Preparing method of paracetamol, caffein, atificial cow-bezoar and chlorphenamine maleate capsule content functional pellet
A technology of Kahuangmin capsules and contents, which can be applied in the directions of capsule delivery, medical preparations with non-active ingredients, and medical preparations containing active ingredients, etc. Threshold value, uneven mixing of raw materials, etc., to achieve the effect of favorable disintegration and dissolution, good drug dissolution rate and small weight difference
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] 1. Micronize 100 kg of raw materials paracetamol, caffeine, chlorpheniramine maleate, and artificial bezoar until the particle size is 200 mesh.
[0030] 2. Preparation of API pellets
[0031] 2.1 Preparation of paracetamol instant pellets
[0032] According to the weight ratio, take 23kg of micronized acetaminophen, add 0.46kg of hydroxypropylmethylcellulose, 0.23kg of microcrystalline cellulose, 3.45kg of lactose, and 1.15kg of starch paste, mix evenly, granulate, and heat at 78°C Down drying, granulation, get particle diameter and be 22 order acetaminophen-based cores, put into the sugar-coated pan, use the mixed solution that concentration is 1% sodium carboxymethylcellulose aqueous solution and 0.308kg citric acid to do spray, Use a spray gun to spray 77kg of micronized paracetamol. When spraying the package, the temperature of the material is room temperature, the rotation speed of the sugar coating pan is 52 rpm, the air inlet temperature is 30°C, and the pressu...
Embodiment 2
[0044] 1. Micronize 100 kg of raw materials paracetamol, caffeine, chlorpheniramine maleate, and artificial bezoar until the particle size is 200 mesh.
[0045] 2. Preparation of API pellets
[0046] 2.1 Preparation of paracetamol instant pellets
[0047] According to the weight ratio, take 27kg of micronized acetaminophen, add 0.675kg of hydroxypropylmethylcellulose, 0.54kg of microcrystalline cellulose, 4.59kg of lactose, and 1.485kg of starch paste, mix evenly, and then granulate, at 82°C Drying, granulation, the particle size is 23 mesh acetaminophen-based nucleus, put into the sugar-coated pan, use the mixed solution of 1% sodium carboxymethyl cellulose aqueous solution and 0.438kg citric acid as spray, adopt Spray gun with 73kg of micronized acetaminophen, when spraying, the temperature of the material is room temperature, the rotation speed of the sugar coating pan is 52 rpm, the air inlet temperature is 40°C, the pressure of the spray gun is 0.3 MPa, and the cladding ...
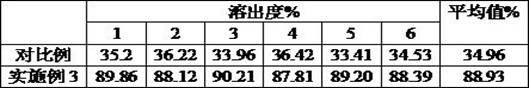
Embodiment 3
[0059] 1. Micronize 100 kg of raw materials paracetamol, caffeine, chlorpheniramine maleate, and artificial bezoar until the particle size is 200 mesh.
[0060] 2. Preparation of API pellets
[0061] 2.1 Preparation of paracetamol instant pellets
[0062] According to the weight ratio, take 25kg of micronized acetaminophen, add 0.55kg of hydroxypropylmethylcellulose, 0.375kg of microcrystalline cellulose, 4kg of lactose, and 1.5kg of starch paste, mix evenly, and then granulate it. Drying, granulation, the particle diameter is 22 mesh acetaminophen-based cores, put into the sugar-coated pan, use the mixed solution of 1% sodium carboxymethyl cellulose aqueous solution and 0.375kg citric acid as spray, adopt Spray gun to spray 75kg of remaining micronized paracetamol. When spraying the package, the material temperature is room temperature, the rotation speed of the sugar coating pan is 52 rpm, the air inlet temperature is 35°C, and the spray gun pressure is 0.3MPa. The number ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com