Preparation method of chlorpheniramine maleate
A technology of chlorpheniramine and chlorophenylacetonitrile, which is applied in the field of drug synthesis and can solve the problems of high equipment requirements and harsh conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1. Method for synthesizing chlorpheniramine and chlorpheniramine maleate of the present invention
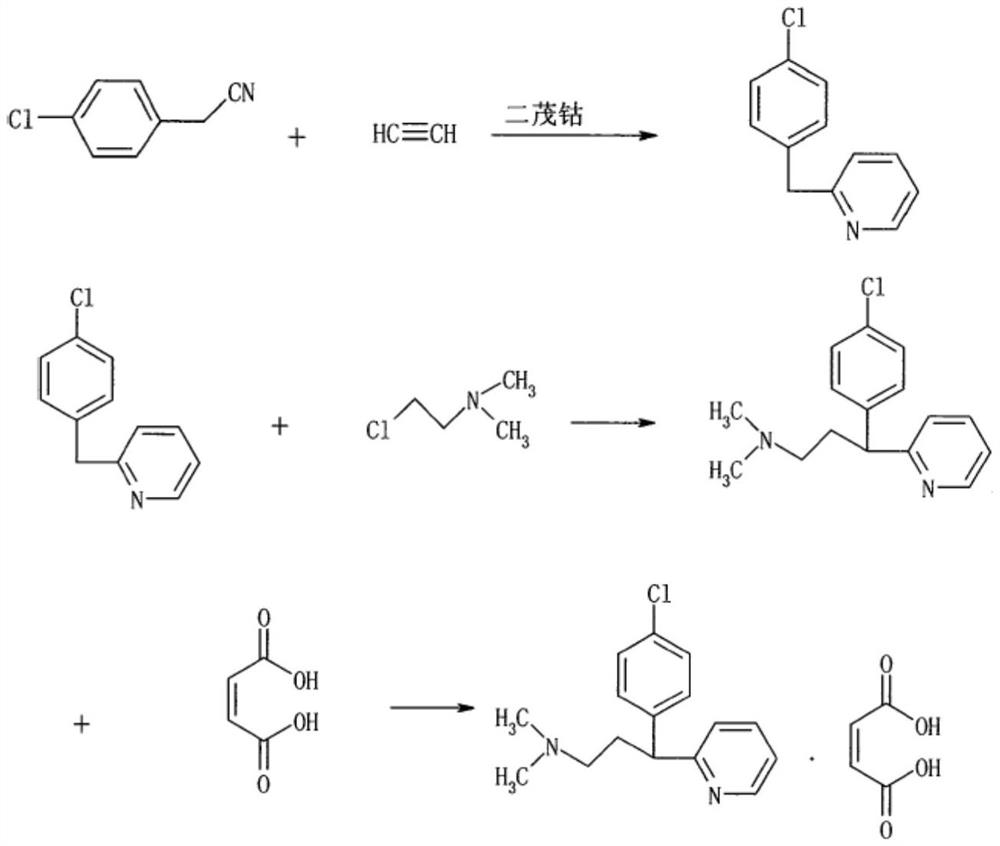
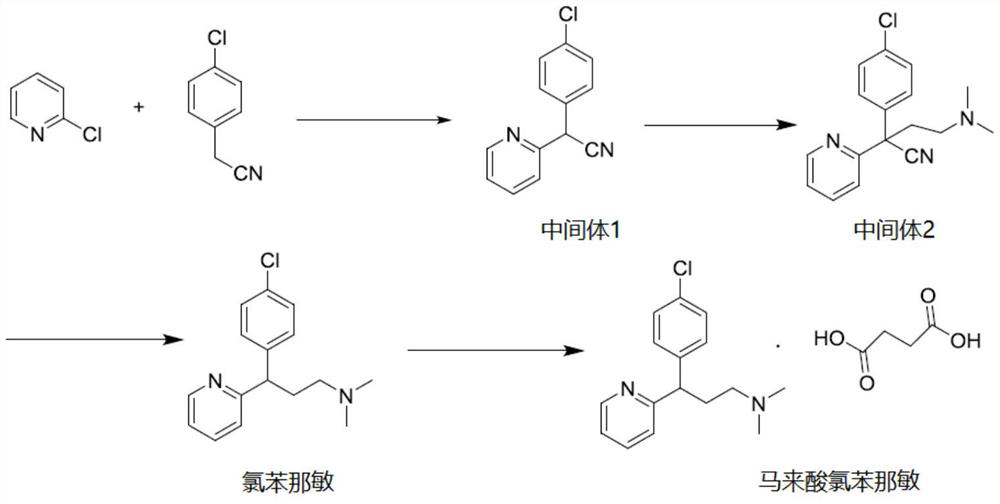
[0034] According to the following synthetic route, chlorpheniramine and chlorpheniramine maleate were prepared.
[0035]
[0036] (1) Preparation of Intermediate 1
[0037] Add 200 g of p-chlorobenzeneacetonitrile, 100 g of 2-chloropyridine, and 600 ml of toluene into the reaction flask, cool with ice water to 15° C., add 51.5 g of sodium amide, and react at room temperature for 2 to 5 hours after the addition. TLC monitors that the reaction is complete.
[0038] The reaction was quenched by adding water to the reaction solution, the aqueous layer was separated, the organic layer was washed with 200 ml of brine, and the organic phase was concentrated to obtain 190 g of intermediate 1 oil with a yield of 95%.
[0039] (2) Preparation of Intermediate 2
[0040] 2.1 Preparation of dimethylamino chloroethane solution:
[0041] Weigh 95g of 2-dimethylaminochloroethane hydrochlorid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com