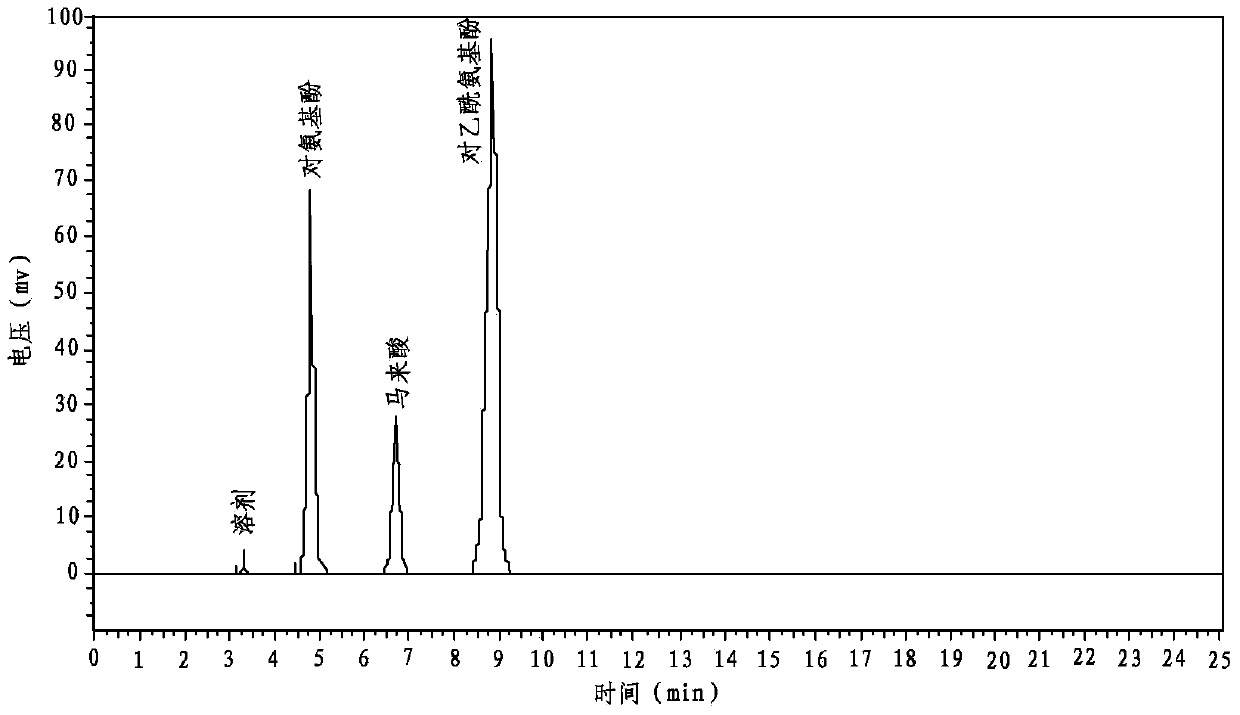
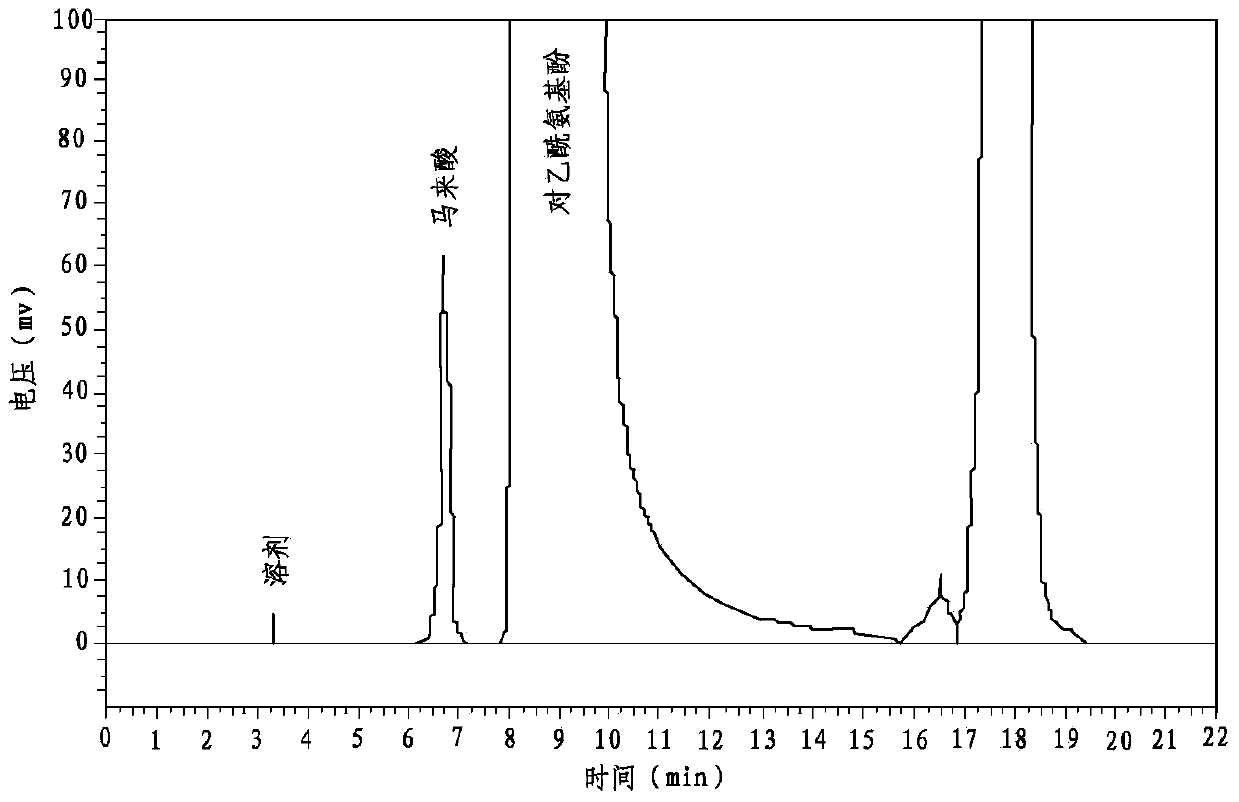
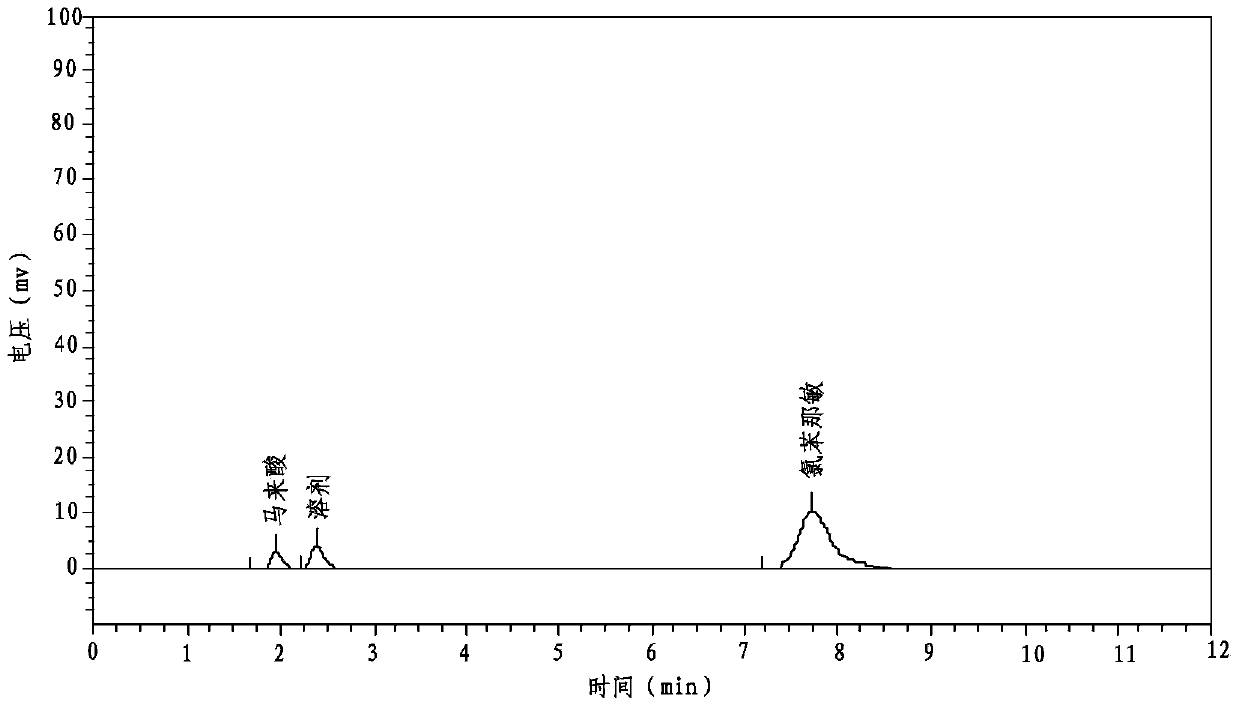
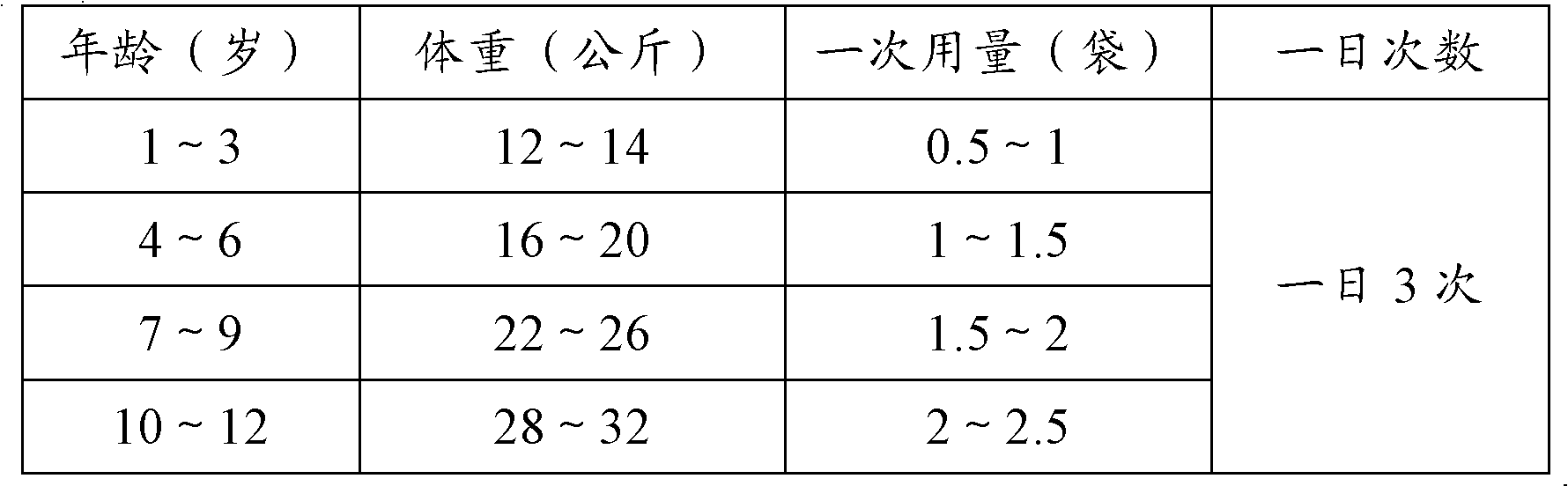
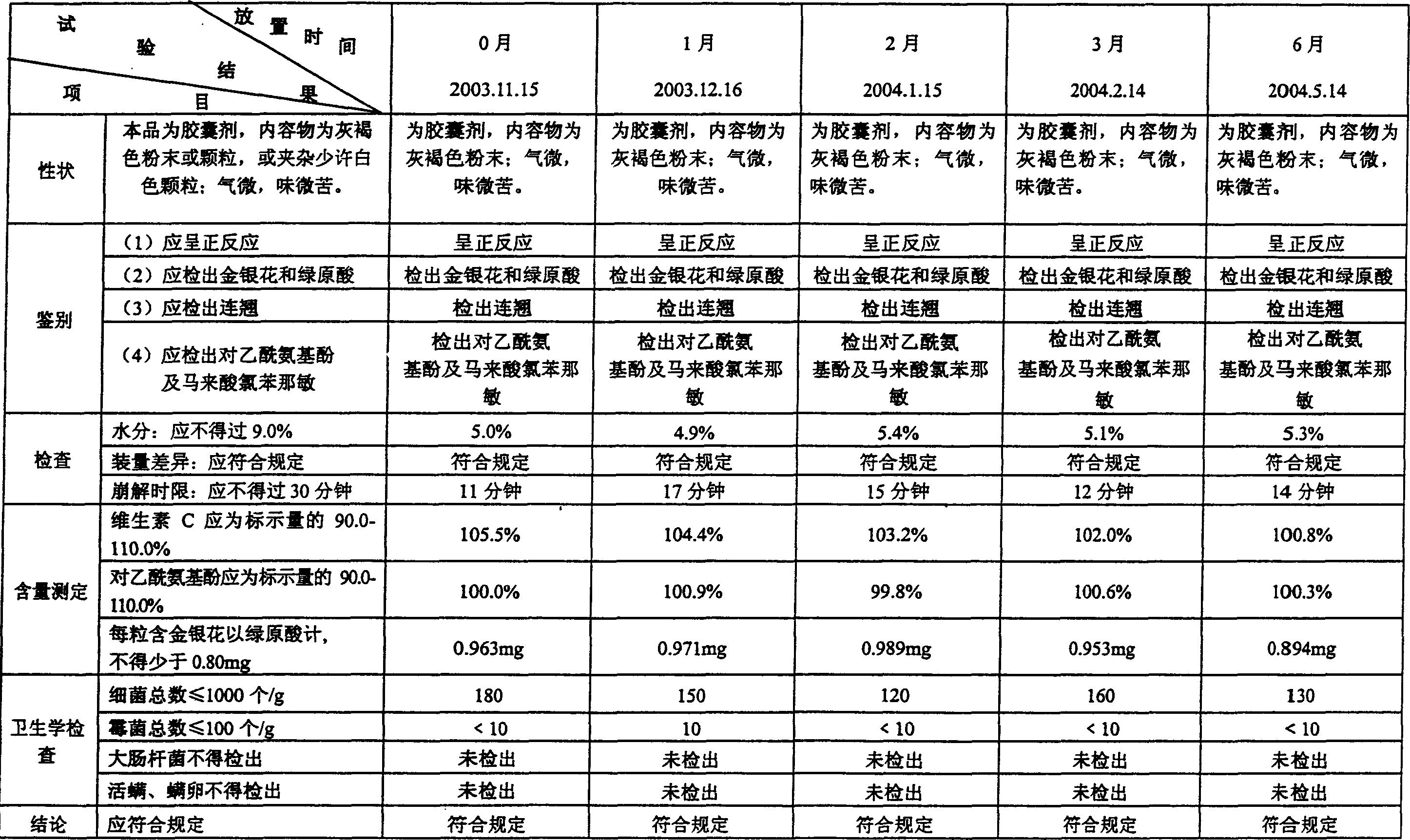
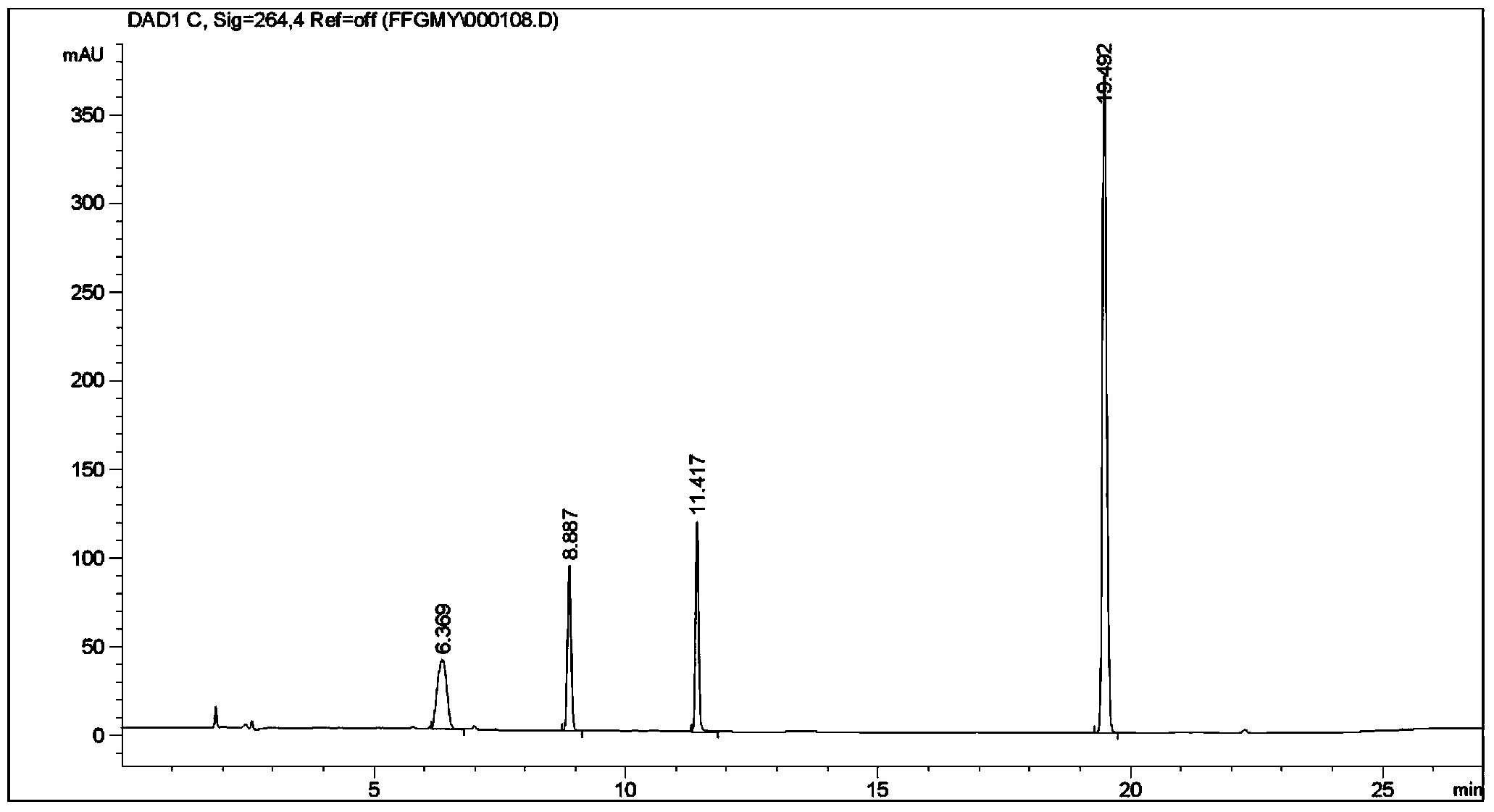
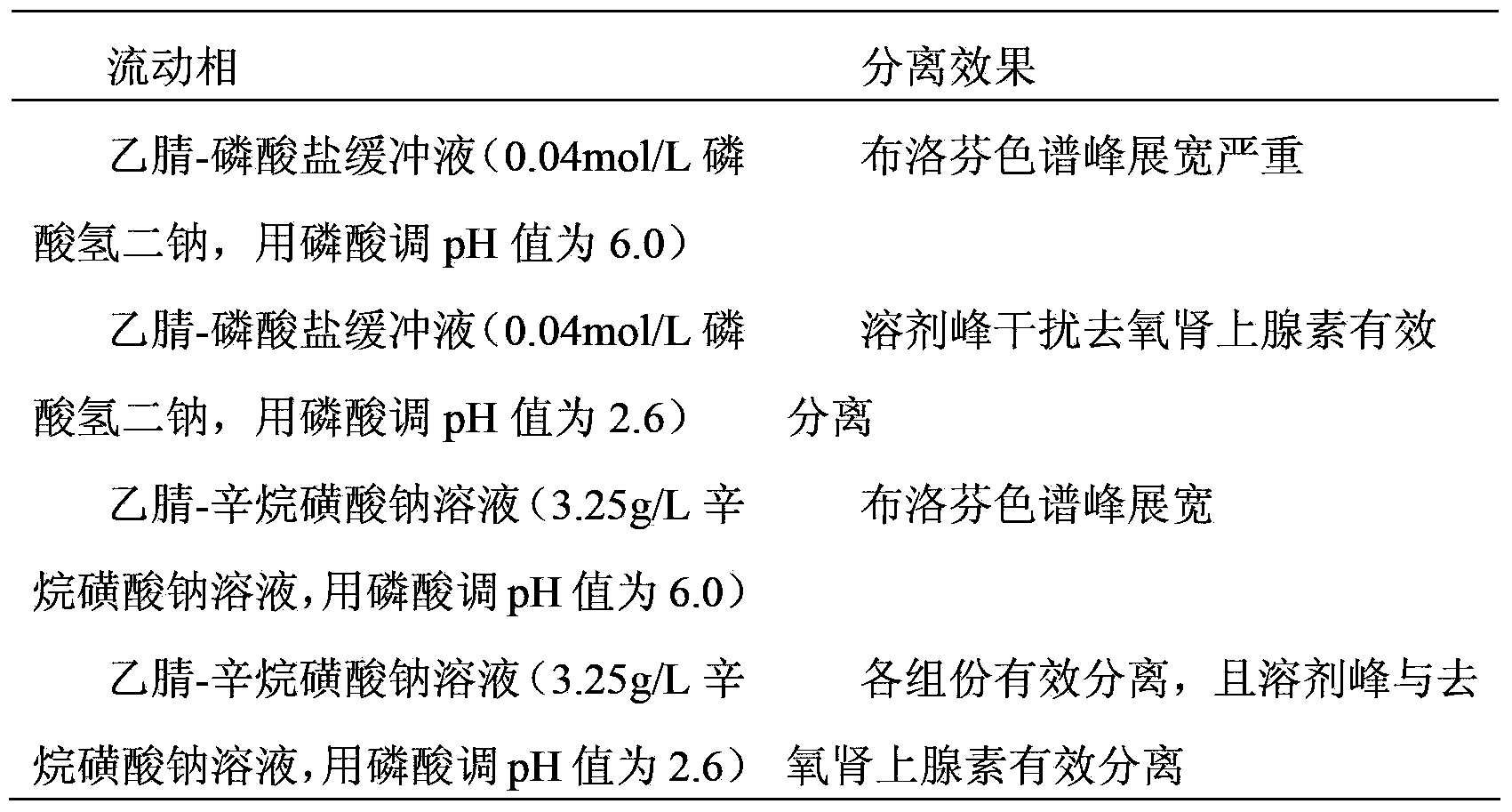
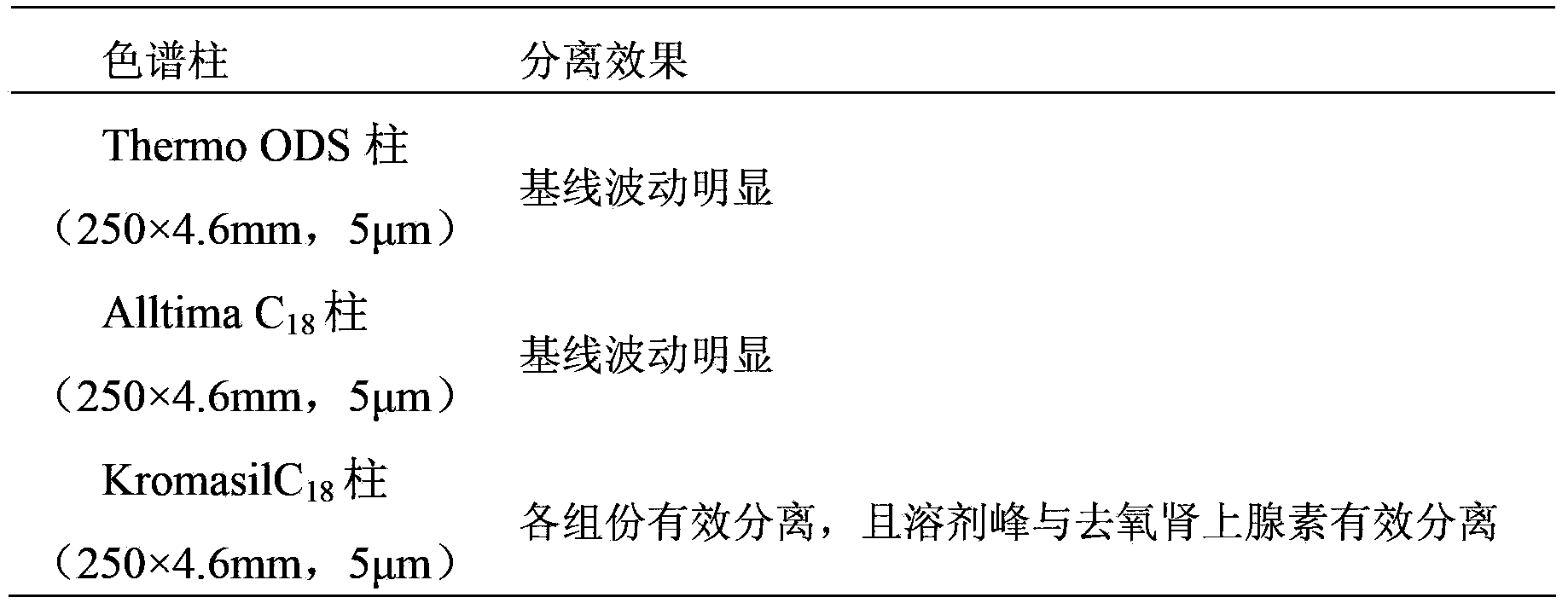
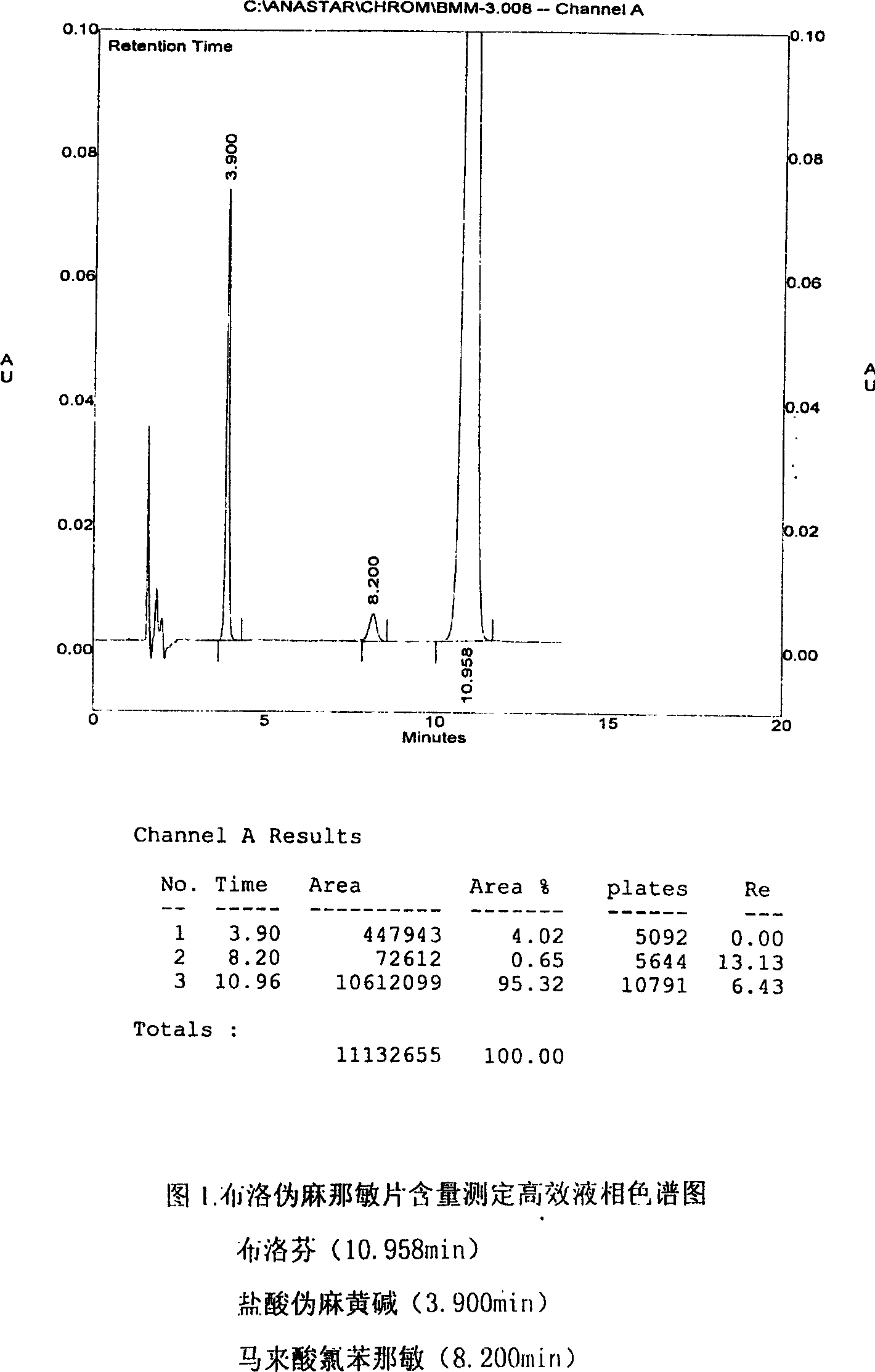
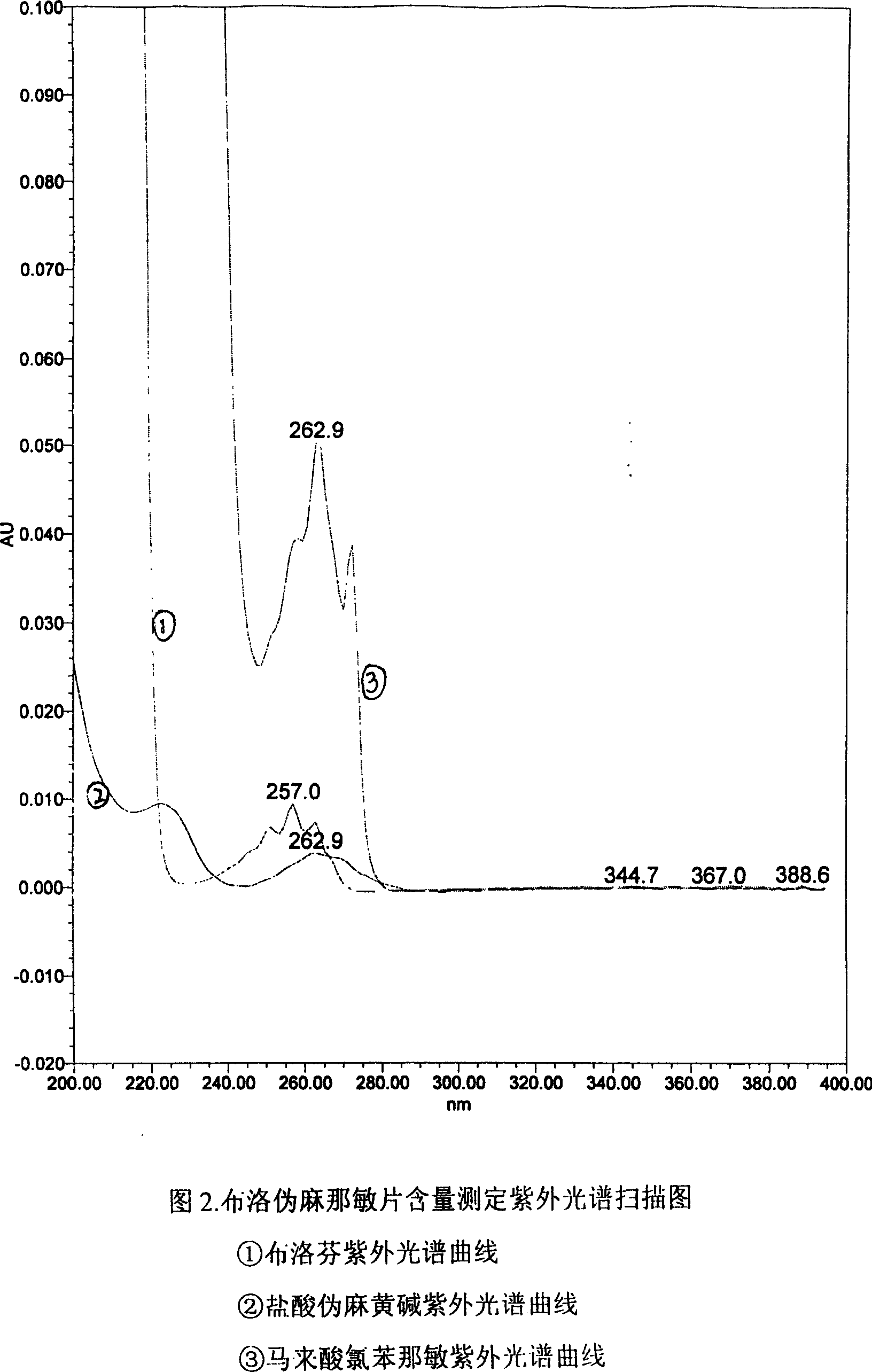
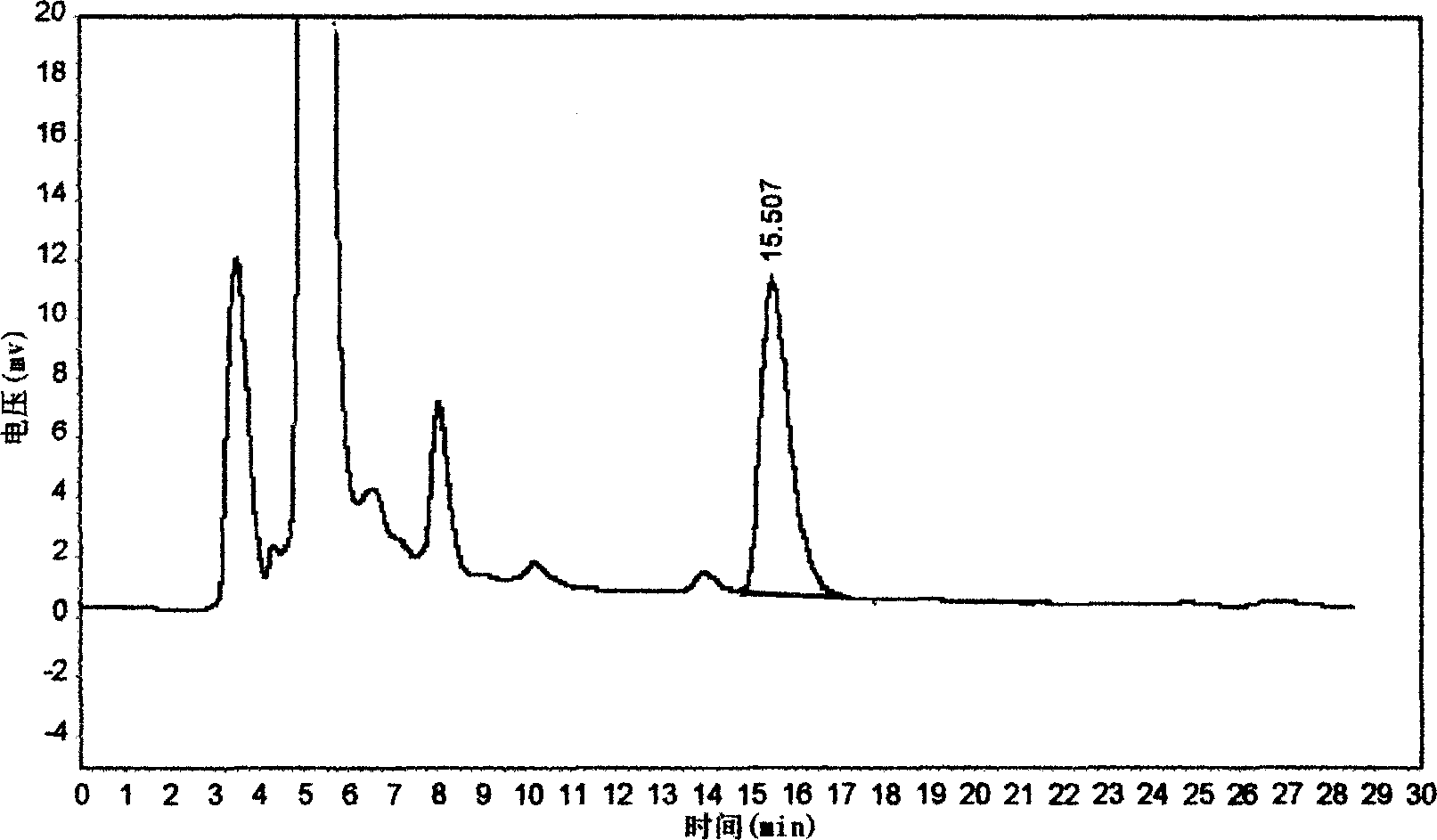
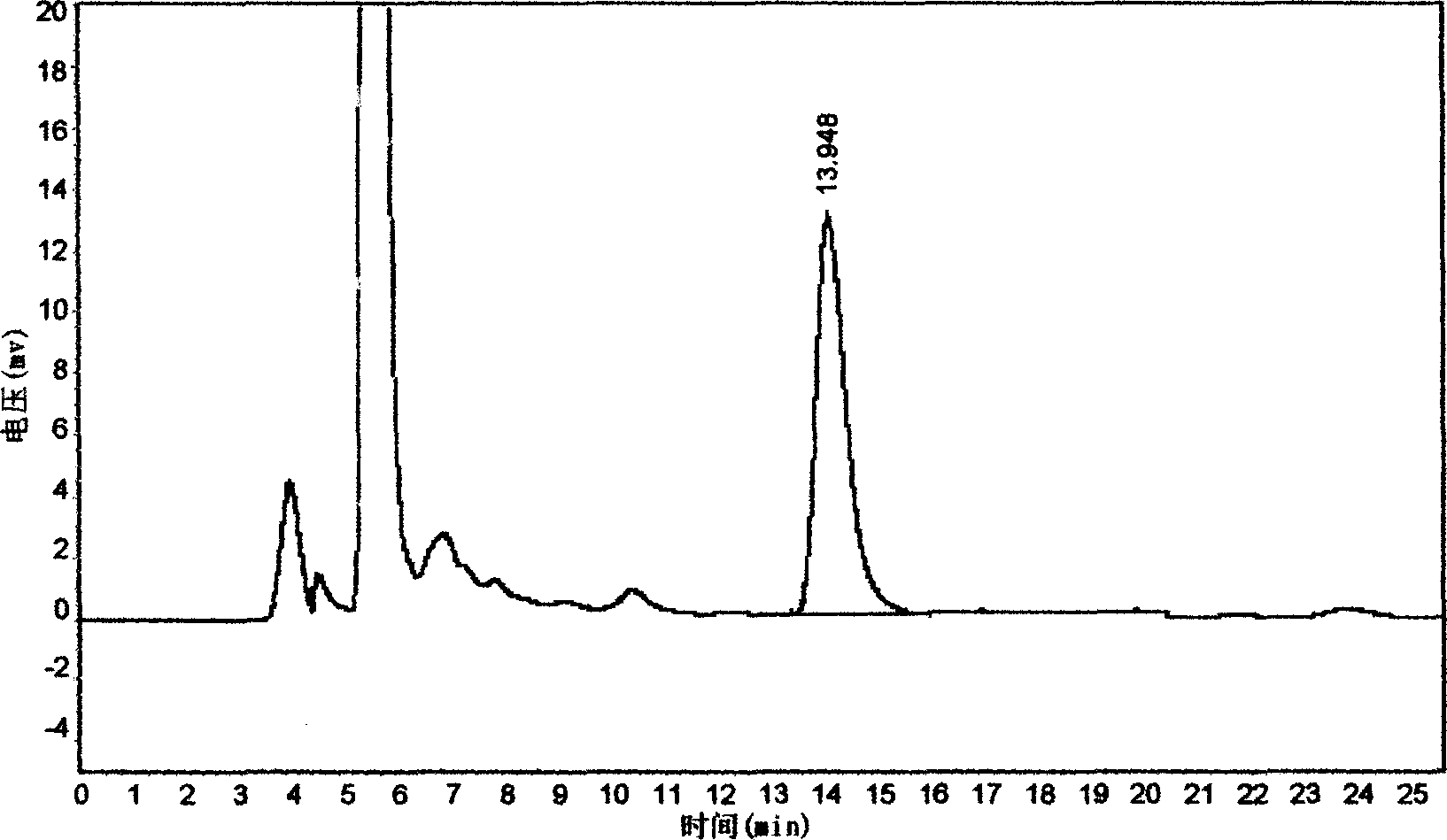
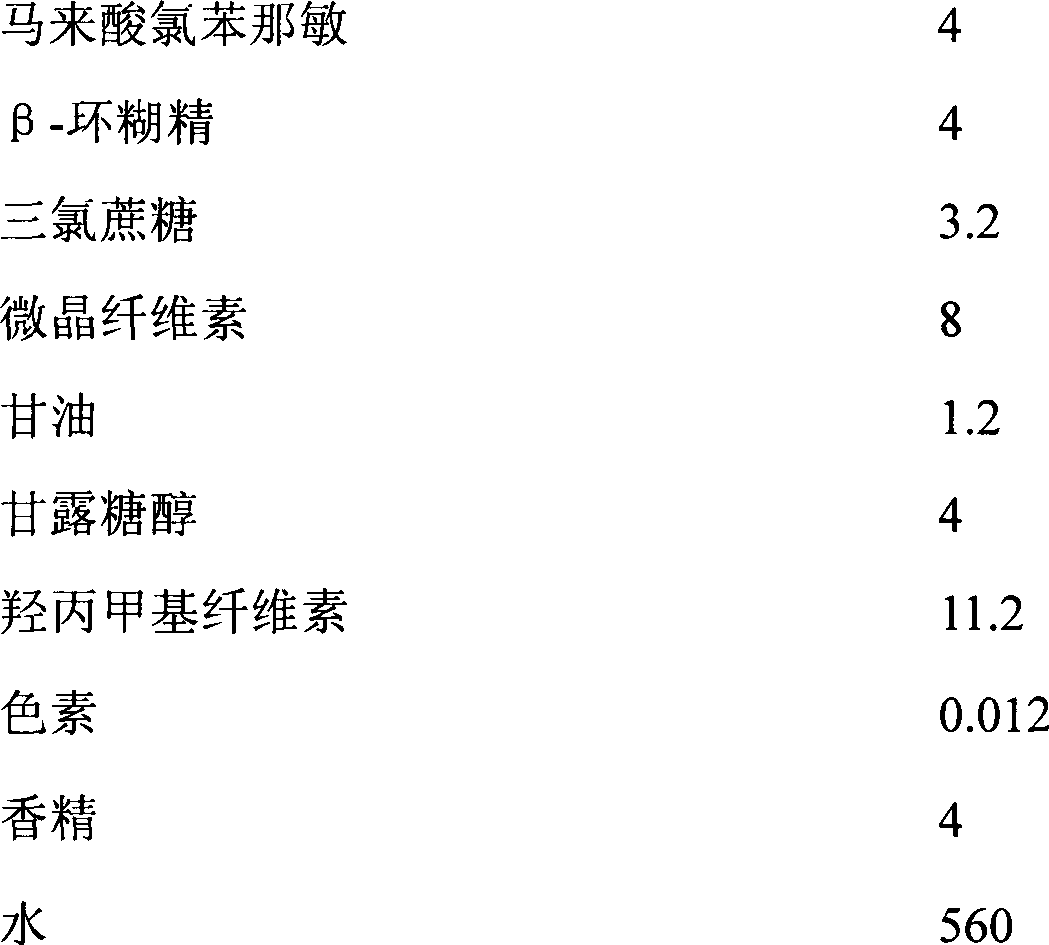
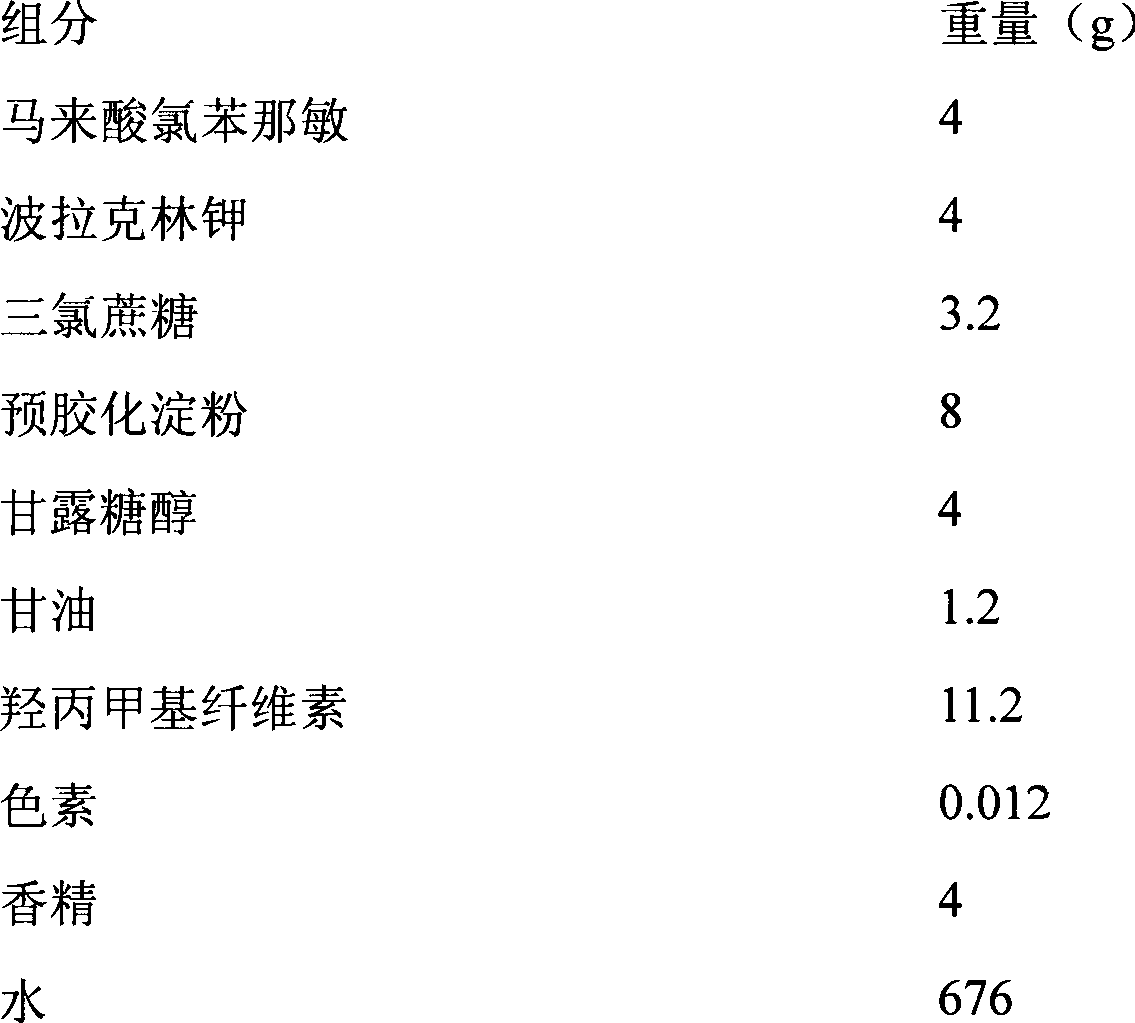
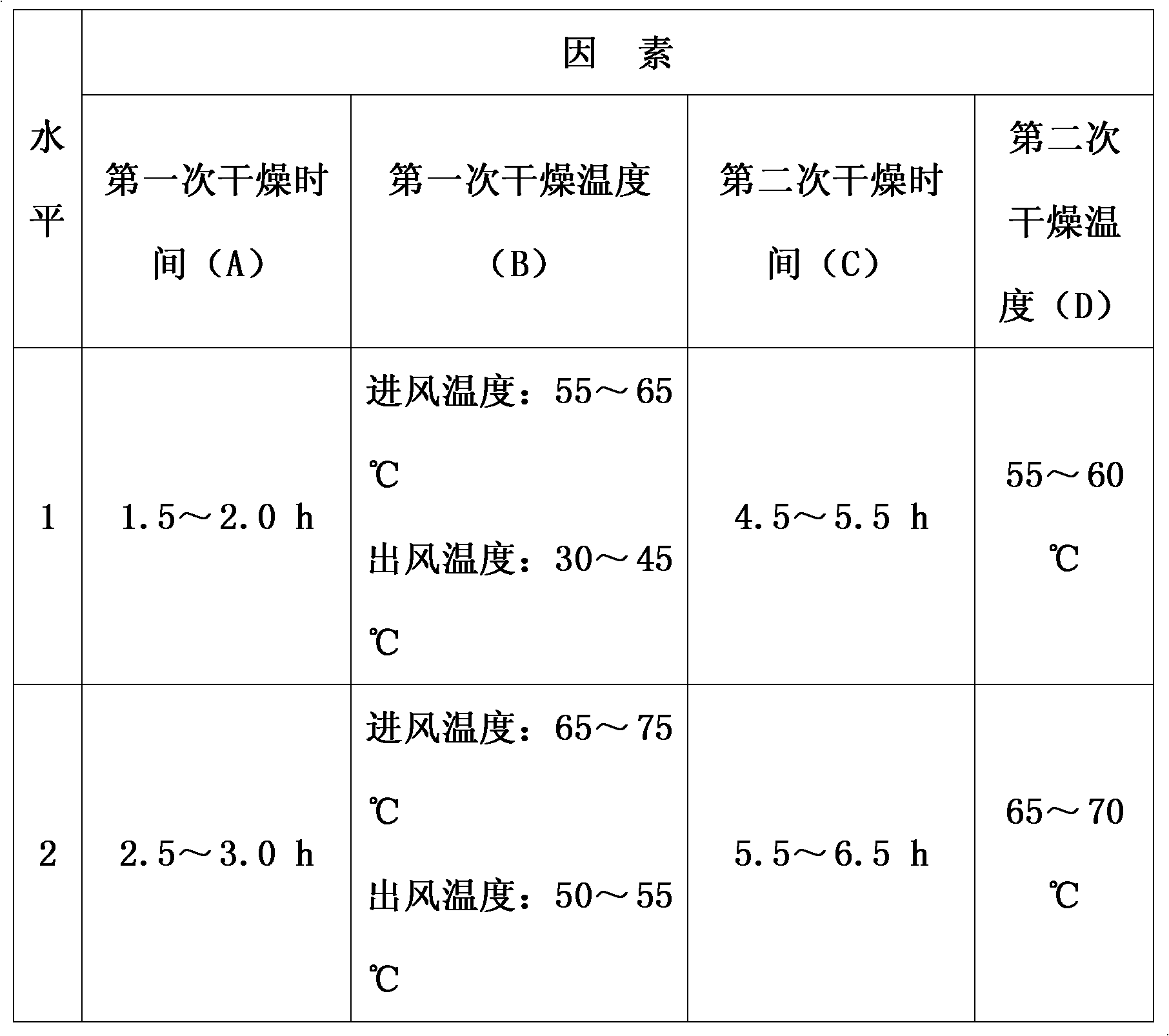
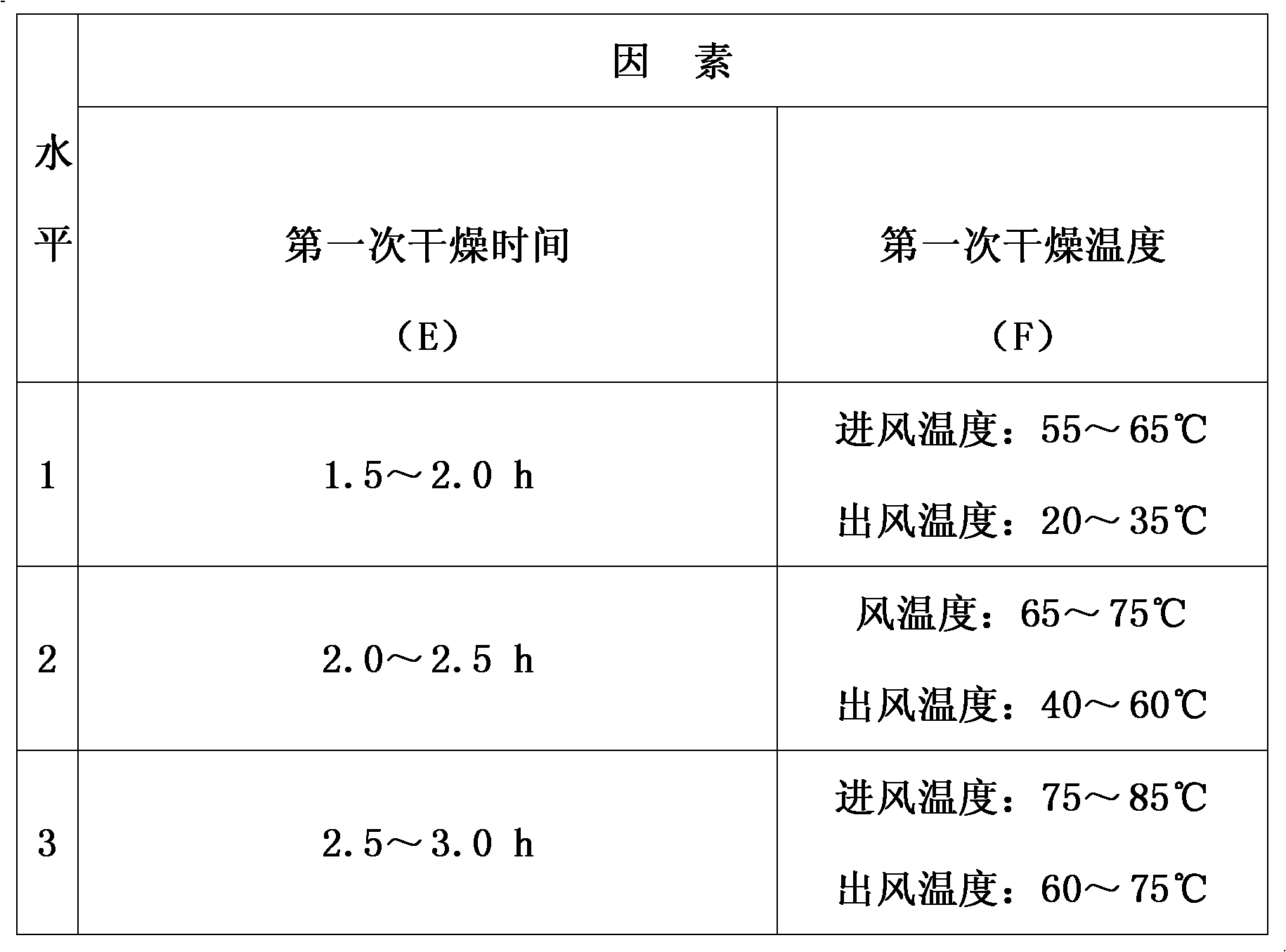
Patents
Literature
139 results about "Chlorphenamine maleate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Oral disintegrant of compound paracetamol
InactiveCN1679525ASimple preparation processDisintegration has little adverse effectOrganic active ingredientsAntipyreticChlorphenamine maleateOrally disintegrating tablet
An oral disintegrating tablet of compound paracetamol is prepared from paracetamol, pseudoephedrine hydrochloride, dextromethorphan HBr, chlorphenamine maleate, filler, disintegrant, adhesive or moistening agent, lubricant, flavouring and pigment through direct tabletting.
Owner:FUDAN UNIV
Solid preparation taken through oral cavity of compound MEthoxyphEnaminE and preparation method
ActiveCN1660107ANot easy to absorb moisture and change colorImprove heat resistanceRespiratory disorderImmunological disordersChlorphenamine maleateHydrogen phosphate
An orally-taken composite solide medicine containing methoxyphenamine is prepared from the methoxyphenamine hydrochloride, noscapine, euphyllin, chlorphenamine maleate and diluent chosen from 13 compounds including stearic acid, calcium hydrogen phosphate, microcrystalline cellulose, etc.
Owner:ADVENCHEN LAB NANJING
Compound paracetamol and amantadine hydrochloride dripping pills and preparation method
The present invention discloses a compound paracetamol amantadine hydrochloride dripping pills preparation and its preparation method. Said compound preparation is made up by using paracetamol, amantadine hydrochloride, chlorphenamine maleate, artificial cow-bezoar, caffeine and matrix for making dripping pills.
Owner:陈茜
Preparation method of compound paracetamol and amantadine hydrochloride capsule
The invention relates to the technical field of the pharmacy, and especially relates to a preparation method of a compound paracetamol and amantadine hydrochloride capsule. The method is characterized in that smaller amounts of components comprising dextrin, calculus bovis factitious, chlorphenamine maleate and caffeine in a prescription are uniformly mixed to obtain mixed powder I, and larger amounts of compounds comprising viregyt hydrochloride and paracetamol in the prescription are uniformly mixed with the mixed powder I to realize uniform mixing and the uniformity of the content of the chlorphenamine maleate in the whole capsule. Double-tapered rotary vacuum drying is adopted, the physical and chemical properties of a material are not changed after the drying of the material, and the content of relevant substances in a finally finished product is low.
Owner:HAINAN ASIA PHARM CO LTD
Xiao er Anfen Huang Namin granule and preparation method thereof
ActiveCN101982179AQuality assuranceReduce dosageOrganic active ingredientsPharmaceutical product form changeChlorphenamine maleateSoft materials
The invention discloses a Xiao er Anfen Huang Namin granule, which is prepared from the following raw materials in terms of 5000g to 5600g of Xiao er Anfen Huang Namin granules through the wet-method granulation: 125 to 375g of acetaminophen, 0.5 to 1.5g of chlorphenamine maleate, 5 to 15g of artificial cow-bezoar, 4500 to 5200g of filler, 3 to 5g of adhesive, and proper amounts of essence and ethanol. The content uniformity and the stability of the granule both meet the requirement, and the product quality is stable and controllable. The invention further discloses a preparation method of the Xiao er Anfen Huang Namin granule, comprising the following steps: dissolving the chlorphenamine maleate in a proper amount of ethanol, adding the adhesive and mixing with the acetaminophen, the artificial cow-bezoar and the filler uniformly, producing the mixture into a soft material, drying, and subpackaging to prepare the Xiao er Anfen Huang Namin granule. The preparation method has simple process flow, can save the energy and the cost, and is suitable for the industrial production.
Owner:HAINAN HULUWA PHARMA GRP CO LTD
A composite bergenin dispersible tablet and preparation method thereof
InactiveCN1864679AHigh dissolution rateGood dissolution uniformityOrganic active ingredientsPill deliveryCarboxymethyl starchChlorphenamine maleate
The compound bergenin disperser tablet consists of bergenin in 82.0-90.0 wt%, chlorphenamine maleate 1.0-2.0 wt%, carboxymethyl starch sodium 5.0-10.0 wt%, magnesium stearate 0.5-2.0 wt%, superfine silica gel powder 0.5-1.5 wt%, sodium dodecyl sulfate 0.1-0.5 wt%, polyvinyl pyrrolidone 1.0-3.0 wt% and aspartame 0.1-0.5 wt%. The compound bergenin disperser tablet is prepared through micronizing the components, mixing, wet pelletizing and tabletting. It may be disintegrated fast within 2 min and has high bergenin and chlorphenamine maleate leaching speed.
Owner:马晶
Refined polyvalent anti-snake poison lyophilized blood serum and using method
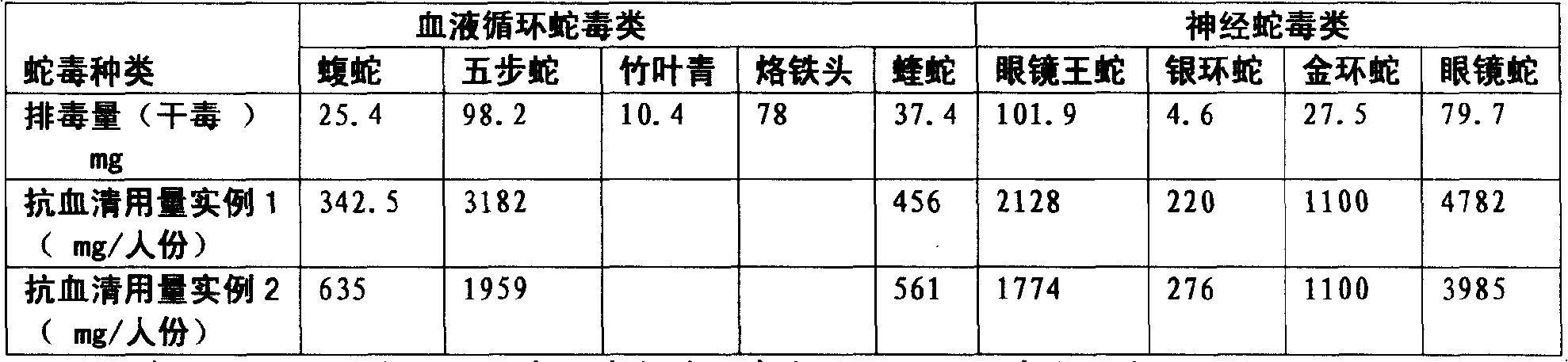
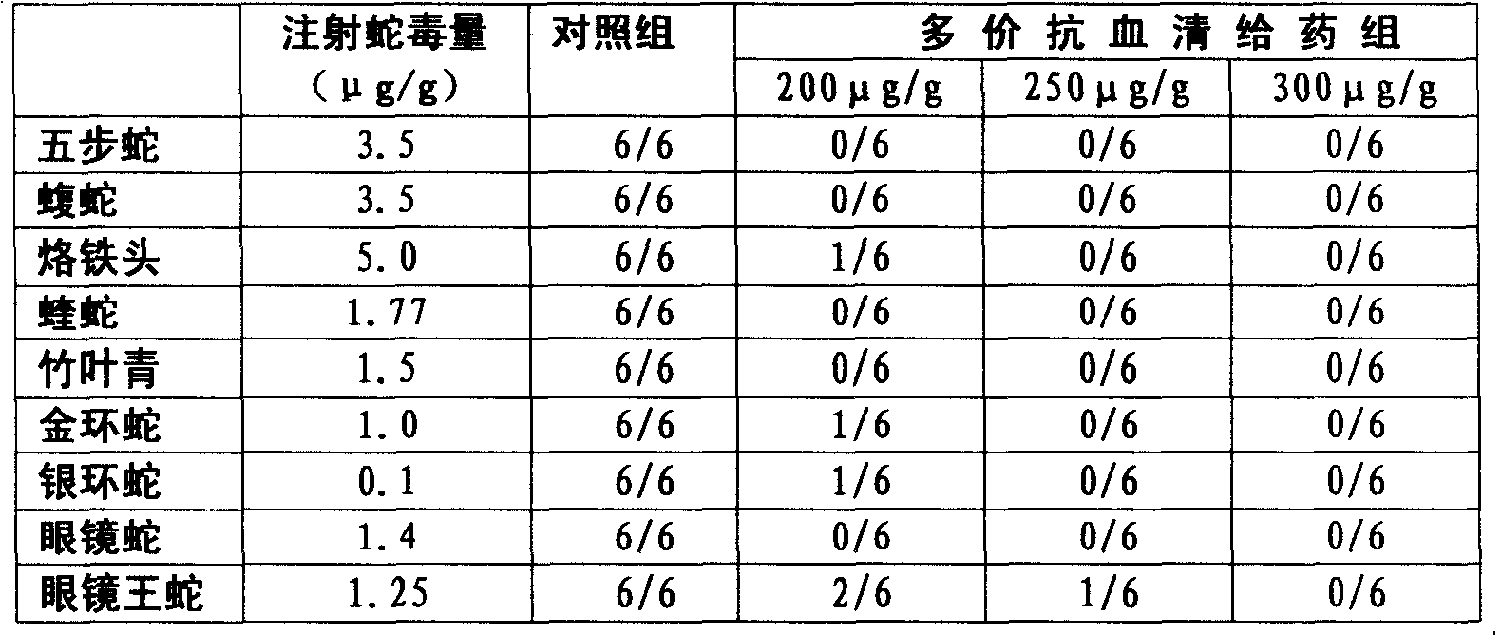
ActiveCN101347617ATimely treatmentEffective treatmentAntinoxious agentsAntibody ingredientsAntigen Binding FragmentSnake venom
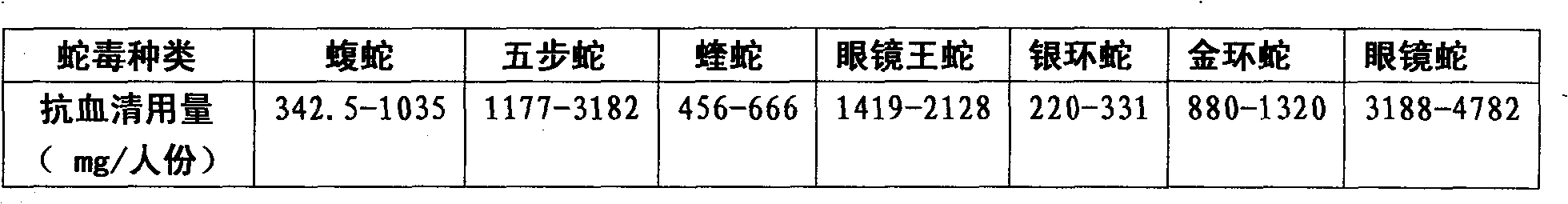
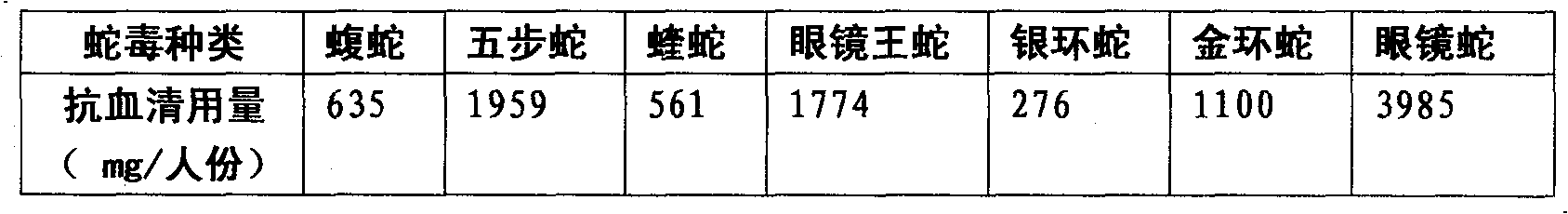
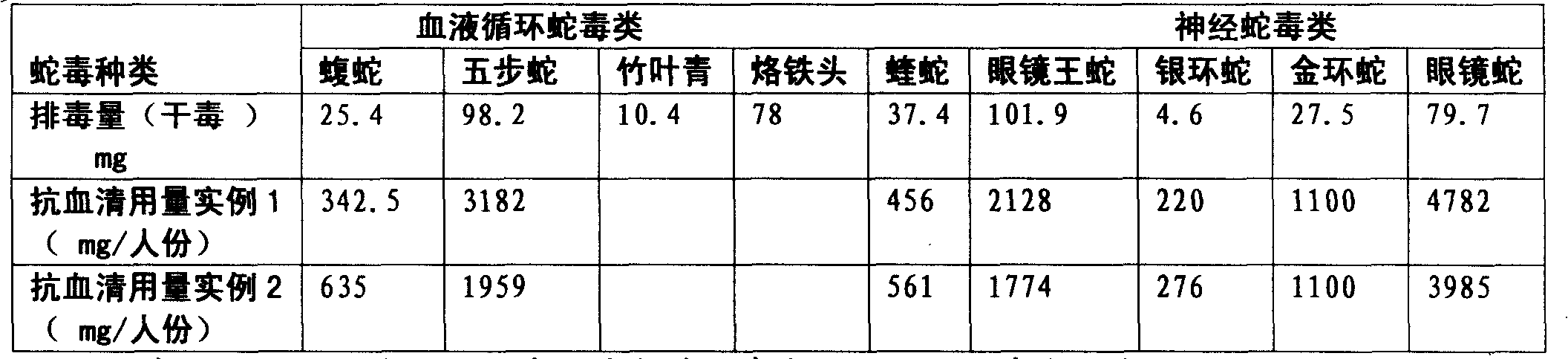
Purified multivalent and lyophilized antivenin and a use method belong to antibody immunity serums that aim at more than two antigens. The invention aims at preparing the multivalent antivenin by a plurality of lyophilized antivenins and the use method. The invention is characterized in that: the antigen-binding fragment F(ab)2 has 60 to 80 percent of lyophilized antiserum, the unit is mg per person, 342.5 to 1035 of vipers, 1177 to 3182 of agkistrodon acutus, 456 to 666 of adders, 1419 to 2128 of king cobras, 220 to 331 of coral snakes, 880 to 1320 of gold banded kraits, 3188 to 4782 of cobras are combined; the optimized combination is as follows: 635 of vipers, 1959 of agkistrodon acutus, 561 of adders, 1774 of king cobras, 276 of coral snakes, 1100 of gold banded kraits and 3985 of cobras. Blood circulation snake venom antiserum and nerves snake venom antiserum are respectively filled and measured. The use method is as follows: the antivenin that is diluted by sodium chloride and an anti-allergic agent, such as chlorphenamine maleate, are injected into muscle or a vein. The antivenin is prepared by only seven antivenins, and can effectively and in time treat injuries caused by various poisonous snakes in our country when the antiserum is directly injected in condition of the undetermined variety of poisonous snakes, and the invention reaches the advanced world level of the antivenin.
Owner:浙江健博生物科技股份有限公司
Soft capsule composition containing zine gluconate, Ibuprofen and chlorphenamine maleate
The present invention relates to medicine preparation, and especially a kind of soft capsule composition containing zinc gluconate, Ibuprofen and chlorphenamine maleate as active components. The composition consists of active component 1 weight portion, dispersant medium 0.8-3.0 weight portions, surfactant 0.02-0.3 weight portions and suspending agent 0.01-1.0 weight portion. The composition has homogeneous dispersion of medicine component and stable quality.
Owner:ZHEJIANG WANLIAN PHARMA IND +1
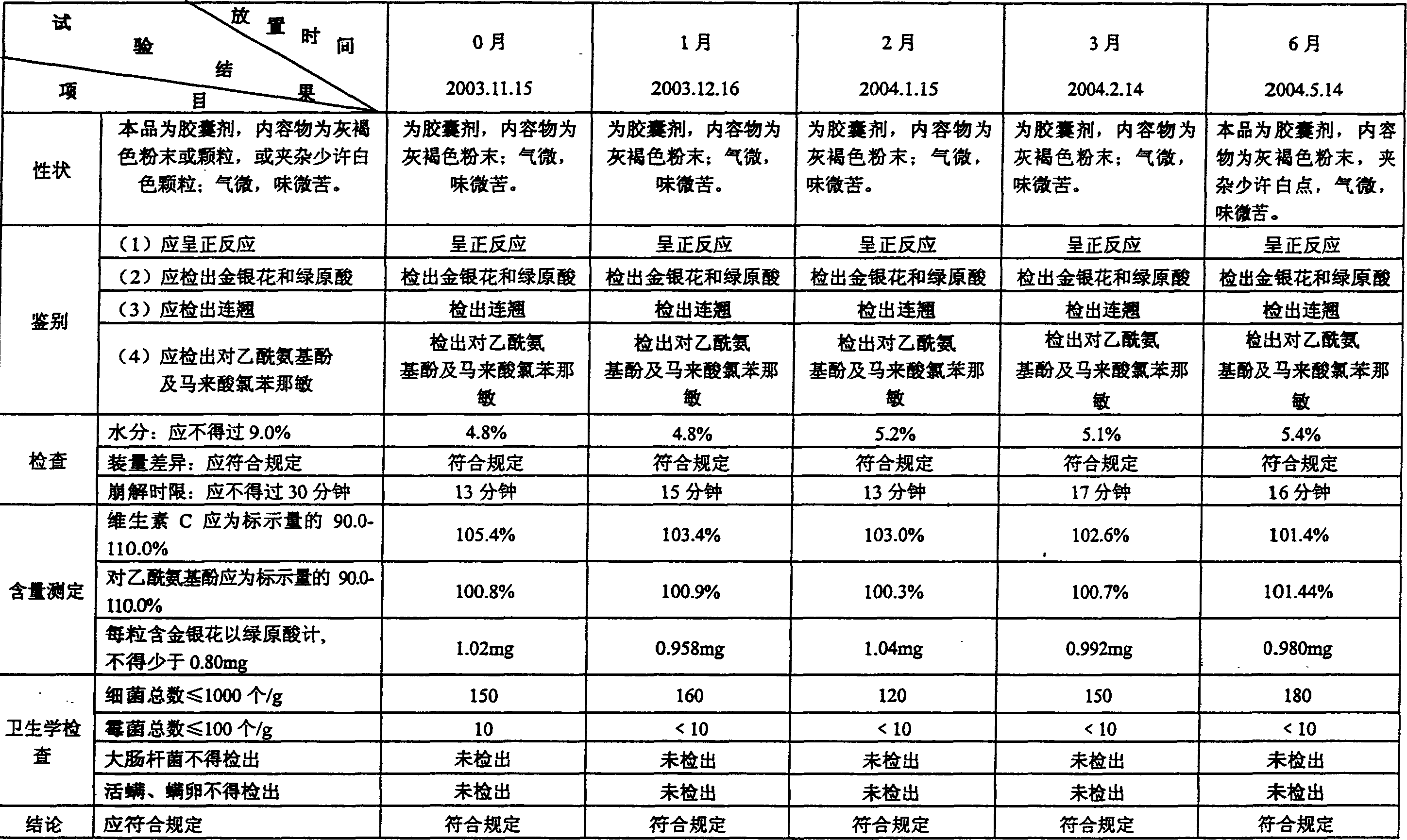
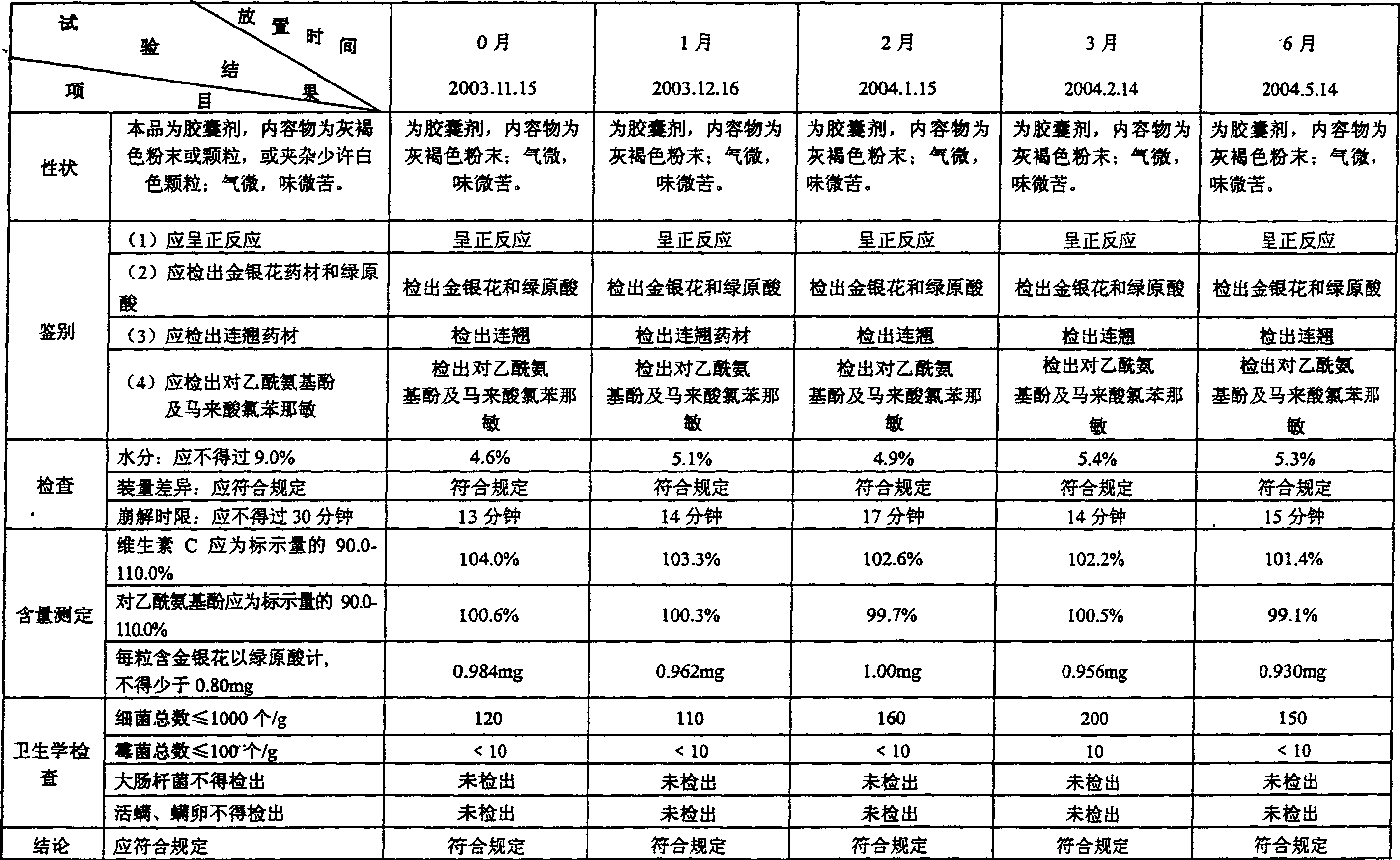
Method for detecting Jingan capsule
ActiveCN101797277AIncrease assayImprove quality controlComponent separationCapsule deliveryChlorphenamine maleateMedicine
The invention discloses a method for detecting a capsule for treating cold. The capsule is prepared from honeysuckle, common andrographis herb, isatis root, dandelion, acetaminophen, amantadinehydrochloride and chlorphenamine maleate. The invention increases the content detection of the amantadinehydrochloride, the honeysuckle and the dandelion on the basis of the traditional quality standard, improves a content detecting method of the acetaminophen, can carry out more effective control on main medicament components of a Jingan capsule, and enables the quality monitoring level of the Jingan capsule to be greatly improved. The application of the invention is more beneficial to manufacturers and the supervisory management department to monitor the product quality and can provide better guarantee for the medical department and the treatment of patients.
Owner:GUIZHOU BAILING GRP PARMACEUTIAL CO LTD
Compound drug for curing colds and preparation technology thereof
ActiveCN101700245AControl uptakeImprove efficacyOrganic active ingredientsAntipyreticImmediate releasePseudoephedrine Hydrochloride
The invention relates to a compound drug for curing colds and a preparation technology thereof. The compound drug consists of active components of Ibuprofen, pseudoephedrinehydrochloride, chlorphenamine maleate with effective doses and proper amount of pharmaceutic adjuvants, which is characterized by preparing lbuprofen with a partial effective dose and pseudoephedrinehydrochloride with an effective dose into a sustained-release preparation; preparing ibuprofen with a residual effective dose and chlorphenamine maleate with an effective dose into an immediate-release preparation; and preparing the sustained-release preparation and the immediate-release preparation into various drug preparations according to conventional methods. The invention adopts the sustained-release technology and the immediate-release technology, effectively controls absorption and use of the three different drug active components in vivo, enables the blood concentration of the three drug active components to achieve effective curative concentration and coincidence in decreasing time, thus enhancing the pesticide effect of the compound drug in the invention cooperatively and improving bioavailability of the compound drug in the invention.
Owner:TIANSHENG PHARMA GROUP
Paracetamol-caffeine-artificial cow-bezoar-chlorphenamine maleate medicinal composition and preparation method thereof
The invention discloses a paracetamol-caffeine-artificial cow-bezoar-chlorphenamine maleate medicinal composition and a preparation method thereof. The paracetamol-caffeine-artificial cow-bezoar-chlorphenamine maleate medicinal composition medicinal composition comprises paracetamol, caffeine, chlorphenamine maleate and artificial cow-bezoar in the weight ratio (90-160):(3-7):(0.1-0.6):(5-10); and a filling agent, a disintegrating agent and a flavoring agent serving as auxiliary materials can be added. The method for preparing the paracetamol-caffeine-artificial cow-bezoar-chlorphenamine maleate medicinal composition comprises the following steps of: weighing paracetamol, artificial cow-bezoar, chlorphenamine maleate and caffeine; mixing uniformly; adding 10-20 percent of auxiliary materials; and pelletizing, drying and straightening. The prepared paracetamol-caffeine-artificial cow-bezoar-chlorphenamine maleate medicinal composition has high disintegrating and dissolving speeds and a good medicament effect, and is used for treating nasal obstruction, headache, sore throat, fever and the like.
Owner:JILIN AODONG GROUP DALIAN PHARMACEUTICAL CO LTD
Refined polyvalent anti-snake poison lyophilized blood serum and using method
ActiveCN101347617BTimely treatmentEffective treatmentAntinoxious agentsAntibody ingredientsAntigen Binding FragmentNaja
Purified multivalent and lyophilized antivenin and a use method belong to antibody immunity serums that aim at more than two antigens. The invention aims at preparing the multivalent antivenin by a plurality of lyophilized antivenins and the use method. The invention is characterized in that: the antigen-binding fragment F(ab)2 has 60 to 80 percent of lyophilized antiserum, the unit is mg per person, 342.5 to 1035 of vipers, 1177 to 3182 of agkistrodon acutus, 456 to 666 of adders, 1419 to 2128 of king cobras, 220 to 331 of coral snakes, 880 to 1320 of gold banded kraits, 3188 to 4782 of cobras are combined; the optimized combination is as follows: 635 of vipers, 1959 of agkistrodon acutus, 561 of adders, 1774 of king cobras, 276 of coral snakes, 1100 of gold banded kraits and 3985 of cobras. Blood circulation snake venom antiserum and nerves snake venom antiserum are respectively filled and measured. The use method is as follows: the antivenin that is diluted by sodium chloride and an anti-allergic agent, such as chlorphenamine maleate, are injected into muscle or a vein. The antivenin is prepared by only seven antivenins, and can effectively and in time treat injuries caused by various poisonous snakes in our country when the antiserum is directly injected in condition of the undetermined variety of poisonous snakes, and the invention reaches the advanced world level of the antivenin.
Owner:浙江健博生物科技股份有限公司
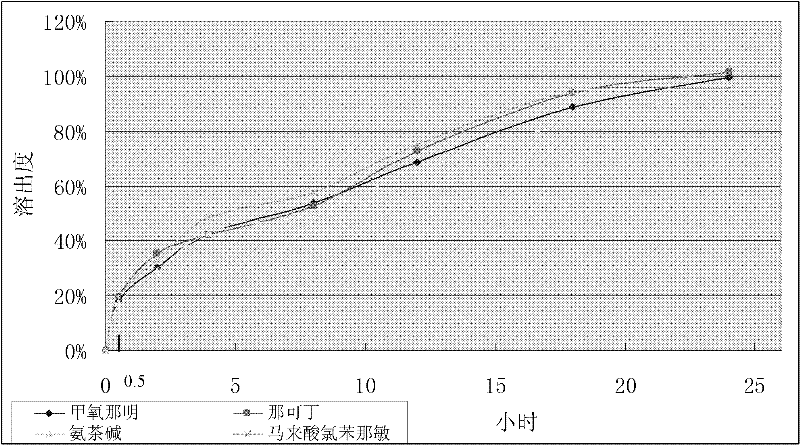
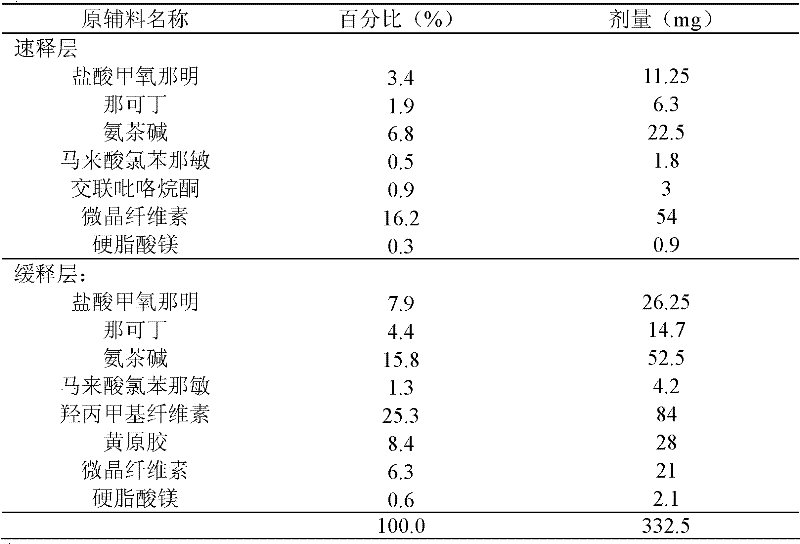
Compound methoxyphenamine quick-release and sustained-release preparation
The invention discloses a compound quick-release and sustained-release preparation containing pharmaceutical ingredients including methoxyphenamine or hydrochloride thereof, narcotine, aminophylline and chlorphenamine maleate. A quick-release part contains 1 to 70 percent of active ingredients and a sustained-release part contains 30 to 99 percent of active ingredients. The active ingredients canbe released constantly for 8 to 24 hours. The compound quick-release and sustained-release preparation may be quick-release and sustained-release tablets, quick-release and sustained-release capsulesand quick-release and sustained-release mixed suspensions.
Owner:SALUS PHARMA TECH SHANGHAI
Preparation method of paracetamol pseudoephedrine hydrochloride and cholrphenamine maleate oral disintegration tablet
InactiveCN1803136AGood content uniformity of active ingredientsImprove bioavailabilityOrganic active ingredientsAntiinfectivesChlorphenamine maleateAdjuvant
The invention provides a process for preparing paracetamol pseudoephedrine hydrochloride and chlorphenamine malease orally disintegrating tablets, mixing paracetamol with bulking agent for granulating, making solution from pseudoephedrine hydrochloride, chlorphenamine maleate and spray packaging material, coating onto obtained paracetamol particles, then dressing to obtain flavor masked particles, finally mixing the particles with other adjuvant and tabletting.
Owner:HONGGUAN BIO PHARMA CO LTD
Pediatric paracetamol, cow-bezoar and chlorphenamine maleate granules and quality control method thereof
InactiveCN102125583AFix security issuesLow raw material costOrganic active ingredientsAntipyreticChlorphenamine maleateBiology
The invention discloses pediatric paracetamol, cow-bezoar and chlorphenamine maleate granules and a quality control method thereof. The granules are prepared from paracetamol, in-vitro cultivated cow-bezoar and chlorphenamine maleate in a ratio of 125:5:0.5. The quality control method combines identification, thin-layer chromatography, thin-layer scanning, and ultraviolet spectroscopy. In the granules, in-vitro cultivated cow-bezoar is used to replace natural cow-bezoar, so as to greatly reduce raw material cost of medicine while ensuring pesticide effect. In addition, the replacement of in-vitro cultivated cow-bezoar for artificial cow-bezoar so that the medicament safety and pesticide effect problems can be solved, and is favorable for comprehensive quality control of the pediatric paracetamol, cow-bezoar and chlorphenamine maleate granules.
Owner:文永盛 +2
Vitaminc lonicera and forsythia capsule and preparing method
The invention discloses a Vitamin C Yinqiao capsule, prepared ofHoneysuckle Flower, weeping forsythia, Fineleaf Schizonepeta Herb, Fermented Soybean, Common Lophatherum Herb, Great Burdock Achene, Reed Rhizome, Platycodon Root, Liquorice Root, Chlorphenamine Maleate, Paracetamol, Vitamin C, and menthence in weight shares. It contains traditional Chinese medicinal components and also menthence, Chlorphenamine Maleate and Vitamin C coated with auxiliaries, and as prepared, adopts step extraction, having functions of cooling and inducing sweat and clearing heat and detoxifying, and able to cure fever and headache, cough, thirst, and throat ache. It is convenient to take, treats both principal and secondary aspect of disease, having obvious effect and able to meet the requirements of the people.
Owner:南宁市言如其保健食品有限公司
Method for determining content of three components comprising phenylephrine hydrochloride, chlorphenamine maleate and ibuprofen in compound cold treatment tablet
ActiveCN104251889AComprehensive quality inspection indicatorsGood repeatabilityComponent separationChlorphenamine maleateSulfonate
he invention discloses a method for simultaneously determining phenylephrine hydrochloride, chlorphenamine maleate and ibuprofen in a compound cold treatment medicine. The method comprises the following steps: respectively preparing a phenylephrine hydrochloride reference substance solution, a chlorphenamine maleate reference substance solution and an ibuprofen reference substance solution; preparing a compound cold treatment medicine sample solution; and determining through high performance liquid chromatography, wherein octadecyl silane-bonded silica gel (2504.0mm, 5mum) is used as a filler, a sodium octane sulfonate solution is used as a mobile phase A, acetonitrile is used as a mobile phase B, gradient elution is carried out, the column temperature is 35DEG C, the flow velocity is 1ml / min, and the detection wavelength is 264nm.
Owner:SHENZHEN NEPTUNUS PHARMA RES INST CO LTD
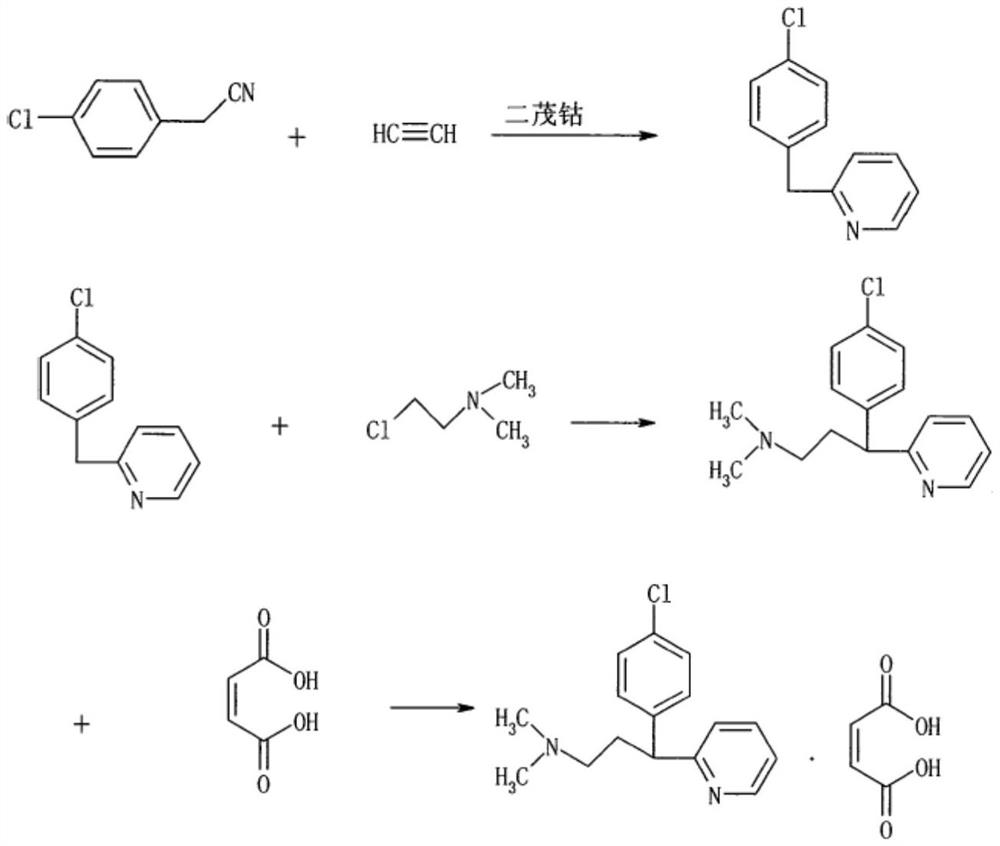
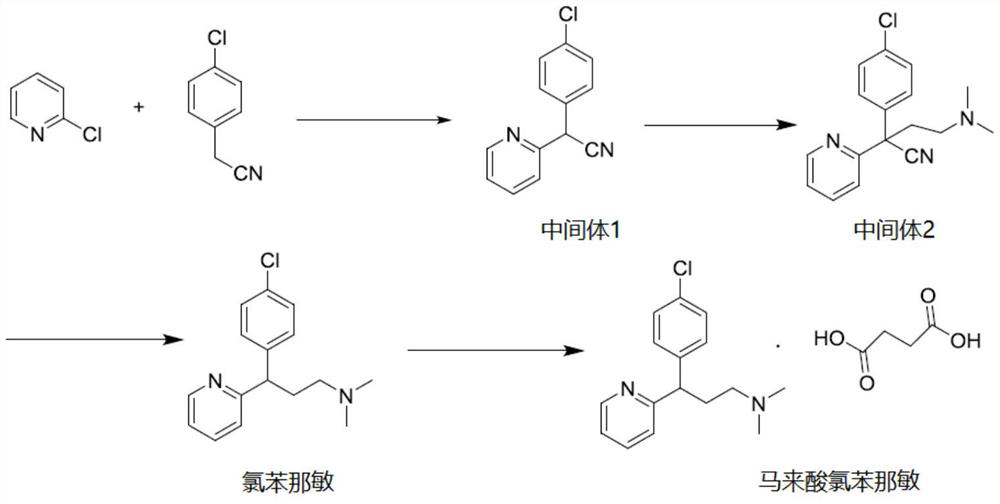
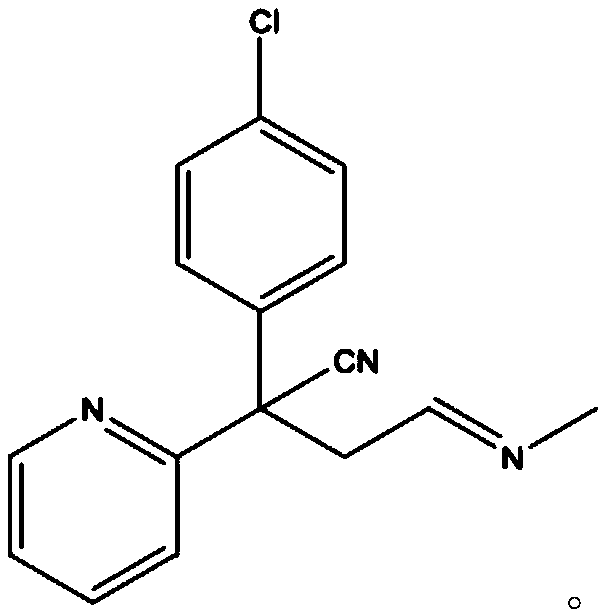
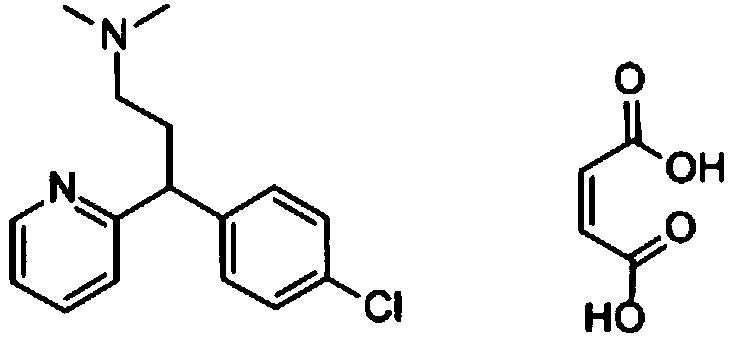
Preparation method of chlorpheniramine maleate
ActiveCN111747887AShort synthetic routeRaw materials are easy to getOrganic compound preparationCarboxylic acid salt preparationChlorphenamine maleateChlorobenzene
The invention provides a chlorpheniramine preparation method, which comprises: (1) reacting p-chlorophenylacetonitrile and 2-chloropyridine to obtain an intermediate 1; (2) reacting the intermediate 1with 2-dimethylaminochloroethane to obtain an intermediate 2; and (3) reacting the intermediate 2 with an alkali to obtain chlorpheniramine. The invention further provides a preparation method of chlorpheniramine maleate. The methods are short in synthetic route, easily available in raw materials and low in cost; the total yield is high, and the product quality is controllable; reaction conditions are mild, requirements on equipment are low, and industrial production is facilitated.
Owner:CHENGDU JIANJIANG PHARMA FACTORY
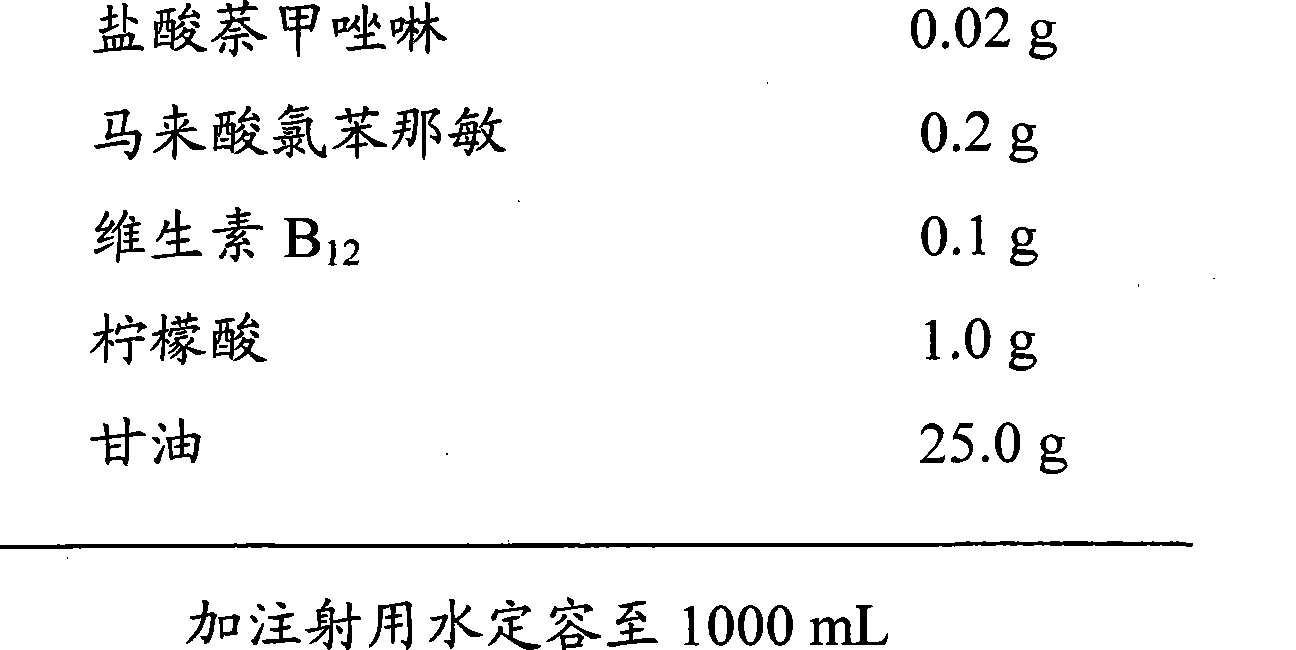
Naphazoline hydrochloride, chlorphenamine maleate and vitamin B12 eye drops without bacteria inhibitor and preparation method thereof
InactiveCN101455636AAvoid side effectsAvoid potential dangerOrganic active ingredientsSenses disorderChlorphenamine maleateVitamin B12
The present invention discloses a naphazoline hydrochloride, chlorphenamine maleate and vitamin B12 eye drops which do not contain bacteriostat and a preparing method thereof, wherein the naphazoline hydrochloride, chlorphenamine maleate and vitamin B12 eye drops comprise naphazoline hydrochloride, chlorphenamine maleate, vitamin B12, pH modifying agent, isoosmotic agent, stabilizing agent, thickening agent, etc. The preparing method adopts an aseptic manipulation filling technique. Furthermore the eye drops of the invention adopt a disposable single-dose independent packaging. The sterilized performance of product is guaranteed. The product is safer, more reliable, easier and more sanitary.
Owner:HEBEI UNIVERSITY OF SCIENCE AND TECHNOLOGY
Compound mequindox injection
InactiveCN101433543AReduce chance of drug resistanceLower resistanceAntibacterial agentsSalicyclic acid active ingredientsChlorphenamine maleateAtropine sulfate
The invention discloses compound mequindox injection, and belongs to medicine preparation containing organic components, or anti-infective medicament for livestock. The injection of each 1, 000 liters is prepared by the following raw materials: 100 to 400 kilograms of sodium salicylate, 10 to 150 kilograms of mequindox, 5 to 110 kilograms of gentamicin sulphate, 1 to 20 kilograms of atropine sulfate, 1 to 20 kilograms of chlorphenamine maleate, and the balance being water for injection. The injection adopts combined compound preparation of the mequindox and the gentamicin sulphate, has efficiency and high efficiency for pig bacterial diarrhea, treats both symptoms and root causes, and relieves stress reaction of sick livestock body.
Owner:TIANJIN SHENGJI GRP CO LTD
Content detecting method for Ibuprofen, chlorphenamine maleate and Pseudoephedrine Hydrochloride compound preparation
InactiveCN1888891AThe detection method is accurateEasy to operateComponent separationSpecial data processing applicationsColloidal silicaPhosphate
A content detecting method for pro-pseudo-chlorpheniramine compound preparation uses octadecyl silance bonding colloidal silica as packing agent. The mobile phase is monopotassium phosphate, methanol and triethyl-amine and the detecting wavelength is 215-225nm. Dissolve suitable pro-pseudo-chlorpheniramine preparation in the need testing solution with phosphate buffer solution under ultrasonic sound condition. Dissolve suitable ibuprofen, hydrochloric acid pseudoephedrine and chlorphenamine Maleate in the comparison solution with phosphate buffer solution under ultrasonic sound condition. Take each 10-20 mul of the need testing solution and the comparison solution accurately and inject into liquid chromatograph spectrometer and record chromatogram graph. Use peak acreage to calculate the content to ibuprofen, hydrochloric acid pseudoephedrine and chlorphenamine Maleate with the external standard method. It can confirm the content to three kind of component in compound preparation by using one mobile phase with the sententious and fast virtues to inspect the stability to the production and control the procreative quality of industrialization.
Owner:SHANDONG INST OF PHARMA IND
Eyedrops
InactiveCN1623549ATo promote metabolismEliminate fatigueOrganic active ingredientsSenses disorderChlorphenamine maleateAlcohol
Owner:赵文达
Method for detecting chlorphenamine maleate content in Chinese-western compound preparation
InactiveCN1865985AEliminate the effects ofEliminate distractionsComponent separationSolid solvent extractionChlorphenamine maleateOrganic solvent
The disclosed detection method for Chlorphenamine Maleate content in preparation comprises: with HPLC, using mixed solvent with alcohol and weak-polar organic solution as extraction solution, octadecylsilicane chemically bonded silica as filler, and the mobile phase with brine, aminoethane and acetonitrile. This invention is simple and has well detection effect.
Owner:蔡炜
Quality control method of composite prepn. of dry mango tree leaves extract
InactiveCN1785219AStrong specificityGood reproducibilityComponent separationRespiratory disorderChlorphenamine maleateSoftgel
A compound medicine in the form of tablet, capsule or softgel is prepared from the dried extract of mango leaf, sodium houttuyfonate and chlorphenamine maleate. Its quality control method features that a thin-layer chromatography is used to identify said three components and an efficient liquid-phase chromatographe is used to measure their contents.
Owner:广西南宁允上医药科技开发有限公司
Chlorphenamine maleate oral fast dissolving film and preparation method thereof
InactiveCN103860523ARapid disintegrationQuick effectOrganic active ingredientsPharmaceutical non-active ingredientsChlorphenamine maleatePlasticizer
The invention belongs to the technical field of medicines, and relates to a chlorphenamine maleate oral fast dissolving film and a preparation method thereof. The chlorphenamine maleate oral fast dissolving film comprises an effective amount of the active component chlorphenamine maleate, an odor mask, a film forming material and a disintegrating agent, and necessary pharmaceutically acceptable filler, sweetener and plasticizer, and a coloring agent and essence both added according to need. The oral fast dissolving film is capable of rapidly disintegrating and dissolving in oral cavity, is good in mouthfeel, fast in drug release speed and rapid in effect onset, and is beneficial for improving medicine compliance of patients especially child patients and elderly patients. Also the oral fast dissolving film is convenient to take, and the problem that medicine taking is not timely in the absence of water is solved.
Owner:天津市聚星康华医药科技有限公司
Method for preparing Jingan capsules
The invention discloses a method for preparing Jingan capsules. The Jingan capsules are prepared from honeysuckle, common andrographis herb, indigowoad root, dandelion, acetaminophen, amantadinehydrochloride and chlorphenamine maleate. Adopting a secondary drying method during preparation, the method completes drying operation quickly at a low temperature that does not causes component change, makes the particles uniformly dried and the water content reach a standard, makes the particles reach required standards in both properties and various quantized indexes under the condition of ensuring high compactness of the particles of the capsules and meet the requirements of a subsequent process procedure, and ensures the overall quality of the products.
Owner:GUIZHOU BAILING GRP PARMACEUTIAL CO LTD
Novel chlortrimeton impurity and preparation process thereof
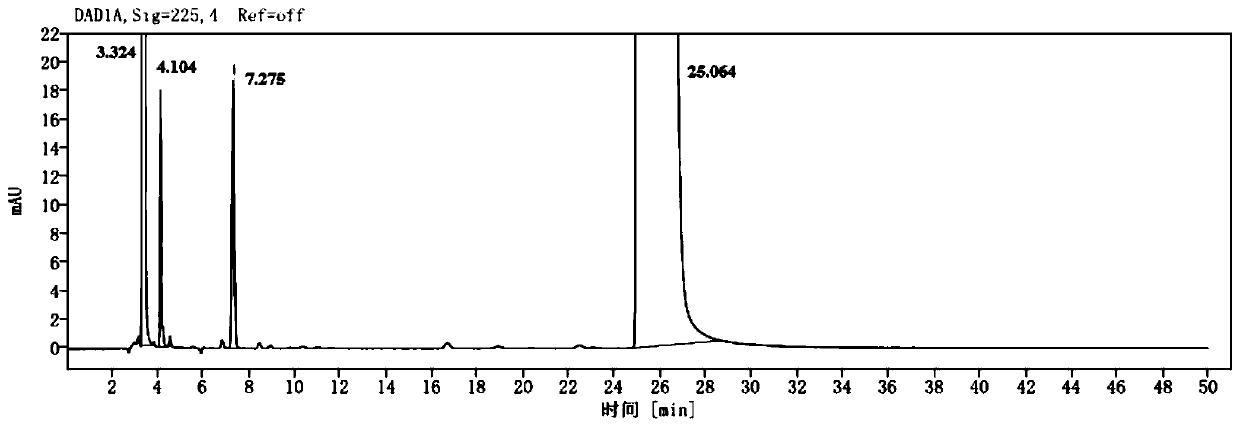
The invention provides a chlortrimeton impurity. The structure of the chlortrimeton impurity is shown in the specification. The invention also provides a preparation process of the chlortrimeton impurity. Experiments prove that the chlortrimeton impurity exists in finished products of chlorpheniramine maleate, and the structure of the chlortrimeton impurity is close to that of chlorpheniramine maleate, so that the separation difficulty is high. However, the chlortrimeton impurity is prepared by a simple process, can be used as a reference substance for quality control of chlorpheniramine maleate, and has a very good application prospect.
Owner:CHENGDU JIANJIANG PHARMA FACTORY
Dispersible tablet of Vitamin C and Lonicera and Forsythia and preparing method thereof
ActiveCN1850241ADrug stabilityImprove stabilityOrganic active ingredientsNervous disorderVitamin CFruit juice
The prevent invention discloses a vitamin C Yinqiao dispersion tablet and its preparation method. It is made up by using Chinese medicinal materials of lonicera flower, forsythia fruit, schizonepeta, bamboo leaf, arctium seed, phragmites root, platycodon root, licorice and menthanol, chlorphenamine maleate, paracetamol and vitamin C as raw material and adding proper auxiliary material through a certain preparation process. Said invention also provides the concrete steps of its preparation method.
Owner:GUIZHOU BAILING GRP PARMACEUTIAL CO LTD
Pharmaceutical composition for treating cold in children and preparation method thereof
InactiveCN102038704AEasy to understandOrganic active ingredientsInorganic active ingredientsSolubilityAllergic reaction
The invention provides a pharmaceutical composition for treating cold in children and a preparation method thereof. The pharmaceutical composition comprises the following components and medicine prepared in ratio: dextromethorphan hydrobromide, chlorphenamine maleate, ammonium chloride, seasoning agent, antiseptic and water, wherein the dextromethorphan hydrobromide is coated by beta-cyclodextrin and is prepared into preparation; the clathrate compound improves the mouthfeel and the water solubility of the dextromethorphan, and realizes delayed release effect so that, after being administrated, the dextromethorphan continuously develops functions of relieving cough in 2-6h, and the ammonium chloride is rapidly released for dispelling phlegm within 1h, thereby effectively solving the problem of antagonism between the dextromethorphan and the ammonium chloride. The pharmaceutical composition can be prepared into syrup, oral solution and drop which are easily accepted by the children. The pharmaceutical composition is simple in preparation technique, easy in production and operation, good in producing stability, reliable in product quality, sweet in taste, good in compliance, and acceptable by children; the dose is accurately measured according to age of the children; and the pharmaceutical composition can safely and rapidly eliminate or relieve cold symptoms of the children, such as cough, excessive and thick phlegm, pharyngalgia, rhinobyon, rhinorrhoea, sternutation and the like caused by infection of upper respiratory tract and allergic reaction.
Owner:BEIJING YIYUE MEDICAL TECH
Preparation method of compound capsule for treating cold
ActiveCN102349900AControl uptakeImprove efficacyOrganic active ingredientsAntipyreticChlorphenamine maleateBlood concentration
The invention relates to a preparation process of a compound capsule for treating cold. The compound capsule contains effective dose of active components, namely ibuprofen, pseudoephedrine hydrochloride and chlorphenamine maleate as well as a proper amount of pharmaceutic adjuvant. The preparation process comprises the following steps of: preparing partial effective dose of ibuprofen and pseudoephedrine hydrochloride into a sustained release preparation; preparing allowance of ibuprofen and effective dose of pseudoephedrine hydrochloride into a rapid release preparation; and preparing the sustained release preparation and the rapid release preparation into various medicinal preparations. According to the preparation process disclosed by the invention, a sustained release technology and a rapid release technology are adopted to effectively control the absorption action of three different medical active components in the body, and thus the blood concentrations of the three different medical active components achieve the effective treatment concentration which is consistent with the disappeared time, further the medical effect of the compound medicament is synergically enhanced and the bioavailability of the compound medicament disclosed by the invention is improved.
Owner:TIANSHENG PHARMA GROUP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com