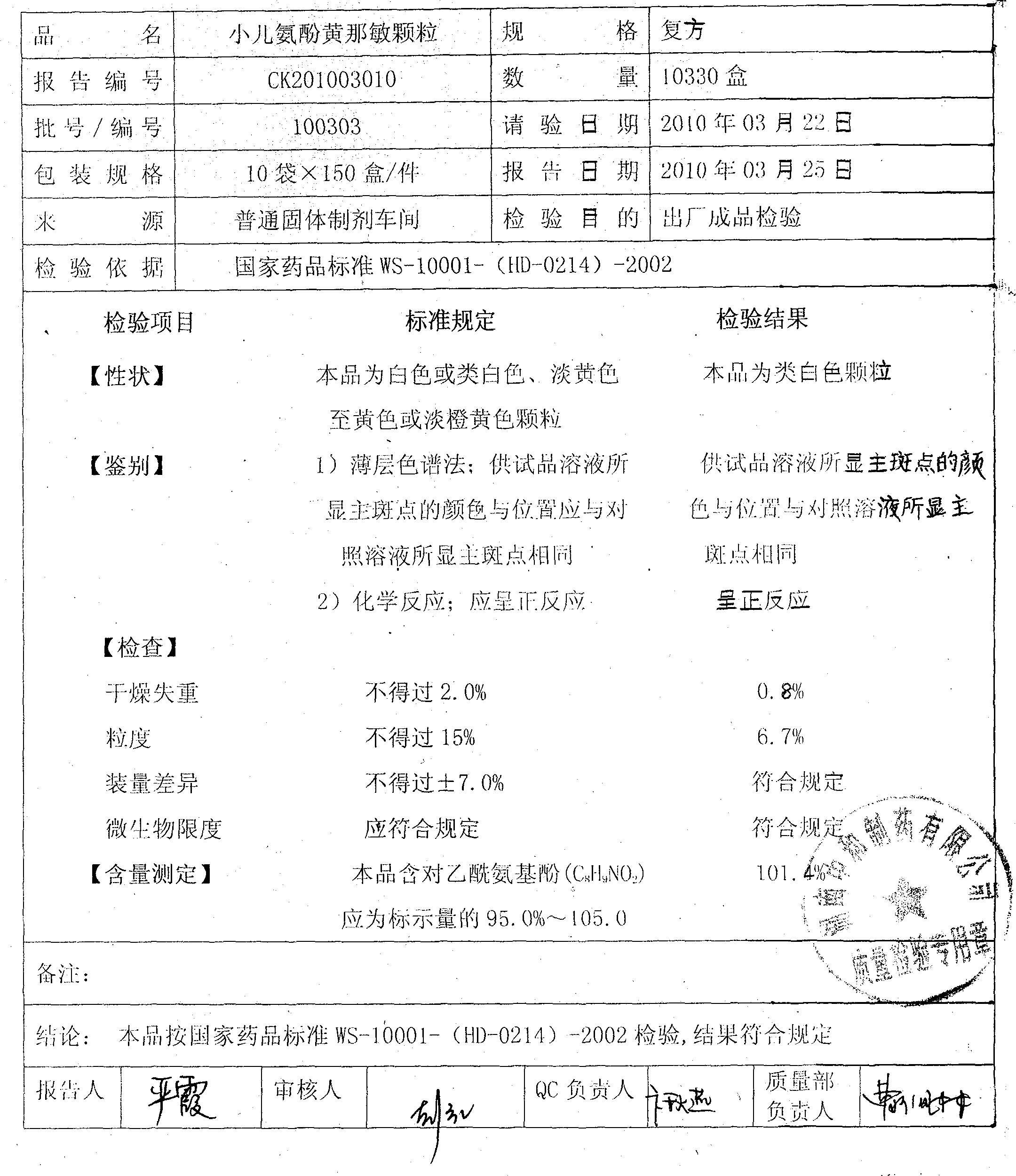
Preparation method of Huang children paracetamol-particles
ActiveCN101940598AImprove bioavailabilitySimple methodOrganic active ingredientsAntiviralsSucroseSaccharin
The invention relates to a preparation method of Huang children paracetamol-particles. The Huang children paracetamol-particles are prepared from the following raw materials in percentage by weight: 1.8-2.2% of paracetamol, 0.08-0.1% of Calculus Bovis Artifactus, 0.008-0.01% of chlorpheniramine maleate and 95-99% of other auxiliary material; and the auxiliary material comprises one or more of sucrose, steviosin, soluble saccharin, aspartame, vanillin, strawberry essence, tangerine essence and the like. The materials are mixed, and slushed with purified water and chlorpheniramine maleate as wetting agent. The preparation method comprises the following steps: (1) mixing and pulverizing part of sweetening agents and Calculus Bovis Artifactus, pulverizing and adding the rest of sweetening agents in an equally progressive increase mode, and mixing the mixture and pulverized paracetamol in a trough type mixing machine; (2) mixing with the wetting agent to prepare soft material, screening to obtain particles; and (3) fluidized-drying, adding essence particles, totally mixing, testing to obtain a qualified product, packaging into small bags, and packaging into exterior bags. The invention has the advantages of favorable content uniformity, high bioavailability and low production cost.
Owner:HUNAN ZHONGHE PHARMA
Traditional Chinese medicinal preparation for treating acute upper respiratory infection and preparation method of traditional Chinese medicinal preparation
InactiveCN104666535ANot easy to absorb moisture and deteriorateImprove stabilityAntibacterial agentsAntiviralsToxic materialBULK ACTIVE INGREDIENT
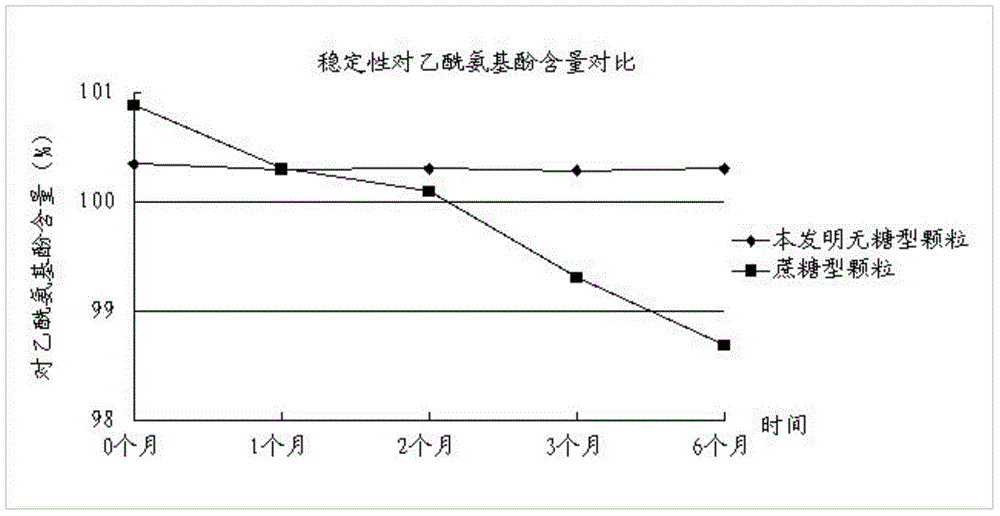
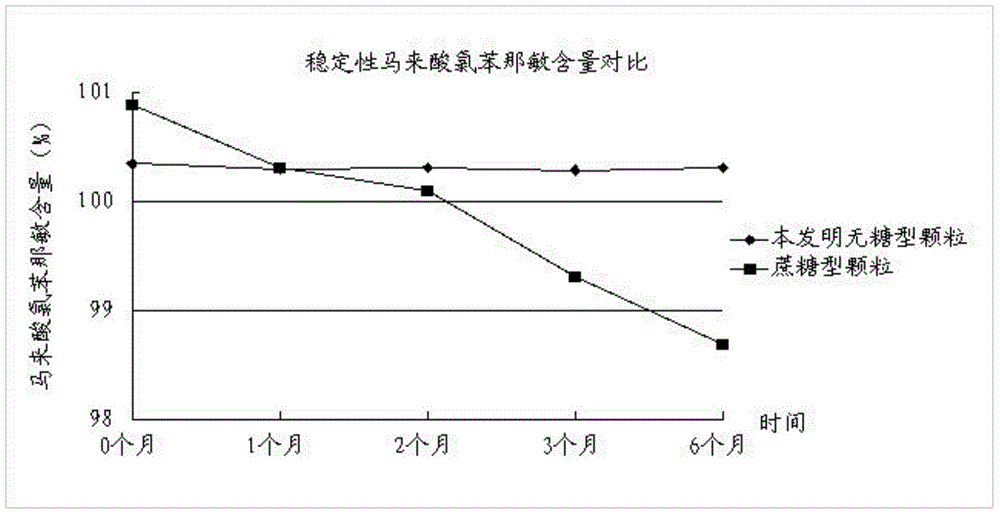
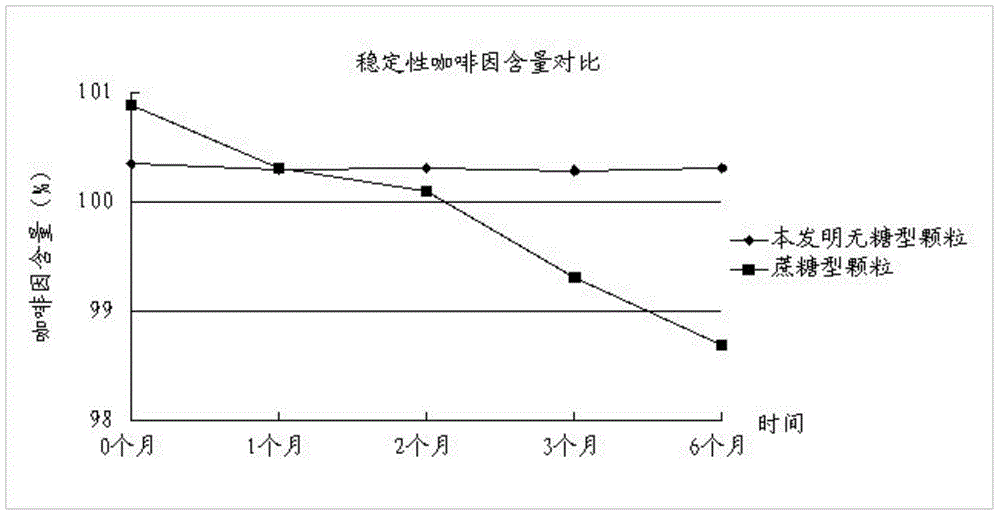
The invention discloses a traditional Chinese medicinal preparation for treating acute upper respiratory infection and a preparation method of the traditional Chinese medicinal preparation and belongs to the field of traditional Chinese medicines. The traditional Chinese medicinal preparation for treating the acute upper respiratory infection is prepared from extracts of honeysuckle, folium vilicis negundo, evodia lepta, wild chrysanthemum flower, south isatis root and holly root, acetaminophen, chlorpheniramine maleate, caffeine and beta cyclodextrin. The traditional Chinese medicinal preparation for treating the acute upper respiratory infection is based on basic pathogenesis of wind, warmness, heat and toxin, medicines with dispelling, relieving, eliminating and promoting effects are selected and combined for jointly realizing the effects of dispelling wind, relieving symptoms and clearing away heat and toxic materials, and the traditional Chinese medicinal preparation for treating the acute upper respiratory infection is sucrose-free granules and has the advantages of high stability, low dosage of auxiliary materials and wide application range. The preparation method of the traditional Chinese medicinal preparation for treating the acute upper respiratory infection has the advantage that active ingredients in each component of the traditional Chinese medicinal preparation are distinguished, processed and refined; and the preparation method of the traditional Chinese medicinal preparation for treating the acute upper respiratory infection is simple, has a broad market prospect and is applicable to large scale production.
Owner:GUANGXI DOUBLE ANT PHARMA
Babu far infrared medicinal plaster for treating arthralgia and myalgia, and wind-cold syndrome and numbness
InactiveCN101301339AReduce adverse reactionsEasy to useSalicyclic acid active ingredientsHeavy metal active ingredientsMyrrhSide effect
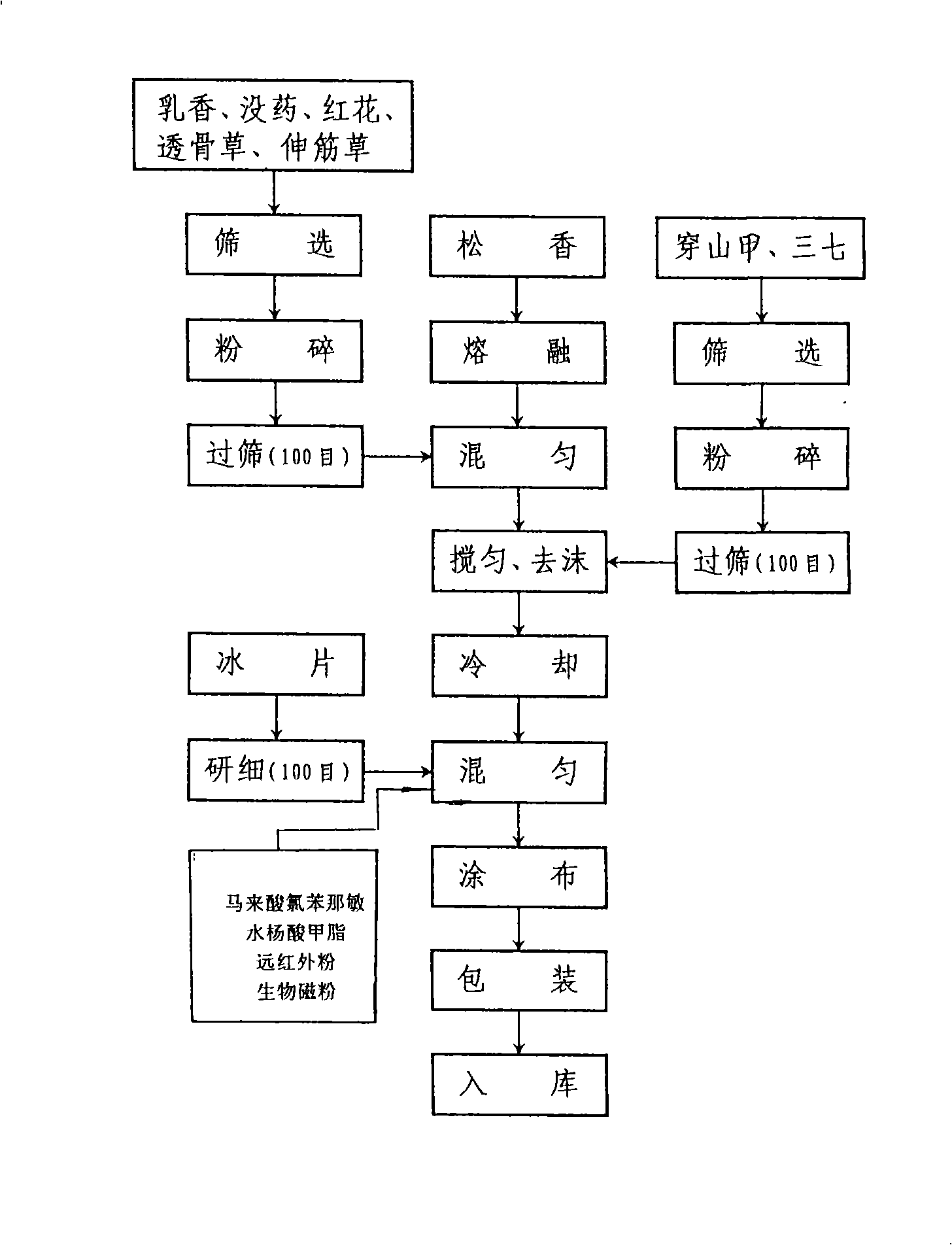
The invention aims to provide a cataplasm far-infrared medical application for treating arthralgia, myalgia, wind cold and numbness, which is elaborately developed by adopting rare pure traditional Chinese medicinal materials, a scientific novel method and a novel process. The application takes a formula in portion by weight: 40 g of borneol, 20 g of frankincense, 20 g of myrrh, 10 g of safflower, 10 g of panax notoginseng, 20 g of pangolin, 60 g of garden balsam stem, 20 g of common clubmoss herb and 400 g of colophony. The main composition of the formula is the pangolin, namely pangolin scales which is salty in taste and slightly cold in property, mainly treats wind cold and limbs numbness, and has the efficacy of dehumidifying, dissolving turbidity, diminishing inflammation and removing pain. The medical application has chlorpheniramine maleate and methyl salicylate which are effective compositions of western medicine added in preparation process, and uses far-infrared powder and biological magnetic powder which are the latest scientific achievements. The application has the advantages of clear effective compositions, obvious efficacy, few side effects, stable preparation and strict quality standard, thereby meeting the requirements of modern Chinese medicine on safety, high efficiency, stability and controllability.
Owner:牛天卓
Ganmaoling granules and preparation method thereof
ActiveCN107669780ALow moisture absorptionHigh dissolution ratePharmaceutical non-active ingredientsGranular deliveryPeppermintsBeta-Cyclodextrins
The invention belongs to the technical field of traditional Chinese medicine, and specifically relates to ganmaoling granules and a preparation method thereof. The ganmaoling granules include, by weight parts, 450-530 parts of evodia lepta, 300-350 parts of spanishneedles herb, 200-300 parts of wild chrysanthemum, 700-780 parts of holly root, 15-25 parts of acetaminophen, 0.1-0.7 parts of chlorpheniramine maleate, 0.2-0.6 part of caffeine, 0.1-0.3 part of peppermint oil, 200-300 parts of lactose, 100-200 parts of debranched starch, 60-120 parts of microcrystalline cellulose, 5-10 parts of beta-cyclodextrin, 3-5 parts of polyvinylpyrrolidone and 80-120 parts of ethanol. The ganmaoling granules prepared by the method have low hygroscopicity, are easy to disintegrate, have high bioavailability, good stability and high safety, and have significant effects on the inhibition of pneumonia and death protection of influenza virus-infected mice.
Owner:GUANGDONG YILI GROUP PHARMA
Allergy and congestion relief and preparation method thereof
ActiveCN104983732AImprove stabilitySimple preparation processOrganic active ingredientsPharmaceutical non-active ingredientsAllergySilicon dioxide
The invention discloses an allergy and congestion relief and a preparation method thereof. The prescription of the allergy and congestion relief comprises ibuprofen, phenylephrine hydrochloride, chlorpheniramine maleate, microcrystalline cellulose, pregelatinized starch, hydroxypropyl methylcellulose, sodium carboxymethyl starch, silicon dioxide, a film coating premixed agent, lactic acid and propyl gallate. Compared with the prior art, the prepared allergy and congestion relief has the beneficial effects that stability is good, the stability of key indexes such as impurities is obviously superior to that of launched products abroad, and the dissolution rate of medicines, especially the dissolution rate of the indissolvable medicine ibuprofen is equivalent to or slightly rapider than that of the launched products abroad.
Owner:CHINA RESOURCES SANJIU MEDICAL & PHARMA +1
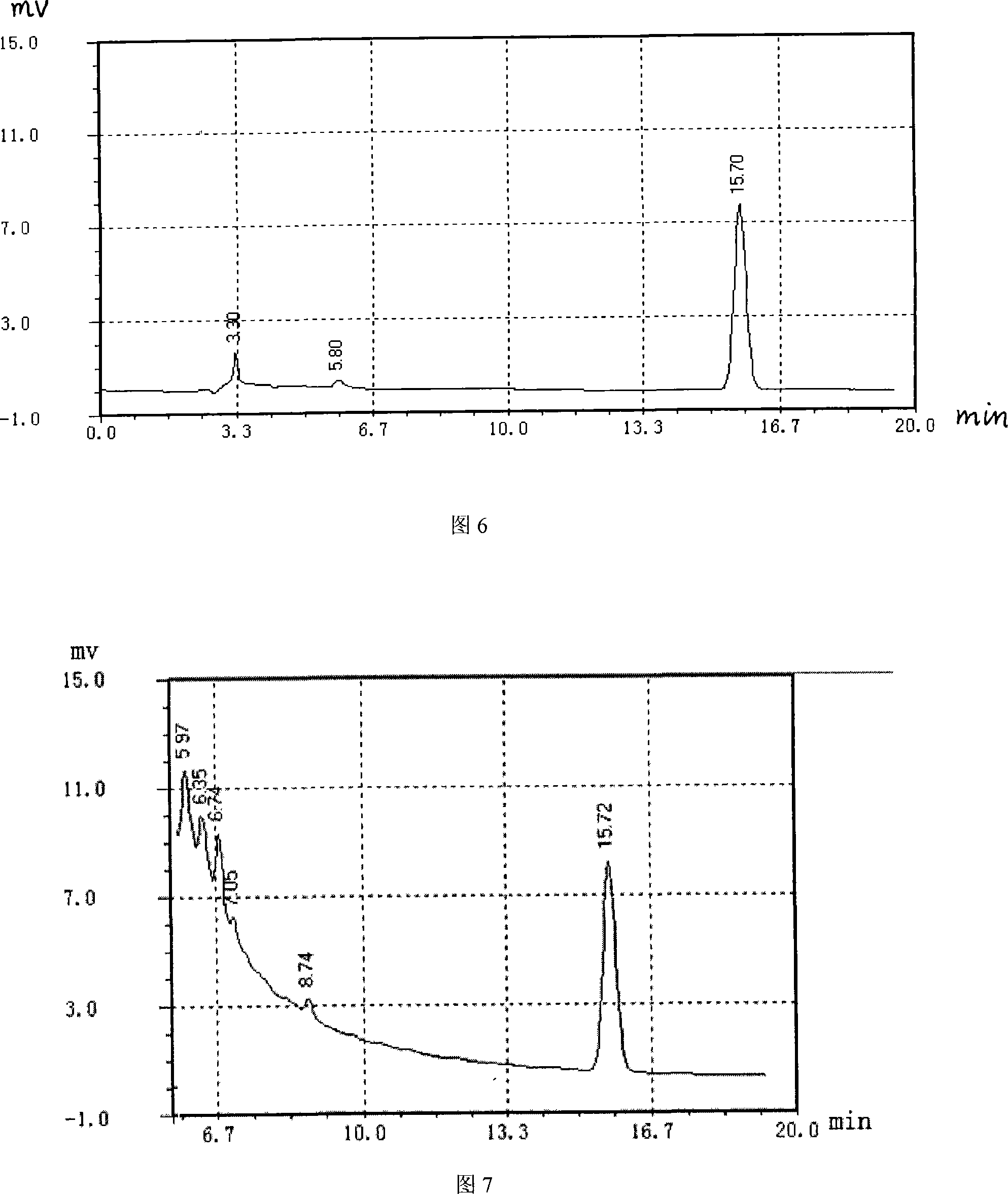
Pediatric paracetamol granule and quality control method
InactiveCN101606950AFix security issuesSolve the efficacy problemOrganic active ingredientsComponent separationQuality controlCalculus bovis
The invention discloses a pediatric paracetamol granule and a quality control method. The formulation is as follows: acetaminophen: in-vitro-cultured calculus bovis: chlorpheniramine maleate (125:5:0.5); the quality control method which combines a character method, thin-layer chromatography, thin layer chromatography scanning, and ultraviolet spectrophotometry is adopted; in the invention, in-vitro-cultured calculus bovis replaces natural calculus bovis to be used for the pediatric paracetamol granule, thus greatly reducing raw material cost of medicines while guaranteeing drug effect; in addition, in-vitro-cultured calculus bovis is used for replacing natural calculus bovis to be used for pediatric paracetamol granule, thus solving medicinal safety and drug effect problem, and simultaneously being beneficial to roundly controlling the quality of the pediatric paracetamol granules.
Owner:文永盛
Quality testing method for compound paracetamol and amantadine hydrochloride granules
InactiveCN102879517AQuality improvementGuaranteed clinical efficacyComponent separationClinical efficacyDissolution
The invention discloses a quality testing method for compound paracetamol and amantadine hydrochloride granules. The method mainly comprises the steps of appraising the quality of the compound paracetamol and amantadine hydrochloride granules by using a thin layer chromatography method, performing content uniformity measuring and content measuring for acetaminophen, amantadine hydrochloride, caffeine, chlorpheniramine maleate and artificial bezoar by using a liquid chromatography method, measuring the dissolution rate, and measuring the content of bilirubin to achieve the artificial bezoar content measuring. The quality testing method of micro samples of the compound paracetamol and amantadine hydrochloride granules is built, researches on appraisal, content measuring and content uniformity measuring of the compound paracetamol and amantadine hydrochloride granules are achieved, the accuracy is high, good in reproducibility, and the quality of the compound paracetamol and amantadine hydrochloride granules can be controlled comprehensively and effectively, so that clinical effects of the preparation are guaranteed.
Owner:葵花药业集团(衡水)得菲尔有限公司
Dispersion tablets for treating and preventing upper respiratory tract infection
InactiveCN101112362AImprove medication complianceSolve the taste problemAntiviralsPharmaceutical non-active ingredientsDrug compoundPseudoephedrine Hydrochloride
The present invention discloses a drug compound which has good taste, contains acetaminophen, chlorpheniramine maleate, cloperastini hydrochloride, pseudoephedrine hydrochloride, caffeine and bromelain and can be dispersed uniformly and is used for curing the upper respiratory tract Infection; the invention further contains fillers, disintegrating agents, flow agents, lubricants, sweeteners and other pharmaceutical excipients, and the single dose thereof is the dispersible tablet form.
Owner:BEIJING D VENTUREPHARM TECH DEV
Dispersible tablet for treating cold and its preparing process
InactiveCN1850083AOrganic active ingredientsAntiinfectivesCross-linkLow-substituted hydroxypropylcellulose
The present invention relates to a dispersion tablet for curing common cold and its preparation process. Said invention is formed from main medicine including paracetamol, pseudoephedrine hydrochloride, dextromethorphan and chlorpheniramine maleate and auxiliary medicine including avicel, low-substituted hydroxypropyl cellulose, povidone K30, cross-linked povidone, aspartame, aspartame, micropowder silica gel and magnesium stearate.
Owner:江西聚仁堂药业有限公司
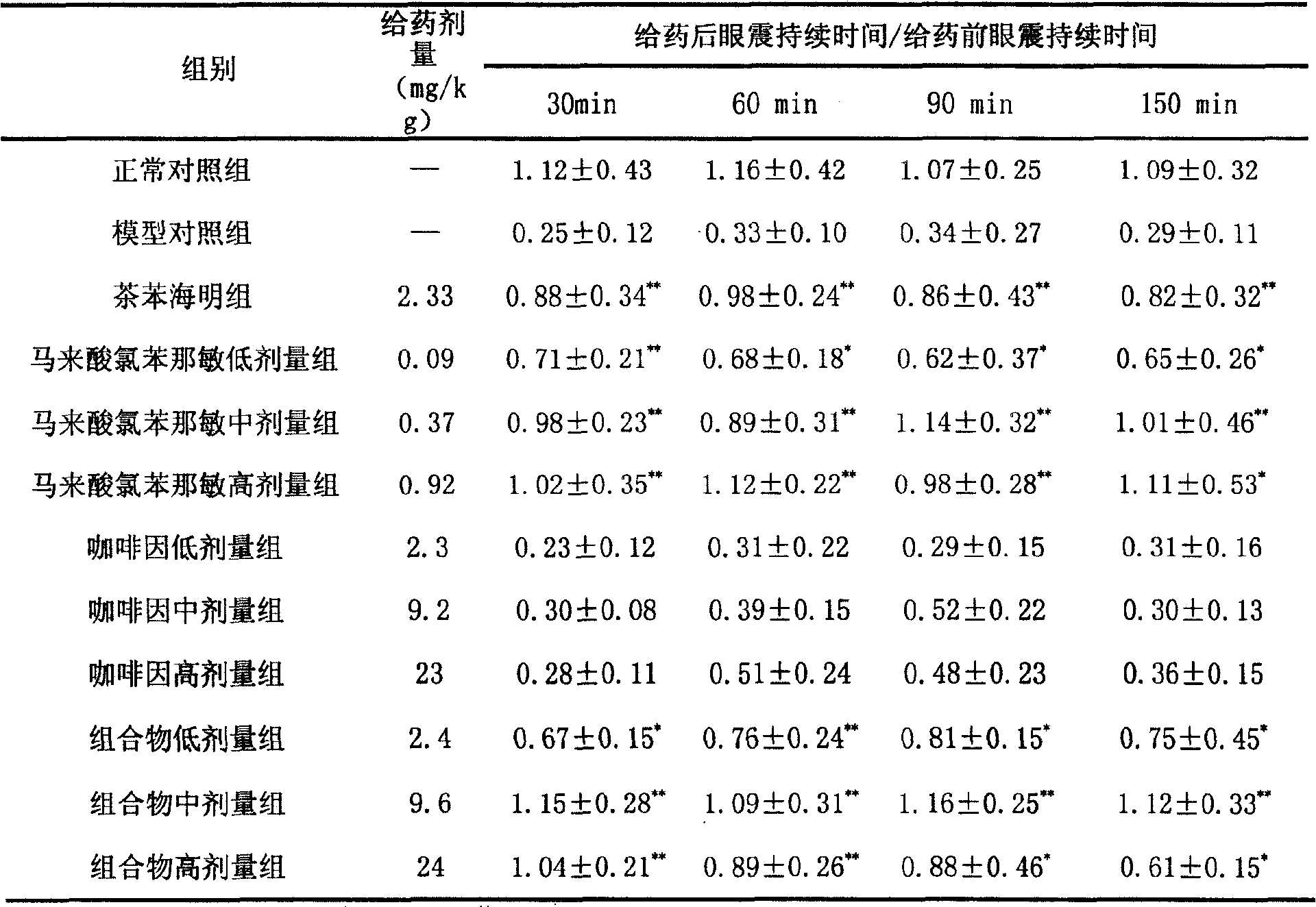
Pharmaceutical combination for preventing and treating motion sickness and preparations thereof
ActiveCN101244064APrevent and treat motion sicknessNo motion sicknessDigestive systemPill deliveryMotion sicknessAdjuvant
The invention relates to a drug composition for the treatment of motion sickness, as well as the preparation, wherein the drug composition comprises caffeine and chlorpheniramine maleate; 50 to 500 mg caffeine and 2 to 20 mg chlorpheniramine maleate are contained in each preparation unit; the preferred mass ratio of caffeine to chlorpheniramine maleate in each composition is 50:2 and the preferred amount of each component is 200 mg caffeine and 8mg chlorpheniramine maleate. The drug composition has an advantage of effectively treating and preventing motion sickness without side effect of sleepiness or lassitude; besides, conventional adjuvant can be added in the drug composition to make drugs through any conventional method for motion sickness treatment; controlled release technologies can also be adopted to make controlled release preparations.
Owner:SUN YAT SEN UNIV +1
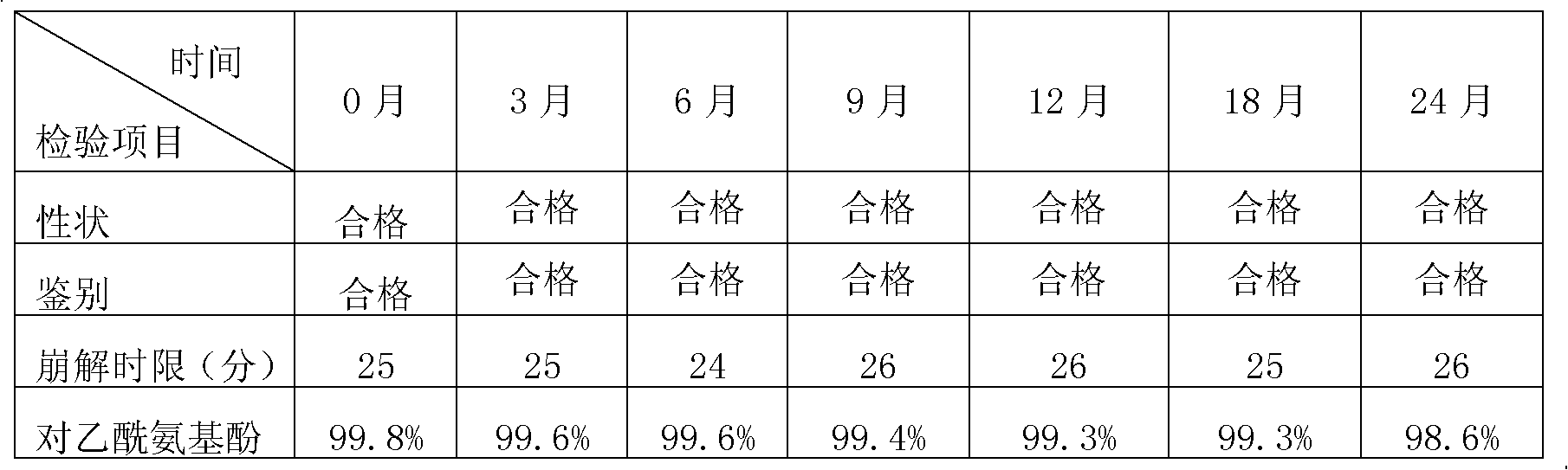
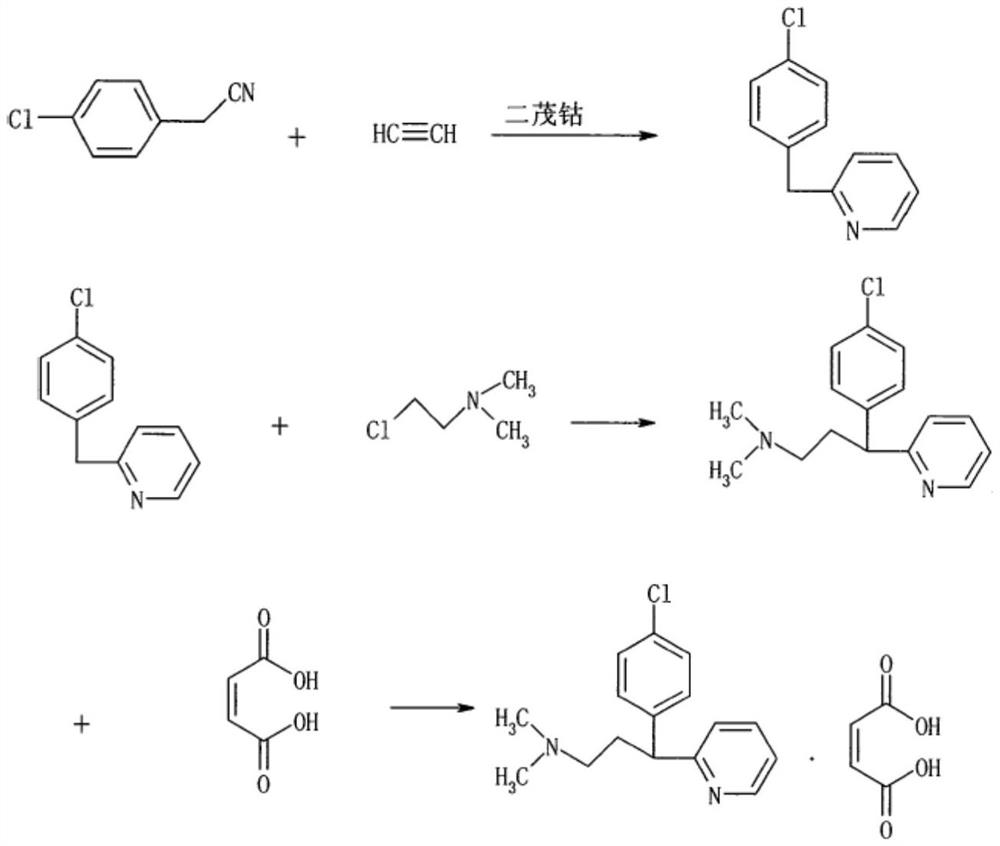
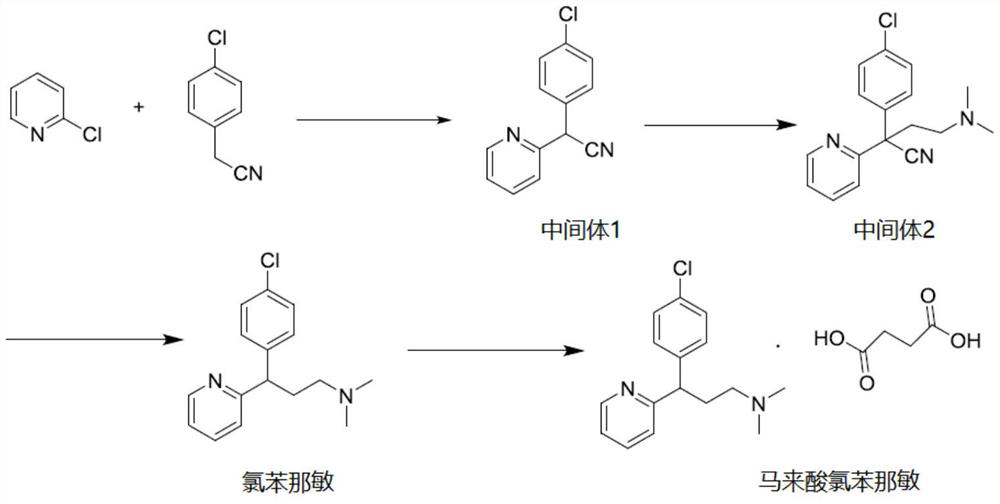
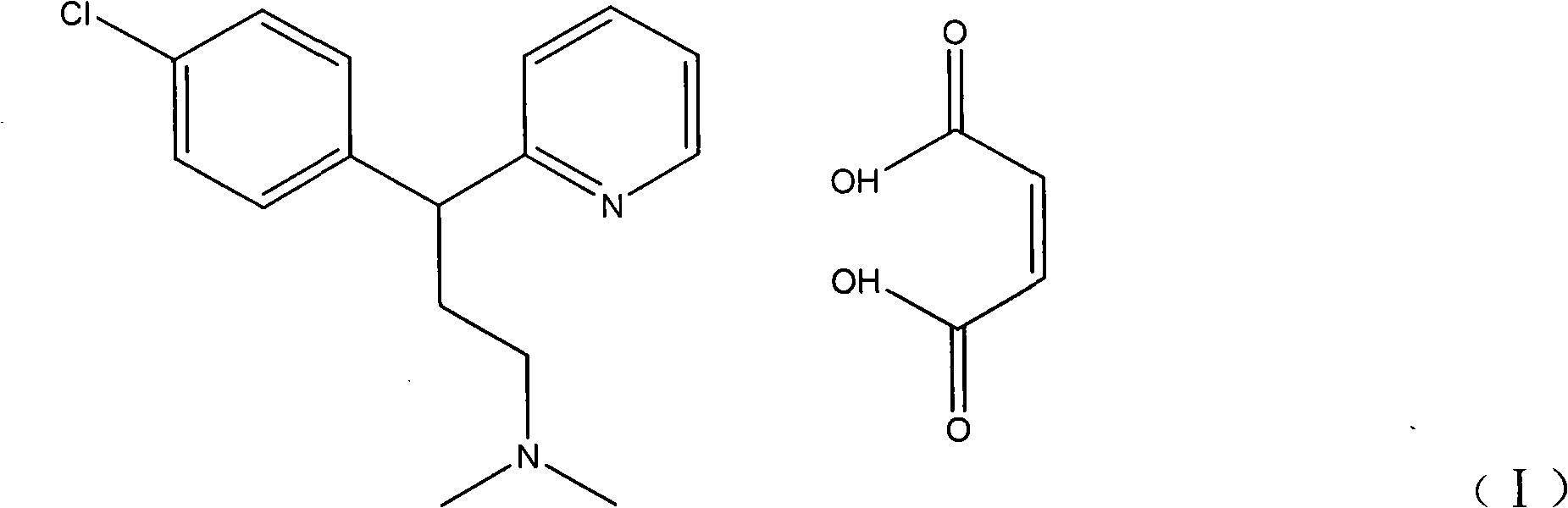
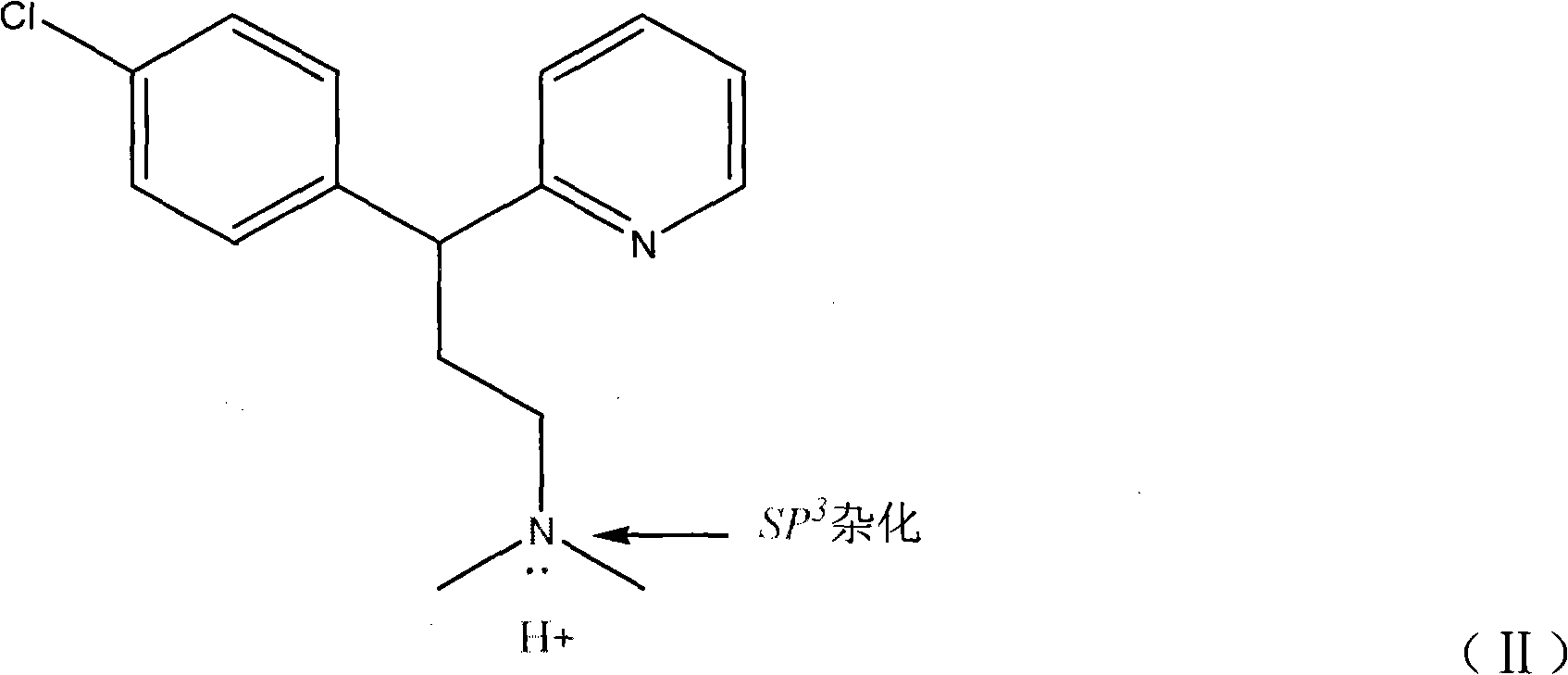
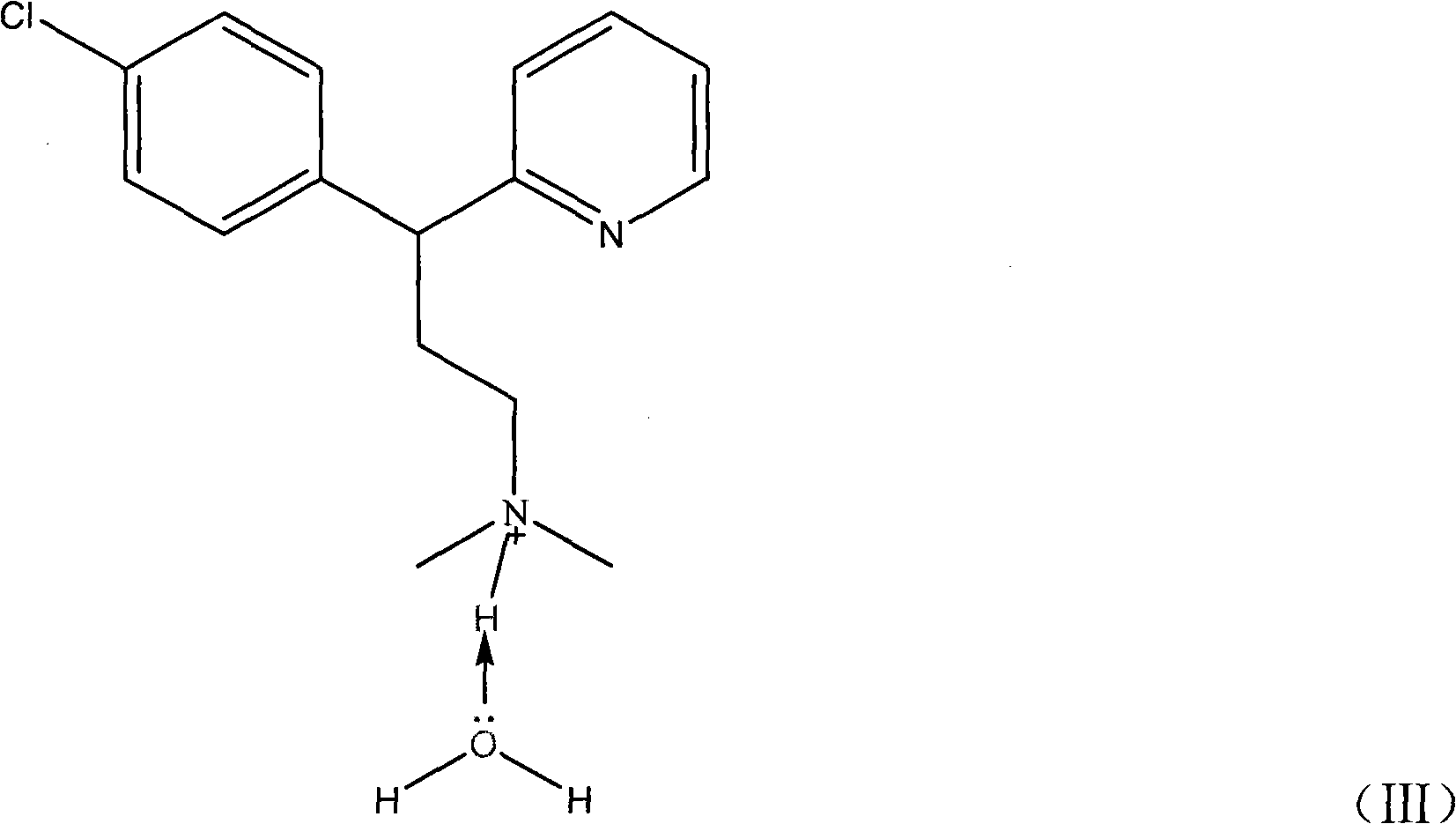
Preparation method of chlorpheniramine maleate
ActiveCN111747887AShort synthetic routeRaw materials are easy to getOrganic compound preparationCarboxylic acid salt preparationChlorphenamine maleateChlorobenzene
The invention provides a chlorpheniramine preparation method, which comprises: (1) reacting p-chlorophenylacetonitrile and 2-chloropyridine to obtain an intermediate 1; (2) reacting the intermediate 1with 2-dimethylaminochloroethane to obtain an intermediate 2; and (3) reacting the intermediate 2 with an alkali to obtain chlorpheniramine. The invention further provides a preparation method of chlorpheniramine maleate. The methods are short in synthetic route, easily available in raw materials and low in cost; the total yield is high, and the product quality is controllable; reaction conditions are mild, requirements on equipment are low, and industrial production is facilitated.
Owner:CHENGDU JIANJIANG PHARMA FACTORY
Method for preparing 'youkadan' granule
InactiveCN101057865AGuaranteed content uniformityAvoid adverse reactionsAntipyreticAnalgesicsCommon coldGallstones
The invention discloses a process for preparing pediatric paracetamol and amantadine hydrochloride granules for treating children's common cold, wherein each unit of the preparation comprises Paracetamol 100mg, amantadine hydrochloride 40mg, artificial ox gallstone 4mg, caffeine 6mg and chlorpheniramine maleate 0. 8mg.
Owner:杨文龙
Method for detecting chlorpheniramine maleate
InactiveCN101865857AStrong specificityReduce dosageMaterial analysis by observing effect on chemical indicatorPhosphoric acidColor reaction
The invention discloses a rapid detection method of chlorpheniramine maleate doped in drugs, Chinese patent medicines and health products, which comprises the following steps: (1) taking a solid sample, adding 3-10 times the weight of water, shaking and dissolving, and then taking 3mL of extract, adding 2 drops of 2% (w / v) of sodium hydroxide solution and uniformly shaking to be used as a solution to be detected; or taking 3mL of liquid sample, and then adding 2 drops of 2% (w / v) of sodium hydroxide solution and uniformly shaking to be used as a solution to be detected; (2) adding 3mL of ethyl acetate in the solution to be detected to extract, standing and layering; (3) taking 2mL of upper ethyl acetate extract, adding 2mL of phosphoric acid water solution of 3% of volume concentration to extract, standing and layering; and (4) taking 1mL of lower phosphoric acid stripping solution, and adding 1-2 drops of trinitrophenol saturated water solution drop by drop, if a yellow precipitate is generated, then judging that the sample contains chlorpheniramine maleate. The detection method of the invention has simple operation, strong specificity and rapid color reaction, and can be suitable for rapidly detecting whether the drugs, the Chinese patent medicines and the health products are doped with chlorpheniramine maleate or not.
Owner:广东省药品检验所
Solid preparation of compound ammonia phenol renin medicine composition liposome
The invention discloses a solid preparation of a compound ammonia phenol renin medicine composition liposome and a preparation method thereof. The method comprises that the compound ammonia phenol renin medicine composition liposome is prepared by acetaminophen, anhydrous caffein, phenylephrine hydrochloride, chlorpheniramine maleate, vitamin B1, egg yolk lecithin acyl serine, phosphatidyl ethanolamine and octadecylamine which are selected according to specified weight ratio, and then the solid preparation is prepared by the compound ammonia phenol renin medicine composition liposome through an ordinary preparation method. The solid preparation of the liposome is high in encapsulation and even in particle size, improves quality of a preparation product, reduces toxic and side effects, andis suitable for industrialized production. In addition, the preparation method is simple, and the medicine is reserved in blood circulation for a long time.
Owner:HAINAN MEIDA PHARMA
Method for determining content and content homogeneity degree of chlorpheniramine maleate in antitussive and expectorant particles
InactiveCN102645505AComprehensive quality inspection indicatorsImprove stabilityComponent separationContent determinationRepeatability
The invention discloses a method for determining the content and the content homogeneity degree of chlorpheniramine maleate in antitussive and expectorant particles, comprising the following four steps of: preparing a comparison product solution, preparing a solution of a sample to be tested under a content determination item, preparing a solution of the sample to be tested under a homogeneity degree determination item, and utilizing a high performance liquid chromatography to determine. The method has the advantages that the specially-prepared solution of the sample to be tested is combined with the high performance liquid chromatography so that the method with good stability and repeatability for determining the content and the content homogeneity degree of the chlorpheniramine maleate in the antitussive and expectorant particles is provided for detecting the content and the content homogeneity degree of the chlorpheniramine maleate in the antitussive and expectorant particles; and therefore, the quality detection index of the antitussive and expectorant particles is more comprehensive and the product quality can be better controlled.
Owner:ZHONGXING PHARM CO LTD JIANGSU
Preparation method for pediatric paracetamol
InactiveCN104189011AQuality improvementGood content uniformityOrganic active ingredientsAntipyreticSucroseMixed materials
The invention discloses a preparation method for pediatric paracetamol. The pediatric paracetamol comprises main materials and auxiliary materials, and is characterized in that the main materials comprise acetaminophen, calculus bovis factitius and chlorpheniramine maleate; the auxiliary materials comprise sucrose powder, essence and purified water. The preparation method comprises the following steps: weighing the main materials and the auxiliary materials; putting acetaminophen, calculus bovis factitius, chlorpheniramine maleate and sucrose powder into a high-speed mixing granulator for mixing; adding the essence and the purified water into the mixed materials, and putting into a swing granulator for granulating; putting into a boiling granulator after granulating; screening materials taken out from the boiling granulator. According to the preparation method, a condition that the quantity of granule fine powder is reduced on the premise that the identification items of calculus bovis factitius accord with the requirements is guaranteed; the pediatric paracetamol prepared is reliable in quality, good in content uniformity and low in manufacturing cost, and the utilization rate of raw materials is improved.
Owner:安徽安科恒益药业有限公司
Soft capsule composition containing acetaminophen
A composite soft capsule contains proportionally the active component chosen from paracetranol and one or more of pseudoephedrine hydrochloride, chlorpheniramine maleate, dextromethorphen hydrobromate, bagodryl hydrochloride and coffin, polyethanediol, sodium (or potassium) acetate, and polyvidone.
Owner:ZHEJIANG WANLIAN PHARMA IND +1
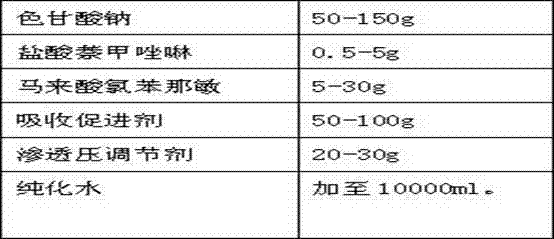
Sodium cromoglycate, naphazoline hydrochloride and chlorpheniramine maleate nasal spray
ActiveCN103751188AGuaranteed pHAvoid side effectsOrganic active ingredientsAntipyreticPreservativeNasal spray
The invention provides a sodium cromoglycate, naphazoline hydrochloride and chlorpheniramine maleate nasal spray for treating allergic rhinitis. The sodium cromoglycate, naphazoline hydrochloride and chlorpheniramine maleate nasal spray comprises sodium cromoglycate, naphazoline hydrochloride, chlorpheniramine maleate, and pharmaceutically acceptable excipients, wherein the pharmaceutically acceptable excipients are selected from a buffering agent, an osmotic pressure regulator, a preservative, an absorption enhancer, a pH (Power of Hydrogen) regulator and the like. According to the sodium cromoglycate, naphazoline hydrochloride and chlorpheniramine maleate nasal spray, the active ingredients can be quickly absorbed through mucosa of nasal cavity and then acts quickly; the sodium cromoglycate, naphazoline hydrochloride and chlorpheniramine maleate nasal spray has the advantages of high bioavailability, outstanding compliance, and convenience in carrying and use, etc.
Owner:SHANDONG TIANSHUN PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com