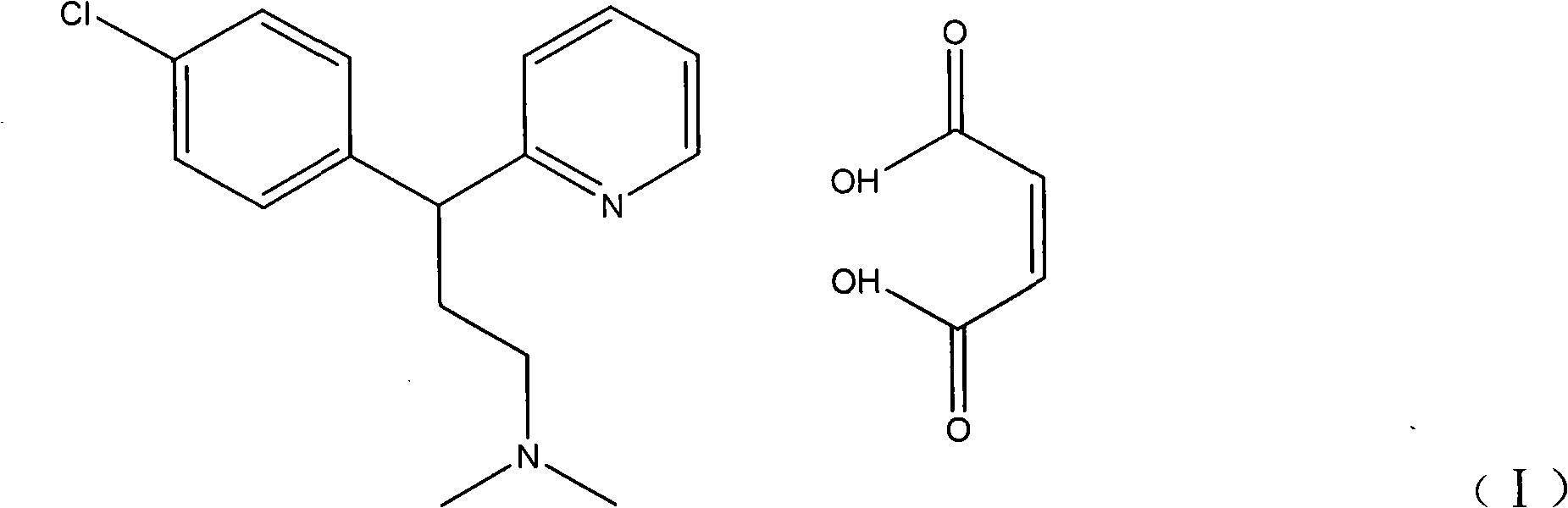
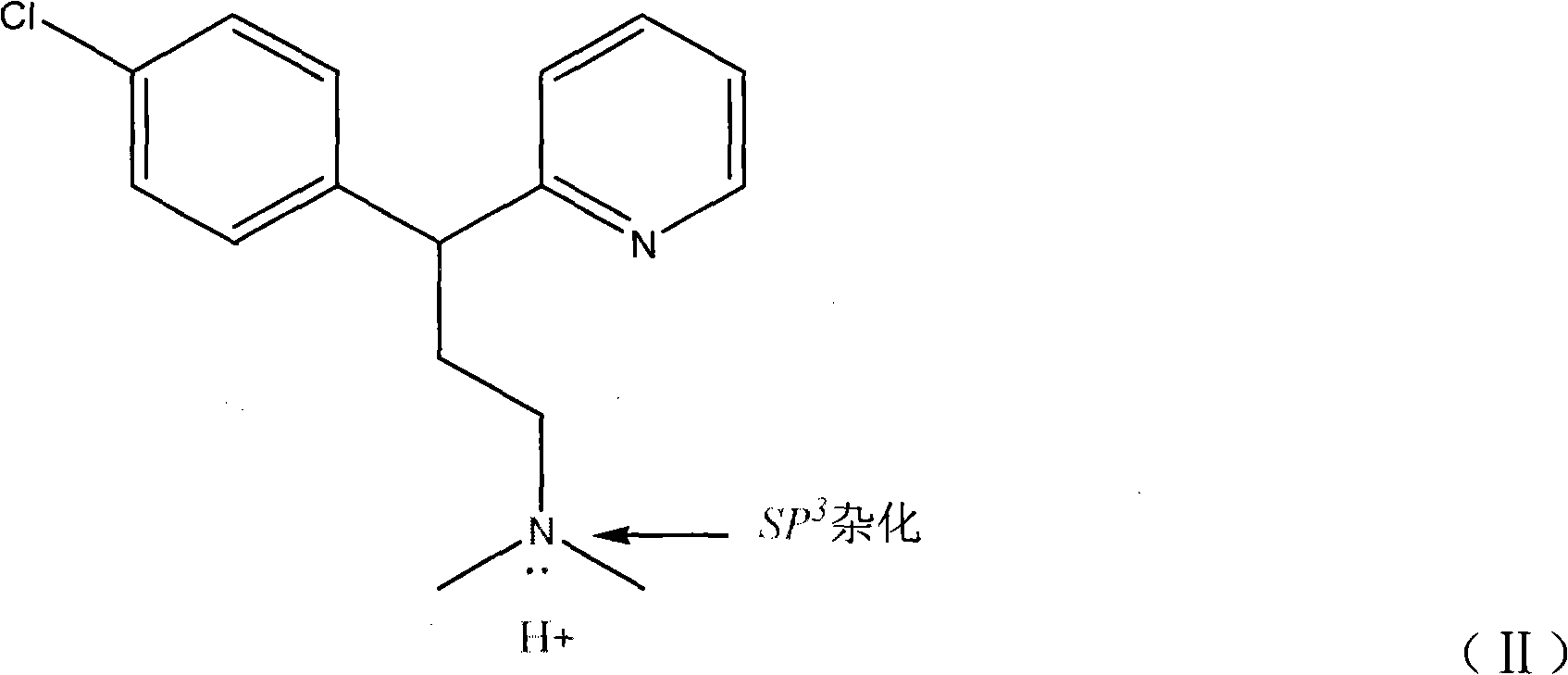
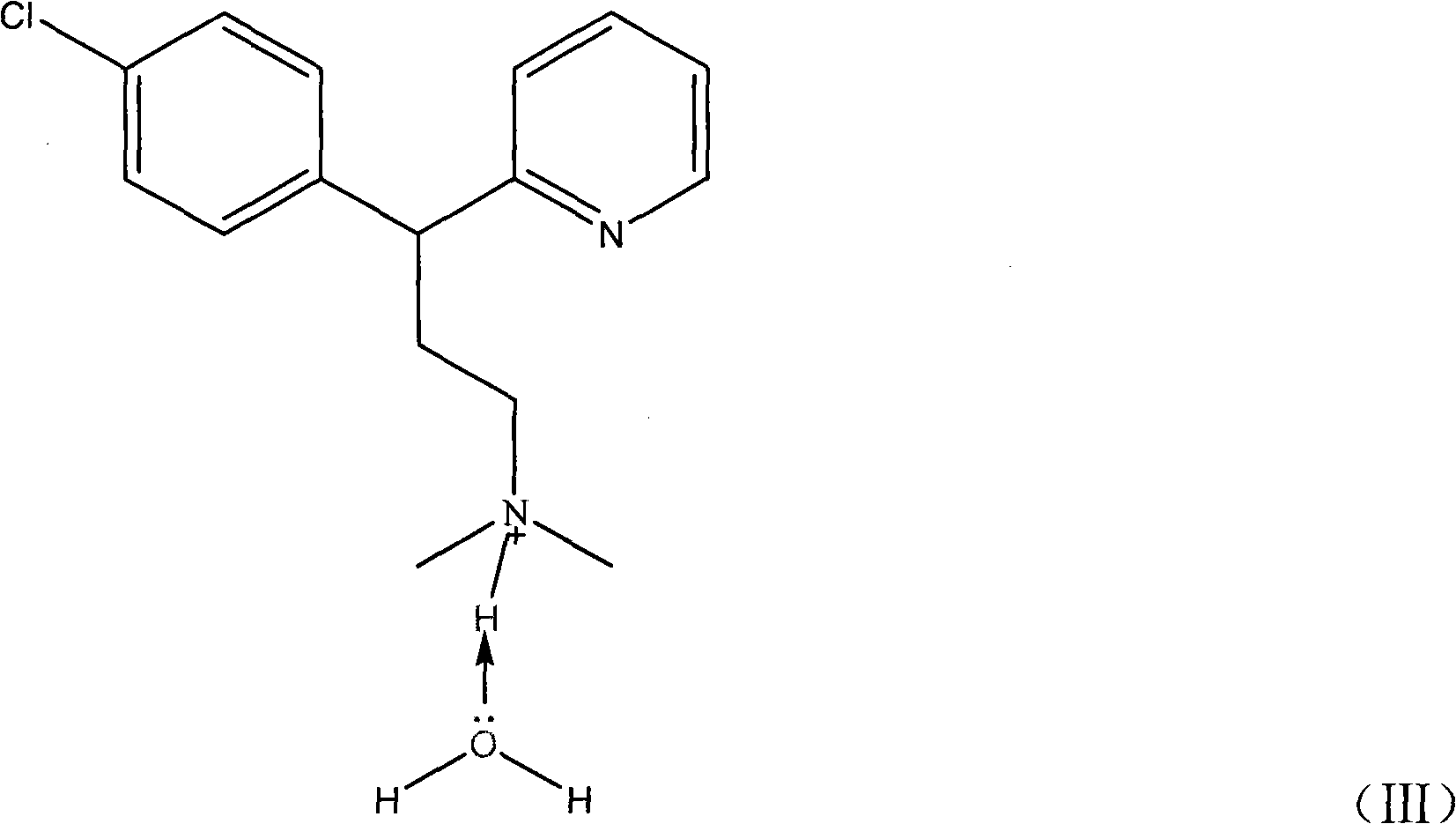
Method for detecting chlorpheniramine maleate
A technology for the detection of chlorpheniramine and its detection method, which is applied in the field of detection of chlorpheniramine maleate in medicine, can solve the problems of high analysis cost, expensive instrument, and great influence on the separation effect, and achieve low analysis cost , strong specificity and high accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Sample: UB Younasal Spray (spray, labeled as produced by Guizhou Miao Ren Tang Biomedical Technology Co., Ltd., batch number: 20091012, as determined by HPLC, this product contains 0.7 mg / ml of chlorpheniramine maleate) .
[0044] Detection steps:
[0045] (1) get sample 3ml, place in 10ml sample bottle 1;
[0046] (2) In the sample bottle 1, dripping mass concentration is 2 drops of aqueous sodium hydroxide solution of 2%, shake up;
[0047] (3) add ethyl acetate 3ml to sample bottle 1, screw cap tightly, shake and make two-phase mixed contact, leave standstill layering, upper liquid is colorless and clear;
[0048] (4) Use a straw to absorb 2ml of the upper layer liquid, place it in a 10ml sample bottle II, add 2ml of phosphoric acid aqueous solution with a volume concentration of 3%, tighten the bottle cap, shake to make the two phases mix and contact, let stand for stratification, and the lower layer of liquid is colorless and clear;
[0049] (5) Use a straw to ...
Embodiment 2
[0051] Sample: UB You Nasal Spray (spray, labeled Guizhou Miao Ren Tang Biomedical Technology Co., Ltd., batch number: 20091020, the result of HPLC determination, this product contains 0.8 mg / ml of chlorpheniramine maleate) .
[0052] Detection steps:
[0053] (1) get sample 3ml, place in 10ml sample bottle 1;
[0054] (2) In the sample bottle 1, dripping mass concentration is 2 drops of aqueous sodium hydroxide solution of 2%, shake up;
[0055] (3) add ethyl acetate 3ml to sample bottle 1, screw cap tightly, shake and make two-phase mixed contact, leave standstill layering, upper liquid is colorless and clear;
[0056] (4) Use a straw to absorb 2ml of the upper layer liquid, place it in a 10ml sample bottle II, add 2ml of phosphoric acid aqueous solution with a volume concentration of 3%, tighten the bottle cap, shake to make the two phases mix and contact, let stand for stratification, and the lower layer of liquid is colorless and clear;
[0057] (5) Use a straw to abs...
Embodiment 3
[0059] Sample: UB Younasal Spray (spray, labeled as produced by Guizhou Miao Ren Tang Biomedical Technology Co., Ltd., batch number: 20091115, as determined by HPLC, this product contains 0.7 mg / ml of chlorpheniramine maleate) .
[0060] Detection steps:
[0061] (1) get sample 3ml, place in 10ml sample bottle 1;
[0062] (2) In the sample bottle 1, dripping mass concentration is 2 drops of aqueous sodium hydroxide solution of 2%, shake up;
[0063] (3) add ethyl acetate 3ml to sample bottle 1, screw cap tightly, shake and make two-phase mixed contact, leave standstill layering, upper liquid is colorless and clear;
[0064] (4) Use a straw to absorb 2ml of the upper layer liquid, place it in a 10ml sample bottle II, add 2ml of phosphoric acid aqueous solution with a volume concentration of 3%, tighten the bottle cap, shake to make the two phases mix and contact, let stand for stratification, and the lower layer of liquid is colorless and clear;
[0065] (5) Use a straw to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com